Effects of Sleep Deprivation on Nocturnal Cytokine Concentrations in Depressed Patients and Healthy Control Subjects
Abstract
Previous studies have reported alterations of cytokine and cytokine-receptor concentrations in psychiatric patient populations, including patients with major depressive disorder (MDD). However, study results are conflicting, and possible causes for these abnormalities are unknown. Since sleep deprivation may induce a rapid improvement of mood in depressed patients, the authors investigated the impact of total sleep deprivation (TSD) for one night, and subsequent recovery sleep, on nocturnal concentrations of interleukin-6 (IL-6), interleukin-1-receptor antagonist (IL-1RA), and soluble IL-2 receptor (sIL-2R) in 15 unmedicated patients with MDD and 16 healthy volunteers. Whereas IL-6 levels normalized again during the recovery night in depressed patients, they were still elevated in control subjects. Serum levels of IL-1RA were higher in depressed patients than in controls, but were not affected by TSD. During recovery sleep, IL-1RA levels increased as compared with the preceding TSD night only in controls. Responders (N=8) differed from nonresponders (N=7) to TSD with regard to IL-1RA, which increased significantly during TSD in responders only. Sleep deprivation therefore seems to significantly affect cytokine levels in both depressed patients and healthy subjects, but does so in different ways. Sleep disturbances in depressed patients could account for the increased levels of cytokines found in these patients in several previous studies. The interaction between antidepressant effects of TSD and alterations of cytokines warrants further investigation.
There is increasing evidence that immune parameters are altered in different psychiatric patient populations, including patients with major depressive disorder (MDD). Such alterations in patients with MDD include functional changes of cellular immune parameters, such as neutrophil and monocyte phagocytosis and cell number of neutrophils and lymphocytes,1 as well as changes in the concentrations of different cytokines and cytokine-receptors.1–3 Among these, interleukin-6 (IL-6) has been examined under several experimental conditions in depressed patients, but results are conflicting. Whereas several studies reported increased concentrations of IL-6 in depressed patients,4–8 others did not find different levels in depressed patients and control subjects9 or found decreased levels in stimulated peripheral blood mononuclear cells.10–12 A recent meta-analysis13 that reviewed all relevant articles published before 2008, found a positive association between levels of IL-1, IL-6, and depression. Inconsistent results were also found for serum concentrations of the soluble receptor for IL-2 (sIL-2R): Some studies reported increased levels4,5,9,14 whereas another study found decreased levels,15 and two others reported no change as compared with normal controls.8,15 Two studies investigated serum levels of the IL-1 receptor antagonist (IL-1RA) in depressed patients: One study found increased levels,7 whereas another study found no alteration.11 A recent study16 found increased IL-1RA levels in a large sample of depressed patients, as compared with healthy controls, and attributed this to an endogenous protection process against depression.
There is increasing evidence that sleep deprivation may affect immune functioning.2,17 Most studies have investigated the effect of partial or total sleep deprivation (TSD) on cytokine levels in healthy subjects and found increased levels of IL-618–21 and IL-1RA22 in response to one-or-more nights of sleep deprivation. However, these results are also conflicting, as Frey et al. found decreased IL-6 levels after 40 hours of total sleep deprivation in healthy subjects.22
Until now, no study has investigated nocturnal cytokine synthesis in patients with MDD in response to TSD. Interestingly, however, increased IL-6 levels on the day before a night of TSD and a night of sleep-phase advance predicted a worse outcome.23 This study is in line with others demonstrating a negative predictive effect of increased IL-624 and increased IL-2R concentrations25 with respect to efficacy of antidepressant treatment.
In the present study, we investigated the effect of one night of TSD, which has been shown to exert potent, albeit short-lasting antidepressant effects,26,27 on the nocturnal synthesis of IL-6, IL-1RA, and IL-2R (inflammatory mediators altered in depressed patients and by sleep deprivation) in 15 unmedicated depressed patients. All had 5 nights in the sleep laboratory, with repetitive blood-drawings during the pre–sleep-deprivation night, the night of TSD, and the following recovery night. A comparison with 16 healthy volunteers gave us the opportunity to investigate whether possible changes in levels of IL-6, IL-1RA, and sIL-2R are a specific response of depressed patients or a rather unspecific response to TSD seen in both patients and healthy volunteers.
Method
Subjects
A group of 19 drug-free patients with MDD according to the Structured Clinical Interview for DSM-IV were recruited for the study; 4 patients dropped out, 1 because of problems with the blood-drawing procedure and 2 because of exclusion criteria that had not been recognized at the time of inclusion. One patient withdrew her consent during the study, before the TSD night. Another patient had a mood deterioration on the morning after the TSD night, and therefore this patient was prematurely retired from the study. Her available cytokine data were included in the analysis. The 16 patients (10 women, 6 men) had a mean age of 34 (SD: 13) years and a mean body mass index (BMI) of 22.6 (SD: 3.8) kg/m2. Among the patients, 40% (6 of 16 patients) were smokers (daily cigarettes >10, except in 1 patient). A group of 17 healthy subjects with no personal or family history of psychiatric disorders was recruited. One subject dropped out because of problems with the blood-drawing procedure. The remaining subjects (11 women, 5 men) had a mean age of 27 (SD: 8) years and a mean body mass index of 22.4 (SD: 1.7) kg/m2; 4 out of 16 subjects were smokers, all of them moderate (<10 cigarettes per day). There were no differences between the groups regarding age, sex, or BMI. The smoker/nonsmoker ratio did not differ significantly between patients and controls. Controls were screened for depressive disorder with the Beck Depression Inventory (BDI) and Hamilton Rating Scale for Depression (Ham-D) questionnaires, but showed no clinical levels. The mean Pittsburgh Sleep Quality Index (PSQI) was 11.9 (SD: 4) for depressed patients and 3.3 (SD: 1.3) in healthy controls (p=0.002). During the study, smoking was not allowed. Exclusion criteria for participation were significant medical disorders or infectious diseases, which were ruled out by a careful physical examination, ECG, EEG, and routine laboratory parameters, including a drug screening test. Additional exclusion criteria were a history of alcohol or drug abuse and any intake of psychotropic medication or anti-inflammatory drugs within 7 days before the investigation, except contraceptives in the group of depressed patients. The drug-free interval had been longer than 4 weeks in all but 1 patient, who stopped doxepine intake 7 days before the study. Restless legs syndrome and sleep apnea syndrome were ruled out by interview and by polysomnography in all subjects and patients. The study was performed according to the Declaration of Helsinki, and the study protocol was approved by the ethical committee of the University of Freiburg. Informed consent was obtained from all subjects and patients before their participation in the study.
Sleep Deprivation Protocol
All subjects spent 5 nights in our sleep laboratory (Figure 1). Nights 1 and 2 served for adaptation to the laboratory conditions. During Nights 3, 4, and 5, blood was sampled every half-hour, beginning at 9:30 P.M., until 8:30 A.M. the next day. Blood samples were obtained through an in-dwelling catheter, which, was placed in a forearm vein 3 hours before the beginning of blood sampling every evening and perfused by isotonic saline at a low speed (25 ml/hr). Blood samples were taken from an adjoining room through a long polyethylene tubing (total amount per night ∼110 ml). At the end of blood sampling in the morning after Nights 3, 4, and 5, the i.v. catheter was removed. In the evening, a new catheter was inserted, in order to avoid local inflammation and increase of cytokines due to the catheter.

A total sleep deprivation (TSD) was performed during Night 4; that is, subjects and patients stayed awake from 7:00 A.M. the morning after Night 3, until 11:00 P.M. the next evening. From the evening of Day 3 until the morning after the recovery night, subjects stayed in the research ward. During the 40 hours of TSD, subjects were strictly advised not to sleep, and, therefore, they were constantly observed by a team of three research fellows. No major physical activities were allowed. Subjects and patients were allowed to remain in a semi-supine position in bed or to sit in a comfortable armchair and were allowed to read or watch TV.
Polysomnographic Assessments
Sleep was recorded by standardized polysomnography for 2 nights before the beginning of the neuroendocrine protocol for adaptation to the sleep laboratory (Night 1) and to get baseline sleep parameters (Night 2), as well as during the catheter Nights 3 and 5 (for the method of polysomnography in our sleep laboratory see Voderholzer et al.28,29).
Psychometric Measurements
Before inclusion into the study, participants completed a 21-item Hamilton Rating Scale for Depression (Ham-D). During Study Days 3, 4, 5, and 6, that is, before and after the night with TSD, a modified Ham-D, with 6 items (Ham-D-630), was filled out twice daily, in the morning and in the evening. Responder status to TSD was defined as a ≥30% reduction of the mean values of morning and evening Ham-D-6 scores after the night of TSD.
Determination of IL-6, IL-1RA, IL-2R, and Cortisol Concentrations
We have chosen to determine IL-6, IL-1RA, IL-2R, and cortisol because of the various studies reporting alteration of those cytokines in depressed patients. IL-1RA and IL-2R were used instead of the respective ligands IL-1 and IL-2, since those cytokines are only occurring in low levels in plasma or serum, approaching the detection level of our ELISA, and thus not providing reliable results. IL-6 was directly measured every 2 hours between 10:00 P.M. and 8:00 A.M. by an ultrasensitive ELISA (GE Healthcare) with a lower detection limit of 0.1 pg/ml. The intra-assay variability is 6.1%; the interassay variability, 19.6%. IL-1RA was determined with an immunoassay by R&D Systems in three samples per night (first sample period: 10:30 P.M.–1:00 A.M.; second sample period: 1:30 A.M. –4:00 A.M.; third sample period: 4:30 A.M. –7:00 A.M., respectively). The lower detection limit is 20 pg/ml; the intra-assay variability ranges between 3.1% and 6.2%; the interassay variability, between 5.0% and 6.7%. Concentrations of sIL-2R were measured by a high-sensitivity ELISA (Immunotech), with a lower detection limit of 5 pMol, in three samples per night, as for IL-1RA. The intra-assay variability ranged between 4.9% and 6.6%; the interassay variability, between 10.6% and 15.1%. Cortisol levels were measured with an immunometric assay (Nichols Institute Diagnostics). All measurements were made in duplicate. Because of the comparatively high interassay variability, all samples of one subject or patient were measured using one ELISA plate.
Statistical Analysis
Results are expressed as mean (standard deviation [SD]). Nocturnal concentrations of IL-6, IL-1RA, and sIL-2R were analyzed by calculating the mean values of all samples of each night and then analyzed using linear mixed-effects analysis with the linear model GROUP*NIGHT. Random effects were modeled as one constant term per subject (repeated-measures design); p values ≤0.05 were considered to be significant. In the case of a significant effect by ANOVA, confirmatory t-tests with Bonferroni adjustment for multiple comparisons were calculated. To evaluate the impact of the clinical response to TSD in depressed patients, the impact of TSD on cytokines was evaluated in Responders and Nonresponders to TSD. For response analysis, repeated-measures 3×3 ANOVA, with the factors Time (baseline, sleep deprivation, recovery night) and Response (responder, nonresponder, healthy control) was calculated.
Results
IL-6
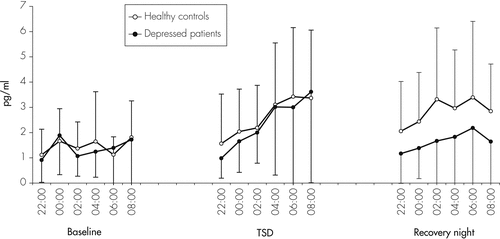
Figure 2 shows IL-6 concentrations in patients and controls at single time-points during the baseline night, the TSD night, as well as the recovery night, and Table 1, the respective mean values per night. As calculated by ANOVA, there was a significant effect for Night, but not for Group or Group × Night interaction (Table 1). In the whole group, during the night of TSD, IL-6 markedly increased with a significant, more than 1.5-fold increase of mean levels as compared with the baseline night (p=0.008). During the recovery night, IL-6 levels increased significantly as compared with the baseline night (p=0.017). In depressed patients, IL-6 showed little variability during the baseline night (Figure 2). A similar increase of IL-6 was found in the group of healthy subjects (Figure 2), with higher mean IL-6 levels on the night of TSD, as compared with the baseline night. Baseline IL-6 was significantly correlated with poor sleep quality in healthy controls (r = −0.76; p=0.01), but not in depressed patients.
| Group | Night 3: Baseline | Night 4: Sleep Deprivation | Night 5: Recovery Sleep | ANOVA | Post-Hoc Tests | |||
|---|---|---|---|---|---|---|---|---|
| Night | Group | Group × Night | ||||||
| IL-6 | Controls | 1.50 (1.10) | 2.56 (1.63) | 2.82 (1.94) | df=2 | df=1 | df=2 | Night 3–Night 4: p=0.008 |
| 10:00 P.M. – | N=15 | F=5.99 | F=1.74 | F=1.18 | Night 3–Night 5: p=0.017 | |||
| 8:00 A.M. | Depressed | 1.14 (0.69) | 2.38 (1.87) | 1.62 (1.87) | p=0.005 | NS | NS | |
| pg/ml | N=13 | |||||||
| IL-1RA | Controls | 189.01 (92.12) | 201.57 (98.25) | 260.12 (90.56) | df=2 | df=1 | df=2 | Night 3–Night 4: NS |
| 10:00 P.M.– | N=16 | F=6.70 | F=16.73 | F=2.93 | Night 3–Night 5: p=0.006 | |||
| 8:00 A.M. | Depressed | 352.03 (128.81) | 376.67 (110.12) | 381.98 (149.89) | p=0.002 | p=0.000 | p=0.061 | |
| pg/ml | N=14 | |||||||
| sIL-2R | Controls | 29.01 (11.51) | 31.51 (13.48) | 30.94 (12.29) | df=2 | df=1 | df=2 | Night 3–Night 4: p=0.007 |
| 10:00 P.M. – | N=15 | F=6.60 | F=1.17 | F=0.07 | Night 3–Night 5: p=0.062 | |||
| 8:00 A.M. | Depressed | 34.73 (11.55) | 37.22 (13.52) | 36.17 (12.86) | p=0.003 | NS | NS | |
| pg/ml | N=10 | |||||||
TABLE 1. Nocturnal Mean (SD) of IL-6, IL-1RAa, and IL-2Rb in Depressed Patients and Controlsc on 3 Consecutive Nights
Cortisol
During TSD, cortisol levels increased significantly in both the depressed patients and in the control group (p=0.0032). Cortisol levels normalized during recovery sleep and returned to baseline levels. No significant differences could be found between Responders and Nonresponders.
IL-1RA
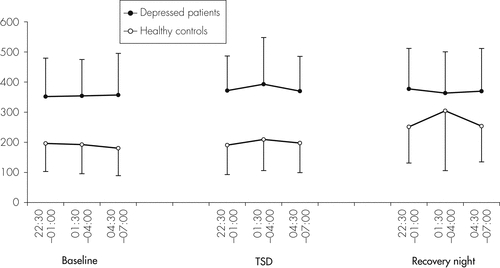
Figure 3 provides IL-1RA serum concentrations of patients and controls at single time-points during the baseline night, the TSD night, as well as the recovery night; Table 1 shows the respective mean values per night. As shown by ANOVA, there were significant main effects for Night (p=0.004) and Group (p<0.001), but only a trend for a Group × Night interaction. In depressed patients, serum IL-1RA levels showed little variability during the baseline night and were not changed in the TSD night and the recovery night as compared with baseline (Figure 3). Similar to the findings in depressed patients, serum IL-1RA levels in controls showed little variability during the baseline night and were not changed in the TSD night (Figure 3). However, in the whole group, during the recovery night, IL-1RA levels increased significantly and were higher as compared with the baseline night (p=0.006). ANOVA detected a significant group effect, and descriptive statistics showed that IL-1RA concentrations were significantly higher in the depressed patients than in the controls on all three nights (p=0.000).
Soluble IL-2R
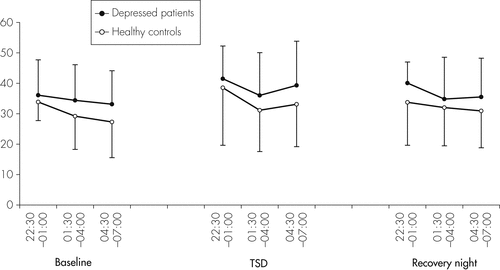
Figure 4 shows sIL-2R serum concentrations of patients and controls at single time-points during the baseline night, the night of TSD, and the recovery night, and Table 1 shows the respective mean values. As shown by ANOVA, there was a significant effect for Night (p=0.003), but not for Group (p=0.291) or Group × Night (p=0.930) interaction (Table 1). sIL-2R levels were higher during TSD as compared with baseline in both groups (p=0.007). During the recovery night, sIL-2R levels were still elevated as compared with the baseline night in both groups (p=0.062). There was a trend toward higher sIL-2R concentrations in depressed patients. This difference, however, was not significant.
Cytokines and Clinical Response to TSD
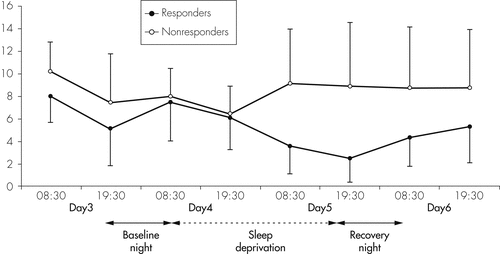
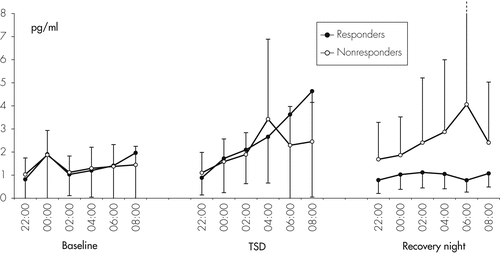
A group of 8 out of 15 depressed patients were responders to TSD; that is, they had a ≥30% reduction on their Ham-D the day after TSD. Figure 5 shows the course of the mean Ham-D 6-item values in Responders and Nonresponders. In controls, Ham-D scores did not change after TSD. At baseline, IL-6, IL-1RA, and sIL-2R were not significantly different between Responders and Nonresponders. We found a significant Time effect, but no Response (responders versus nonresponders versus healthy controls) and Time × Response effects for IL-6; significant effects for Time, Response, and Time × Response for IL-1RA; and a significant Time effect, but no Response and interaction effects for sIL-2R. When comparing total cytokine levels during the baseline and the TSD night in Responders and Nonresponders to TSD, no group differences were found for IL-6 and sIL-2R (Table 2 and Figure 6). However, a difference was found for IL-1RA, which increased significantly during TSD in the Responder, but not in the Nonresponder group (p=0.050). During the recovery night, IL-6 in Responders returned to baseline, whereas the Nonresponders had a nonsignificant trend toward higher concentrations during recovery sleep, as compared with baseline (Figure 6; Table 2). Sleep quality at baseline night was not significantly different between Responders and Nonresponders, and was not correlated with mood after TSD. No significant correlations were found between cytokine concentrations and Ham-D values before therapy or with the Ham-D values after TSD; that is, there was no evidence for a direct association between cytokine levels and treatment response.
| Responder to TSD (IL-6, N=7; IL-1-RA, N=8; sIl-2R, N=6) | Non-Responder to TSD (IL-6, N=6; IL-1-RA, N=6; sIl-2R, N=4) | ANOVA, p | Post-Hoc Tests | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Sleep Deprivation | Recovery Sleep | Baseline | Sleep Deprivation | Recovery Sleep | |||
| IL-6 | 0.95 (0.67) | 2.65 (2.35) | 0.98 (0.57) | 1.33 (0.71) | 2.10 (1.36) | 2.37 (2.55) | Time: p=0.011 | Baseline–Sleep deprivation: p=0.016 |
| pg/ml | Response: | Baseline–Recovery sleep: p=0.069 | ||||||
| NS | ||||||||
| Time × Response | ||||||||
| p=0.097 | ||||||||
| IL-1RA | 297.28 (111.96) | 345.87 (115.39) | 309.87 (112.97) | 414.60 (124.61) | 411.87 (100.25) | 478.13 (145.36) | Time: p=0.025 | Time: |
| pg/ml | Response: | Baseline–Sleep deprivation: NS | ||||||
| p=0.000 | Baseline–Recovery sleep: p=0.038 | |||||||
| Time × Response | Response: | |||||||
| p=0.042 | Responder–Nonresponder: p=0.050 | |||||||
| Responder–Healthy controls: p=0.068 | ||||||||
| Nonresponder–Healthy controls: p=0.000 | ||||||||
| sIL-2R | 31.67 (13.84) | 33.97 (15.92) | 33.41 (14.63) | 38.40 (7.94) | 41.11 (10.28) | 40.32 (10.06) | Time: p=0.012 | Baseline–Sleep deprivation: p=0.020 |
| pg/ml | Response: | Baseline–Recovery sleep: NS | ||||||
| p=0.381 | ||||||||
| Time × Response | ||||||||
| NS | ||||||||
TABLE 2. Impact of Total Sleep Deprivation of 1 Night on Cytokine Concentrations of Depressed Patients as Related to Antidepressant Response (Responders: N=8; Nonresponders: N=7)
Discussion
The main result of this study is a significant but complex impact of TSD on peripheral concentrations of cytokines in humans. We found a significant increase of IL-6 concentrations in both depressed patients and healthy controls during the night of TSD. With regard to IL-1RA, levels were higher in depressed patients than in controls, but did not significantly change during the three consecutive nights, whereas, in controls, IL-1RA levels increased significantly during the recovery night. This result is in line with a recent study16 that also found elevated levels of IL-1RA in depressed patients; sIL-2R concentrations were slightly but significantly higher during TSD in both groups.
A study investigating changes in cytokine levels in sleep-deprived animals showed results comparable to our study. After 36 hours of sleep deprivation, levels of IL-6 and IL-1RA were increased in mice.31 REM sleep deprivation over 72 hours led to elevated levels of IL-1, IL-1β, IL-6, IL-17A, and TNF-α in rats.32 The source of elevated cytokines seem to be mononuclear phagocytes, albeit few data exists on the cellular origin.33 Our results regarding cortisol are in line with several studies showing that sleep deprivation elevates cortisol levels.34
First of all, methodological aspects have to be discussed. Haack et al. reported that peripheral IL-6 concentrations rose within 10 hours in blood samples taken from the forearm where the catheter was placed, whereas control samples taken from the contralateral arm at identical time-points did not demonstrate such elevations.35 Therefore, increased cytokine levels during the night of TSD might be confounded by the blood-drawing procedure itself. However, in our study, a new i.v. catheter was inserted before each of the three consecutive nights, and a strong increase of IL-6 concentrations was only observed during the night of TSD, but not during the nights when subjects slept. Therefore, it seems unlikely that increased IL-6 concentrations during TSD are due to an increased local IL-6 synthesis because of the placement of an i.v. catheter. Secondly, several previous studies only measured single serum samples from each subject. Since serum concentrations of IL-1RA, IL-2R, and IL-6 are significantly affected by age, body mass index, gender, smoking habits, ongoing infectious diseases, or previous medication,11 and may show circadian secretion,36 inconsistent study results may well be due to incomplete matching of patients and controls according to these possibly confounding variables. In the present study, the matching as well the assessment of cytokine levels at several measurement points make it unlikely that the results are due to findings by chance.
Several previous studies investigated changes of cytokine and cytokine-receptor levels by partial sleep deprivation (PSD) or TSD in healthy volunteers. With respect to IL-6, our results are supportive of earlier studies demonstrating increased levels of IL-618–21 in response to one or more nights of sleep deprivation. In contrast, our findings are not in line with studies of Dinges and coworkers, who failed to find significant changes in IL-6 levels after short-term sleep deprivation,37 and Born et al., neither of whom found a significant change in IL-6 during TSD as compared with a control night.38 Frey et al. and Vgontzas et al. even found decreased IL-6 levels after total sleep deprivation in healthy subjects.25,39 The cause of the divergent results is unclear, but differences in methodology, especially the assessment of cytokine levels during day- or nighttime, may account for them. Redwine and colleagues investigated the effect of partial sleep deprivation (PSD) on IL-6 concentrations in healthy volunteers.40 They found a nocturnal increase of IL-6 after sleep onset, which was delayed after PSD until 3 A.M. Their interpretation of this finding was that loss of sleep may serve to decrease nocturnal IL-6 levels. However, their increase of IL-6 concentrations is similar to the increase seen in our study on the night of TSD, which might indicate that the increase in IL-6 levels is not the consequence of recovery sleep after PSD, but of the sleep deprivation period before. Recently, Vgontzas et al. reported that napping during daytime after sleep deprivation41 induced a decrease of IL-6 concentrations for several hours, which fits with our finding of an increase of IL-6 after sleep deprivation.
Contrary to IL-6, IL-1RA concentrations did not increase during one night of TSD. This is in contrast to recent studies showing increased levels of IL-1RA after 40 hours of total sleep deprivation.22 These studies, however, did assess cytokines during daytime, which makes a direct comparison with our study difficult. In the group of healthy subjects, but not in the depressed patients, IL-1RA concentrations were significantly increased during recovery sleep. Since IL-1RA belongs to the IL-1-receptor family, and IL-1RA concentrations correlate with those of IL-1, the increase of IL-1RA after 40 hours without sleep in our healthy subjects may fit with earlier studies demonstrating an increase of IL-1 during periods of sleep deprivation.37,42 Similarly, Born et al. found an increase of IL-1β-production during wakefulness, as compared with a control condition when subjects were allowed to sleep.38
A further finding of our study was a stimulatory influence of a night of TSD on sIL-2R concentrations. Born et al. measured the production of IL-2 during sleep and sleep deprivation and found significantly lower levels during sleep deprivation than in the sleep condition.38 Since sIL-2R and IL-2 are inversely related, our results of a stimulatory effect of sleep deprivation on sIL-2R might be explained by a suppressive effect of sleep deprivation on IL-2.
So far, there is, at least to our knowledge, only one previous study that investigated cytokine levels in relation to TSD in patients with MDD.23 In this study, increased IL-6 levels at the day before a night of TSD and a night of sleep-phase advance predicted a worse response to TSD in depressed patients. However, this study has many shortcomings, such as small sample size (N=10), single determinations of IL-6 levels, and unreliable ratings for treatment response. Therefore, these data can only be interpreted with caution. As far as we know, up to now, no other studies reported on the effect of sleep deprivation on cytokine levels in depressed patients. In our study, TSD led to a significant increase of IL-6 levels in unmedicated depressed patients that was reversed on the recovery night. This finding might be relevant with regard to several earlier reports on elevated IL-6 concentrations in depressed patients,4 postulating that an activation of the immune system with a consecutive activation of the HPA system might be a relevant patho-mechanism of depression. Our results, however, might also be consistent with the idea that elevated IL-6 levels are an epiphenomenon of disturbed sleep, which is usually a part of the depressive symptomatology. This may underline the possible role of disturbed sleep as the cause of increased IL-6 levels found in several studies and may also possibly underline the role of disturbed sleep as the primary pathogenetic factor for the development of depressive disorders, a view that is supported by several epidemiological studies.43
With respect to treatment response to TSD and cytokine levels, no statistically significant differences were found between nonresponders and responders for IL-6 and sIL-2R. There was, however, a nonsignificant trend to lower IL-6 levels on the recovery night in responders compared with nonresponders. Regarding IL-1RA, a significant increase was observed in the responder group, but not in the nonresponder group. Because of the small number of nonresponders and responders, however, these small effects should not be over-interpreted, and conclusions should not be drawn from these effects until they are confirmed by a study in a larger sample of responders and nonresponders.
The underlying mechanisms of increased cytokine concentrations in response to TSD are unknown. One explanation for the elevation of IL-6 might be an increase of catecholamine levels after sleep deprivation,38 since catecholamines have been shown to increase the production of cytokines,44–46 and exercise-induced release of epinephrine and norepinephrine correlates with increases in IL-6.47 Since sleep deprivation also has been shown to increase synthesis of IL-1,21,22,48 and IL-1 is able to stimulate IL-6 in various cell types, for example, as in the findings of Lieb et al., Bauer et al., and Helle et al.,49–51 the increased IL-6 levels during TSD might also be mediated by IL-1. However, in our studies, we were not able to detect IL-1 in our serum samples with our ELISA.
The possible functional consequences of increased cytokine levels during a night with sleep deprivation are unknown. Several issues should be discussed. It has been shown that cytokines such as interleukin-1 (IL-1) and interleukin-6 (IL-6) interact with the central nervous system by activating the hypothalamic-pituitary-adrenal axis (HPA axis).48,52,53 Furthermore, an activation of the HPA axis can induce increased production of IL-6 by itself and can maintain cortisol production from the adrenal glands even in the absence of ACTH.54 An activation of the HPA axis plays a key role for the response of the organism to stress, and the HPA axis is activated in acutely depressed patients. An increase of cytokines after disturbed sleep might therefore trigger an activation of the HPA axis and thus contribute to the increased risk of developing a depressive disorder after chronic insomnia.
Another aspect is the direct behavioral effects of cytokines, which induce a variety of central nervous effects, such as fever, apathy, fatigue, and sleepiness.55,56 It has been proposed that increased cytokine concentrations after sleep disruption might partly account for the fatigue in patients suffering from insomnia.39,57
With respect to the antidepressant effects of sleep deprivation in depressed patients, it seems contradictory that a night of acute sleep deprivation elevates IL-6 and cortisol34 but induces a transient improvement of depressive symptoms.26 However, acute sleep deprivation in depressive patients also causes a transient but almost complete normalization of disturbed sleep on the recovery night. Vgontzas et al. reported that, in healthy subjects, acute sleep deprivation reduces cortisol secretion the next day via an increase of slow-wave sleep and hypothesized that this might also be the mechanism through which sleep deprivation relieves depression temporarily.41 This indicates that the neurobiological effects of acute sleep loss and chronically disturbed sleep may not be the same. In a study of modest sleep restriction from 8 to 6 hours/day for 1 week, IL-6-secretion was increased, whereas peak cortisol secretion was decreased in 25 healthy volunteers.58 Accordingly, a study investigating a more severe sleep restriction to 1 hour/day for 1 week showed similar results, with increased IL-6 levels as a result of an increasing level of activity of leukocytes over the study period, but normal cortisol levels.59 These results show that chronic sleep deprivation leads to increased IL-6 levels, which are also seen in depressed patients.4–8 Inflammatory cytokines interfere with many pathological findings in depression, for example, glial degeneration, altered neurotransmitter release and uptake, neuroendocrine functioning, and neural plasticity.60–62 In summary, our study demonstrates that acute sleep loss may significantly influence peripheral concentrations of cytokines differently in healthy and depressed subjects. With regard to earlier findings in terms of elevated cytokine levels in unmedicated depressive patients, we assume that changes in cytokine levels accompanying psychiatric illness could well be secondary to a severe sleep disorder, rather than reflecting a primary pathogenic mechanism. Our results further indicate that elevated levels of cytokines such as IL-6 may partly explain fatigue and sleepiness occurring after sleep deprivation. The interaction between antidepressant effects of TSD and alterations of cytokines warrants further investigation.
1 : Inflammatory aspects of depression. Inflamm Allergy Drug Targets 2008; 7:19–23Crossref, Medline, Google Scholar
2 : Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 2005; 12:255–269Crossref, Medline, Google Scholar
3 : Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21:374–383Crossref, Medline, Google Scholar
4 : Immunoendocrine aspects of major depression: relationships between plasma interleukin-6 and soluble interleukin-2 receptor, prolactin and cortisol. Eur Arch Psychiatry Clin Neurosci 1995; 245:172–178Crossref, Medline, Google Scholar
5 : Indicators of immune activation in major depression. Psychiatry Res 1996; 64:161–167Crossref, Medline, Google Scholar
6 : Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci 1997; 247:228–233Crossref, Medline, Google Scholar
7 : Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 1997; 9:853–858Crossref, Medline, Google Scholar
8 : Immune-inflammatory markers in patients with seasonal affective disorder: effects of light therapy. J Affect Disord 2001; 63:27–34Crossref, Medline, Google Scholar
9 : Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord 1995; 34:301–309Crossref, Medline, Google Scholar
10 : Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 2007; 21:229–237Crossref, Medline, Google Scholar
11 : Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res 1999; 33:407–418Crossref, Medline, Google Scholar
12 : Plasma concentrations of interleukin-1beta, interleukin-6, soluble interleukin-2 receptor and tumor necrosis factor alpha of depressed patients in Japan. Neuropsychobiology 2001; 43:59–62Crossref, Medline, Google Scholar
13 : Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71:171–186Crossref, Medline, Google Scholar
14 : Cytokines and depression in cases with fibromyalgia. J Rheumatol 2002; 29:358–361Medline, Google Scholar
15 : Aberrant interleukin-2 receptor-mediated blastoformation of peripheral blood lymphocytes in a severe major depressive episode. Psychol Med 1998; 28:481–484Crossref, Medline, Google Scholar
16. : Depressive symptomatology is associated with decreased interleukin-1 beta and increased interleukin-1 receptor antagonist levels in males. Psychiatry Res 2009; 15;167(1-2):73–79Google Scholar
17 : Low levels of circulating inflammatory cytokines: do they affect human brain functions? Brain Behav Immun 2002; 16:525–532Crossref, Medline, Google Scholar
18 : Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 2006; 166:1756–1762Crossref, Medline, Google Scholar
19 : Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab 2004; 89:4409–4413Crossref, Medline, Google Scholar
20 : Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol 2001; 107:165–170Crossref, Medline, Google Scholar
21 : Seven days’ around the clock exhaustive physical exertion combined with energy depletion and sleep deprivation primes circulating leukocytes. Eur J Appl Physiol 2006; 97:151–157Crossref, Medline, Google Scholar
22 : The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun 2007; 21:1050–1057Crossref, Medline, Google Scholar
23 : Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26:1167–1170Crossref, Medline, Google Scholar
24 : Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22:370–379Crossref, Medline, Google Scholar
25 : Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol 2001; 11:203–208Crossref, Medline, Google Scholar
26 : The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry 1990; 147:14–21Crossref, Medline, Google Scholar
27 : Sleep deprivation in depression: what do we know, where do we go? Biol Psychiatry 1999; 46:445–453Crossref, Medline, Google Scholar
28 : Are there gender differences in objective and subjective sleep measures? a study of insomniacs and healthy controls. Depress Anxiety 2003; 17:162–172Crossref, Medline, Google Scholar
29 : Impact of experimentally induced serotonin deficiency by tryptophan depletion on sleep EEG in healthy subjects. Neuropsychopharmacology 1998; 18:112–124Crossref, Medline, Google Scholar
30 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
31 : Sleep-deprived mice show altered cytokine production manifest by perturbations in serum IL-1ra, TNFa, and IL-6 levels. Brain Behav Immun 2003; 17:498–504Crossref, Medline, Google Scholar
32 : REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interferon Cytokine Res 2009; 29:393–398Crossref, Medline, Google Scholar
33 : Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulines as responses to sleep deprivation in laboratory rats. Am J Physiol Regul Integr Comp Physiol 2005; 289:1054–1063Crossref, Google Scholar
34 : Impact of sleep deprivation and subsequent recovery sleep on cortisol in unmedicated depressed patients. Am J Psychiatry 2004; 161:1404–1410Crossref, Medline, Google Scholar
35 : Diurnal variations of interleukin-6 plasma levels are confounded by blood-drawing procedures. Psychoneuroendocrinology 2002; 27:921–931Crossref, Medline, Google Scholar
36 : IL-6 and its circadian secretion in humans. Neuroimmunomodulation 2005; 12:131–140Crossref, Medline, Google Scholar
37 : Sleep deprivation and human immune function. Adv Neuroimmunol 1995; 5:97–110Crossref, Medline, Google Scholar
38 : Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol 1997; 158:4454–4464Medline, Google Scholar
39 : Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 1999; 84:2603–2607Crossref, Medline, Google Scholar
40 : Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab 2000; 85:3597–3603Medline, Google Scholar
41 : Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab 2007; 92:4199–4207Crossref, Medline, Google Scholar
42 : Effects of sleep deprivation on human immune functions. FASEB J 1989; 3:1972–1977Crossref, Medline, Google Scholar
43 : Stress, depression and the activation of the immune system. World J Biol Psychiatry 2000; 1:17–25Crossref, Medline, Google Scholar
44 : The effect of strenuous exercise, calorie deficiency, and sleep deprivation on white blood cells, plasma immunoglobulins and cytokines. Scand J Immunol 1996; 43:228–235Crossref, Medline, Google Scholar
45 : Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J Neurochem 2005; 93:549–559Crossref, Medline, Google Scholar
46 : Effect of exogenous catecholamines on tumor necrosis factor alpha, interleukin-6, interleukin-10, and beta-endorphin levels following severe trauma. Vascul Pharmacol 2008; 48:85–91Crossref, Medline, Google Scholar
47 : Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol 1996; 271:E601–E605Medline, Google Scholar
48 : Regulation of the HPA axis by cytokines. Brain Behav Immun 1995; 9:253–275Crossref, Medline, Google Scholar
49 : Interleukin-1 beta uses common and distinct signaling pathways for induction of the interleukin-6 and tumor necrosis factor alpha genes in the human astrocytoma cell line U373. J Neurochem 1996; 66:1496–1503Crossref, Medline, Google Scholar
50 : Regulation of interleukin-6 expression in cultured human blood monocytes and monocyte-derived macrophages. Blood 1988; 72:1134–1140Crossref, Medline, Google Scholar
51 : Differential induction of interleukin-6 production by monocytes, endothelial cells and smooth muscle cells. Prog Clin Biol Res 1991; 367:61–71Medline, Google Scholar
52 : Interleukin 6 modulates interleukin-1-and stress-induced activation of the hypothalamic-pituitary-adrenal axis in male rats. Neuroendocrinology 1996; 63:227–236Crossref, Medline, Google Scholar
53 : Acute effects of recombinant human interleukin-6 on endocrine and central nervous sleep functions in healthy men. J Clin Endocrinol Metab 1998; 83:1573–1579Medline, Google Scholar
54 : The role of interleukin-6 in the human adrenal gland. Eur J Clin Invest 2000; 30(Suppl 3):91–95Crossref, Medline, Google Scholar
55 : Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol Ther 1996; 69:85–95Crossref, Medline, Google Scholar
56 : Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci 2006; 1083:329–344Crossref, Medline, Google Scholar
57 : Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab 2003; 88:2087–2095Crossref, Medline, Google Scholar
58 : Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 2004; 89:2119–2126Crossref, Medline, Google Scholar
59 : Seven days’ around the clock exhaustive physical exertion combined with energy depletion and sleep deprivation primes circulating leukocytes. Eur J Appl Physiol 2006; 97:151–157Crossref, Medline, Google Scholar
60 : Depression, cytokines, and glial function. Metabolism 2005; 54(Suppl 1):33–38Crossref, Medline, Google Scholar
61 : Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65:732–741Crossref, Medline, Google Scholar
62 : Mechanisms of cytokine-induced behavioural changes: psychoneuroimmunology at the translational interface. Brain Behav Immun 2009; 23:149–158Crossref, Medline, Google Scholar



