Symptom Profile and Etiology of Delirium in a Referral Population in Northern India: Factor Analysis of the DRS–R98
Abstract
Delirium is understudied in developing countries, where there tends to be a lower proportion of older persons and comorbid dementia. The authors assessed 100 consecutive cases of DSM-IV delirium (patients' mean age: 44.4 [standard deviation: 19.4] years; mean DRS–R98 score: 25.6 [3.6]) referred to an adult Consultation–Liaison Psychiatry service in Northern India. Disturbances of attention, orientation, visuospatial ability, and sleep disturbance were the most frequent symptoms, followed by language, thought-process abnormality, and motor agitation. A three-factor solution was identified, representing domains for cognition, higher-order thinking, and circadian rhythm/psychosis. These domains can guide studies addressing the relationship between symptom profile, therapeutic needs, and outcomes and are consistent with core domains previously identified in other countries.
Delirium is a complex, acute, and frequently reversible impairment of consciousness, occurring in response to one or more physiological and/or pharmacological etiological insults. It occurs in approximately one in five hospitalized patients1 and is independently associated with poor outcomes in the form of functional decline, prolonged hospitalization, increased need for institutional care, and elevated mortality and healthcare costs.2 Despite its high prevalence, delirium is often underrecognized and undertreated because of the complex nature of its symptoms, including their variability and fluctuation over time.2 The phenomenological profile of delirium varies somewhat across clinical populations and healthcare settings,3 but recent studies suggest that “core” symptoms (attention deficits, sleep–wake cycle disturbance, motor-activity changes, language and thought disturbances) occur more consistently than other, less-common symptoms (psychosis, affective changes).3,4 Severity of inattention correlates highly with other common features4 and is a cardinal and required symptom of delirium in diagnostic systems such as DSM and ICD. The interrelationship between the various symptoms of delirium has been studied using factor analysis, but most studies have used the Delirium Rating Scale (DRS) or Memorial Delirium Rating Scale (MDAS).5–13 One report used the Delirium Rating Scale, Revised-98 (DRS–R98), which was designed specifically for detailed phenomenological research.14
Studies from developed countries emphasize older populations from geriatric and palliative-care settings where particular etiologies and confounding effects of comorbid dementia are prominent. As such, these findings may not generalize to non-Western populations, where delirium is more common in younger subjects and has different etiological underpinnings.15 Studies from developing countries are few, even though delirium is a common diagnosis among psychiatric referrals.15,16 With this background in mind, we studied the phenomenology and etiological underpinnings of delirium occurring in a large teaching hospital in Northern India by use of standard assessment methods.
METHOD
Subjects and Design
This cross-sectional study was carried out at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, a multispecialty, tertiary-care, teaching hospital that provides services to a population of approximately 40 million persons in Northern India and that includes Union Territory of Chandigarh and the states of Punjab, Haryana, Himachal Bradesh, Jammu and Kashmir, and Rajasthan. The Department of Psychiatry provides a 24-hour Consultation–Liaison psychiatry service to all the wards and the emergency service of the hospital.
Patients consecutively referred (N=420) to the Consultation–Liaison (C/L) team between July and December 2009 who met DSM-IV criteria for delirium17 were recruited for research evaluation; 141 patients (35% of the total referrals) were diagnosed as having DSM-IV delirium without dementia or other Axis I psychiatric disorders (except substance-abuse disorders). Of those referred for research evaluation, 41 are not included in this report: 4 were under 16 years old; 10 did not meet the DRS–R98 cutoff score criterion; 8 caregivers did not give consent; 10 patients were receiving care in the intensive-care setting and were unable to cooperate with assessments; and 9 patients had left the hospital before undergoing DRS–R98 assessment. Delirium patients were evaluated by a trained research psychiatrist (KC), using the Delirium Rating Scale, Revised-98 (DRS–R98).18
The procedures and rationale for the study were explained to all patients and their families, but, because many patients had cognitive impairment at entry into the study, it was presumed that most were not capable of giving informed written consent. Because of the non-invasive nature of the study, ethics committee approval was given to augment patient assent with proxy consent from next of kin (where possible) or a responsible caregiver, in accordance with the Helsinki Guidelines for Medical Research involving human subjects.19
Sociodemographic variables and other medical information were collected by use of standardized data-collection forms. Assessments were based on all available information obtained from the patients, caregivers, medical staff, and medical records.
Cases were included if patients met DSM-IV criteria for delirium and also scored above diagnostic cutoff scores for delirium per the DRS–R98, because DSM-IV criteria are less stringent for full syndromal delirium than DSM-II-R or ICD-10.20,21 Subjects with a documented history of dementia and other Axis I psychiatric disorders (except substance-use disorders) were excluded. According to local practice, pharmacotherapy is usually initiated after psychiatric assessment, and, therefore, this group had received minimal previous exposure to psychotropic agents used to treat delirium.
Assessment Tools
The Delirium Rating Scale, Revised-98 (DRS–R98)18 is designed for broad phenomenological assessment of delirium. It is a 16-item scale, with 13 severity and 3 diagnostic items, with high interrater reliability, sensitivity, and specificity for detecting delirium in mixed neuropsychiatric and other hospital populations. Each item is rated 0 (Absent/Normal) to 3 (Severe Impairment), with descriptions anchoring each severity level. Severity scale scores range from 0 to 39, with higher scores indicating more severe delirium. Delirium typically involves scores above 15 points (Severity scale) or 18 points (Total scale), when dementia is in the differential diagnosis. It has good interrater reliability, validity, sensitivity, and specificity for distinguishing delirium in mixed neuropsychiatric populations that include dementia, depression, and schizophrenia.18
The Delirium Etiology Checklist (DEC)22 was used to standardize attribution of etiology on the basis of all available clinical information into 12 categories of etiological causation, each rated on a 5-point scale for degree of attribution to the delirium episode, ranging from “ruled out/not present/not relevant” (0) to “definite cause”,4 as follows: drug intoxication/drug withdrawal; metabolic/endocrine disturbance; traumatic brain injury; seizures; infection (intracranial); infection (systemic); neoplasm (intracranial); neoplasm (systemic); cerebrovascular; organ insufficiency; other CNS; and other systemic causes. This system allows for the documentation of the presence and suspected role for multiple potential causes of delirium and provides more information specifically relevant to delirium than a listing of current medical conditions.
Data Analysis
Data were analyzed with SPSS Version 14. Descriptive data were described as frequencies, percentage, mean, and standard deviation (SD). Factor analysis was carried out for DRS–R98 item data by principal-components analysis, orthogonal rotation, and varimax technique, so as to identify the minimum number of factors explaining the maximum variance in the data. On the basis of the delirium literature evidence,6 we decided in advance to generate two- and three-factor models. To render the extracted factors meaningful and interpretable, only items with loadings of ≥0.4 and factors with eigenvalue of >1 were identified as significant. The items loading at ≥0.40 on two or more factors were assigned to the factor where their loading was the highest, although all values are shown.
RESULTS
Demographic and Clinical Profiles
We assessed 100 patients with DSM-IV delirium who also met the DRS–R98 cutoff for syndromal delirium. There was a predominance of men (78%). Age range was 16–90 years (mean age: 44.4 [19.4]), with a majority (82%) of subjects younger than age 65. Years of education ranged from 0–20 years, with a mean of 9.2 (4.9). The estimated duration of delirium before assessment ranged from 6 hours to 20 days, with a mean of 2.9 (2.8) days. There was a similar representation of referrals from medical (51%) and surgical wards (49%). At the time of assessment, the number of medications received ranged from 0 to 9, with a mean of 4.11 (1.7) per patient; 13 subjects were already receiving psychotropic medications to treat delirium, with 6 taking benzodiazepines; 4, antipsychotics; and 3, both benzodiazepines and antipsychotics.
In almost all cases, the delirium could be attributed to a definite etiology (92%), with a mean number of etiologies of 3.2 (1.0) per patient (range: 1–6). The frequency of etiological categories of delirium as measured on the DEC were (in descending order): endocrine-metabolic disturbance (N=70), infectious (N=45; CNS:11, and systemic: 34), organ failure (N=28), drug-related (N=29; intoxication: 7, withdrawal: 22), seizure-related (N=16), cerebrovascular (N=6), traumatic brain injury (N=5), and other (N=5).
Phenomenological Profile
The DRS–R98 Severity score was 21.0 (3.4), and the DRS–R98 Total score was 25.6 (3.6); range: 19–34. The ratings for individual DRS–R98 items are shown in Table 1. Mean Severity scores were highest for sleep–wake cycle, orientation, attention, short-term memory, and visuospatial ability; and next-highest for language, thought process, motor agitation, and affective lability.
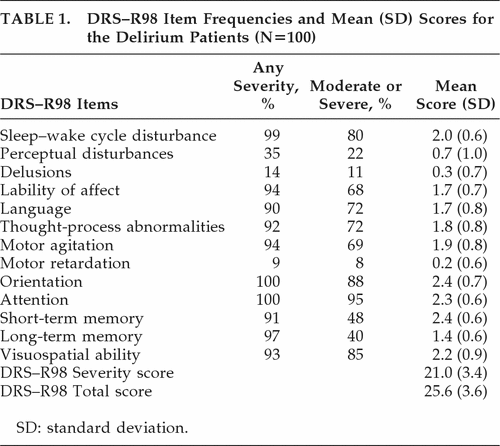 |
The most common delirium features present at any severity level were disturbances in orientation, attention, sleep, and long-term memory, lability of affect, motor agitation, visuospatial disturbance, thought-process abnormality, and language disturbance (90%–100%); and the least-common were perceptual abnormalities (35%) and delusions (14%). For Frequency, using a Severity cutoff score of ≥2 (moderate or severe), the most common symptoms were attention, orientation, visuospatial ability, and sleep disturbance (80%–95%), with language, thought-process abnormality, motor agitation, and lability of affect (68%–72%) moderately frequent, whereas long-term memory, perceptual abnormality, and delusions (11%–40%) were the least common. Thus, the frequencies generally paralleled the pattern for mean severities.
Factor Analysis
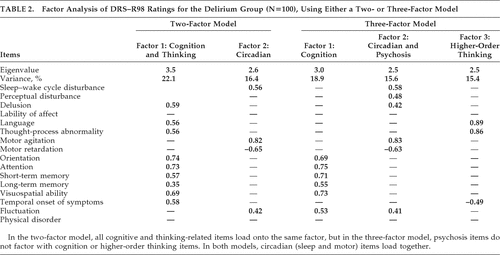
Principal-components analysis initially identified a five-factor solution accounting for 66% of the variance in DRS–R98 scores. These (items, eigenvalues, and variance) were the following: Factor 1 (cognitive symptoms, 3.68; 23%), Factor 2 (language and thought-process abnormality, 2.48; 15.53%); Factor 3 (motor agitation and retardation, 1.83; 11.45%); Factor 4 (sleep disturbance, perceptual abnormalities, and delusions, 1.21; 7.59%), Factor 5 (lability of affect, 1.08; 6.77%).
In order to identify a factor solution accounting for maximum variance with the simplest factor structure, we generated two- and three-factor solutions (Table 2). For both solutions, cognitive items loaded with each other and dominated the major factor. The main difference between the two models was that disturbances of higher-order thinking (language, thought-process abnormalities) occupied a separate factor in the three- factor solution but were subsumed into the Cognitive factor in the two-factor solution. In both solutions, motor items and sleep–wake cycle disturbances dominated a separate factor, although, in the three-factor model, they loaded with the two psychosis items. Overall, the three-factor solution was preferred because it accounted for a higher percentage of variance and was consistent with the literature and the previously proposed model of delirium comprising three core domains; that is, 1) generalized disturbance of cognition; 2) disorganization of higher-order thinking; and 3) alterations to circadian patterns involving sleep–wake cycle and motor activity.
 |
DISCUSSION
We studied the phenomenology of delirium occurring in general-hospital inpatients in a developing-country setting. We used standardized assessment methods, including validated tools for delirium phenomenological assessment. The population studied herein provides a unique insight into the clinical profile of delirium occurring in a relatively younger population, without the influence of comorbid dementia, and with limited previous exposure to delirium treatment with psychotropic agents, both of which can considerably affect delirium profile.
The frequency and severity of long-term memory impairment was lower than that reported in populations that include many elderly patients. The almost-exclusive motor presentation of hyperactivity probably reflects this being a referral population where patients with behavioral disturbance and, especially, agitation are most often referred, but also might reflect the predominance of men and younger persons. We found a predominance of symptoms of attention, visuospatial ability, orientation, sleep–wake cycle, thought-process, language, motor agitation, and lability of affect in our delirium cohort.
Studies of the delirium clinical profile, using well-validated tools in C/L settings, are relatively rare, with most work focusing on elderly medical and surgical or palliative-care populations.3 The frequency of delirium in this group was higher than in other studies, where delirium was noted in 10%–28% of referrals,23–25 but these studies may have been influenced by the fact that they were conducted in a newly-established C/L service23 or used retrospective methods to identify cases.25 Meagher26 reviewed motor-subtype patterns in delirium studies and highlighted the occurrence of relative hyperactivity of clinical presentation in C/L referral versus elderly medical, palliative-care, and ICU populations.
Previous studies suggest that the clinical presentation of delirium is largely similar, but may have subtle difference in pediatric populations. Turkel et al.27 reported that children experience a similar range of symptoms as adults, but with less-frequent delusions and greater symptom fluctuation, sleep–wake cycle disturbance, affective lability, and agitation, although this finding needs to be interpreted with caution because of study-design limitations and comparisons with the adult literature. Leentjens et al.28 found more severe cognitive symptoms in geriatric delirium, as compared with adults, and more severe hallucinations, delusions, sleep–wake cycle disturbance, and lability of mood in pediatric age-groups. Our work in a relatively young adult/late adolescent population with delirium identified a relatively low frequency of overt psychosis, as has been reported across most adult studies, but with prominent affective lability and motor agitation. The reason for this difference is not discernible in our study design, which studied a referral sample and requires an incidence design.
This study also highlights the frequency of disturbances to higher-order thinking, as evidenced by the finding that 93% of patients had evidence of either disturbed language or thought-processes measured on the DRS–R98. This concurs with findings from recent studies in other populations4,14,29 that such disturbances are key elements of delirium and should be included in diagnostic criteria. This consistent expression of thought-process abnormalities suggests that the de-emphasis of disorganized thinking as a diagnostic criterion between DSM-III-R and DSM-IV may warrant reexamination during the development of delirium criteria for DSM-V and/or ICD−11.30 However, any reintroduction of disorganized thinking as a key criterion would require the development of more reliable ways of assessment for nonpsychiatrists, given that this was a key reason for its removal after DSM-III-R.31
Factor-analysis reports to-date mostly used the original DRS and/or MDAS, and mostly identified two- or three-factor solutions, typically with a composite cognitive and one-or-more neurobehavioral factors.6–14 Our findings are not inconsistent with these previous reports, but the DRS–R98 offers advantages for broader phenomenological assessment and delineated a three-factor model that parallels the proposed three core domains of delirium: attention, circadian, and higher-level thinking.4,14,32 Only one previous study14 has reported the factor structure of delirium phenomenology using the DRS–R98, performed in a Columbian general-hospital inpatient population, and identified a two-factor solution, but this study included a comorbid population with relatively smaller number of patients with full syndromal delirium.
Although recent work has reemphasized the central role of attentional disturbance in delirium,4,33 this study affirms the prominence of circadian disruptions of sleep–wake cycle and motor activity, as well as higher-order thinking. This can assist in the development of descriptions that are representative of the breadth of the syndrome of delirium, while also allowing for more consistent detection in clinical practice. The presence of three core and discrete symptom clusters contrasts with the current DSM-IV definition of delirium, which emphasizes attentional disturbance in the context of acute onset and fluctuating course, but does not require circadian and higher-level thinking impairments. This report supports previous findings that these other two domains are core and need to be considered in delirium.
Limitations
Although this population had minimal exposure to psychotropic medications to manage delirium, we were not able to control for beneficial or adverse effects of medications prescribed for this medically ill population on the phenomenology of delirium. We excluded dementia based on existing diagnosis, although not with a formal tool, although very few patients were in a vulnerable age-range for common degenerative dementias.
CONCLUSIONS
This study describes delirium phenomenology occurring in a relatively young patient group from a developing country. This work highlights the complexity of symptom-profile in delirium, where attentional and other cognitive disturbances are accompanied by a high frequency of disturbances to sleep–wake cycle, motor activity, and higher-order thinking—all of which are not well represented in DSM-IV diagnostic criteria. The DRS–R98 allows for uniquely comprehensive evaluation of a wide breadth of disturbances that occur in delirium, and principal-components analysis of these data delineate a three-factor solution supportive of a previously-proposed three-core domain model for delirium phenotype. These core domains may have different neuroanatomical underpinnings and can guide future research addressing the relationship between symptom-profile, endophenotype, genotype, therapeutic management, and outcomes for this complex and serious neuropsychiatric syndrome.
1. : Occurrence and outcome of delirium in medical inpatients: a systematic literature review. Age Ageing 2006; 35:350–364Crossref, Medline, Google Scholar
2. : Delirium, in: Textbook of Psychosomatic Medicine, 2nd Edition. Edited by Levenson JL. Washington, DC, American Psychiatric Press, Inc., 2010Google Scholar
3. : Delirium phenomenology: what can we learn from the symptoms of delirium? J Psychosom Res 2008; 65:215–222Crossref, Medline, Google Scholar
4. : Phenomenology of 100 consecutive adult cases of delirium. Br J Psychiatry 2007; 190:135–142Crossref, Medline, Google Scholar
5. : Delirium after cardiac surgery: a prospective study. Ph.D. thesis, Erasmus University, Rotterdam, Benecke Consultants, Amsterdam, Netherlands, 1994Google Scholar
6. : Further analyses of the Delirium Rating Scale. Gen Hosp Psychiatry 1995; 17:75–79Crossref, Medline, Google Scholar
7. : Is delirium different when it occurs in dementia? a study using the Delirium Rating Scale. J Neuropsychiatry Clin Neurosci 1998; 10:199–204Link, Google Scholar
8. : Clinical utility, factor analysis, and further validation of the Memorial Delirium Assessment Scale in patients with advanced cancer. Cancer 2000; 88:2859–2867Crossref, Medline, Google Scholar
9. : Assessing delirium in cancer patients: the Italian versions of the Delirium Rating Scale and the Memorial Delirium Assessment Scale. J Pain Symptom Manage 2001; 21:59–68Crossref, Medline, Google Scholar
10. : How do delirium and dementia increase length of stay of elderly general medical inpatients? Psychosomatics 2004; 45:235–242Crossref, Medline, Google Scholar
11. : Delirium-O-Meter: a nurses' rating scale for monitoring delirium severity in geriatric patients. Int J Geriatr Psychiatry 2005; 20:1158–1166Crossref, Medline, Google Scholar
12. : Clinical presentation of delirium in patients undergoing hematopoietic stem cell transplantation. Cancer 2005; 103:810–820Crossref, Medline, Google Scholar
13. : Validation of the Memorial Delirium Assessment Scale. J Crit Care 2009; 24:530–534; doi:10.1016/j.jcrc.2008.12.016Google Scholar
14. : Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised-98. Psychosomatics 2009; 50:255–262Crossref, Medline, Google Scholar
15. : Prevalence and clinical profile of delirium: a study from a tertiary-care hospital in north India. General Hosp Psychiatry 2009; 31:25–29Crossref, Medline, Google Scholar
16. : Psychiatric profiles in medical-surgical populations: need for a focused approach to consultation–liaison psychiatry in developing countries. Indian J Psychiatry 1998; 40:224–230Medline, Google Scholar
17.
18. : Validation of the Delirium Rating Scale, Revised '98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–241Link, Google Scholar
19.
20. : The impact of different diagnostic criteria on prevalence rates for delirium. Dementia Geriatr Cogn Disord 2003; 16:156–162Crossref, Medline, Google Scholar
21. : An empirical study of different diagnostic criteria for delirium among elderly medical inpatients. J Neuropsychiatry Clin Neurosci 2003; 15:200–207Link, Google Scholar
22. : Neuropsychiatric aspects of delirium, in American Psychiatric Association Textbook of Neuropsychiatry, 5th Edition. Edited by Yudofsky SHales R. Washington, DC, American Psychiatric Publishing, 2007, chap. 15Google Scholar
23. : Delirium: 100 cases. Can J Psychiatry 1988; 33:375–378Crossref, Medline, Google Scholar
24. : Delirium in a general-hospital psychiatric consultation service. Int Med J 1997; 4:181–185Google Scholar
25. : Prevalence of psychotic symptoms in delirium. Psychosomatics 2000; 41:519–522Crossref, Medline, Google Scholar
26. : Motor subtypes of delirium: past, present, and future. Int Rev Psychiatry 2009; 21:59–73Crossref, Medline, Google Scholar
27. : Comparison of delirium in adults and children. Psychosomatics 2006; 47:320–324Crossref, Medline, Google Scholar
28. : A comparison of the phenomenology of paediatric, adult, and geriatric delirium. J Psychosom Med 2008; 64:219–223Crossref, Medline, Google Scholar
29. . Neuropsychiatric and cognitive profile of patients with DSM-IV delirium referred to an old-age psychiatry consultation–liaison service. Int Psychogeriatr 2011; 23:1167–1174Crossref, Medline, Google Scholar
30. : Defining delirium for the International Classification of Diseases, 11th Revision. J Psychosom Res 2008; 65:207–214Crossref, Medline, Google Scholar
31. : Delirium: background papers for DSM-IV. J Neuropsychiatry Clin Neurosci 1993; 5:154–160Link, Google Scholar
32. : Delirium, in Oxford Textbook of Psychiatry, 2nd Edition. Edited by Gelder MAndreasen NLopez I. Oxford, UK, Oxford University Press, 2009, chap. 4.1.1, pp 325–332Google Scholar



