Concepts and Strategies for Clinical Management of Blast-Induced Traumatic Brain Injury and Posttraumatic Stress Disorder
Abstract
After exposure of the human body to blast, kinetic energy of the blast shock waves might be transferred into hydraulic energy in the cardiovascular system to cause a rapid physical movement or displacement of blood (a volumetric blood surge). The volumetric blood surge moves through blood vessels from the high-pressure body cavity to the low-pressure cranial cavity, causing damage to tiny cerebral blood vessels and the blood–brain barrier (BBB). Large-scale cerebrovascular insults and BBB damage that occur globally throughout the brain may be the main causes of non-impact, blast-induced brain injuries, including the spectrum of traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD). The volumetric blood surge may be a major contributor not only to blast-induced brain injuries resulting from physical trauma, but may also be the trigger to psychiatric disorders resulting from emotional and psychological trauma. Clinical imaging technologies, which are able to detect tiny cerebrovascular insults, changes in blood flow, and cerebral edema, may help diagnose both TBI and PTSD in the victims exposed to blasts. Potentially, prompt medical treatment aiming at prevention of secondary neuronal damage may slow down or even block the cascade of events that lead to progressive neuronal damage and subsequent long-term neurological and psychiatric impairment.
Non-Impact Brain Injuries Resulting From a Volumetric Blood Surge Created by Blast Shock-Wave in the Cardiovascular System
Non-impact, blast-induced brain injuries including traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) may result from parenchymal brain damage, which is caused by the primary blast effect in war fighters exposed to a blast overpressure wave itself, but who do not sustain penetrating and blunt-impact injuries that are caused by secondary and tertiary blast effects.1 Non-impact, blast-induced TBI and PTSD have been considered as the signature injury of the wars in Iraq and Afghanistan.2–5 It is estimated that more than 300,000 veterans returning from Iraq and Afghanistan have suffered a TBI or PTSD or both since 2000.6–10 Blast-induced TBI is strongly associated with the development of PTSD. Many PTSD symptoms (such as depression, anxiety, memory and attention deficits, sleep problems, and emotional disturbances) overlap with symptoms of TBI.11,12 Some studies indicate that approximately 80% of patients with blast-induced and non-blast TBI develop chronic PTSD.13–15 Blast-induced TBI and PTSD seem to have similar pathogenesis.1
A recent study on blast shock-wave mitigation using hydraulic energy redirection and release technology suggested that a volumetric blood surge might be created when the kinetic energy of the blast shock-wave was transferred into hydraulic energy in the cardiovascular system to cause a rapid physical movement or displacement of blood.16 The volumetric blood surge has also been verified by both wound ballistics experiments in animals17 and finite-element simulation of blast loads on the torso.18 It has a strong destructive power, and can cause a non-contact, remote injury to distant organs and tissues. The volumetric blood surge can possibly be the major cause of non-impact, blast-induced brain injuries (TBI and PTSD).1 It may move through blood vessels from the high-pressure ventral body cavity (thoracic cavity and abdominal cavity) to the low-pressure cranial cavity, thus acutely increasing cerebral perfusion pressure and causing damage to both tiny cerebral blood vessels and the blood–brain barrier (BBB) in the global brain.
Global cerebrovascular insults and the BBB damage caused by the volumetric blood surge may result in diffuse cerebral edema, hyperemia, vasospasm, and microhemorrhaging in the brain, thus further triggering secondary neuronal damage (Figure 1[A]).1 The delayed neuronal damage includes excitotoxicity, inflammation, ionic imbalance, oxidative stress, apoptosis, diffuse axonal injury, and neurodegeneration. Secondary neuronal damage is an indirect consequence of initial injury (such as cerebrovascular insults and the BBB damage after exposure of the body to blast shock-wave) and a major contributor to the ultimate neuronal cell death and neural loss in the injured brain. Much of the damage done to the brain does not typically occur at the time of initial injury and does not result directly from the initial injury itself. A cascade of progressive neural injury and neuronal cell death is triggered by the initial injury and continues in the hours, days or weeks following the initial insult.19,20 This delayed secondary neuronal damage has been considered to be largely responsible for serious neurological and psychiatric impairments, including memory loss, inability to concentrate, speech problems, motor and sensory deficits, and behavioral problems (Figure 1[A]).21 However, because blast-induced brain injuries activate the cascade of progressive neuronal damage, it is difficult to separate clinical outcomes of the initial damage to tiny cerebral blood vessels and the BBB from that of secondary neuronal damage, and to separate neuropathophysiologically-based symptoms from neuropsychiatrically-based problems.
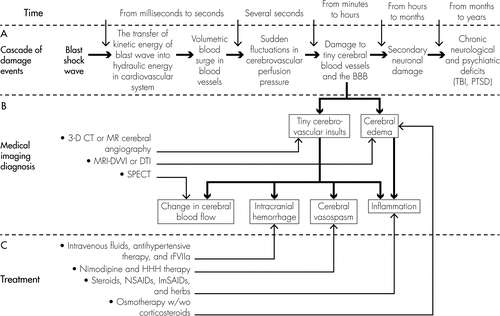
[A]: A secondary cascade of neuronal damage develops with time in the brain after exposure to blast shock-wave; [B]: Clinical imaging technologies such as 3-D CT or MR cerebral angiography, SPECT, and MRI-DWI/DTI may help detect tiny cerebrovascular insults, changes in blood flow, and cerebral edema; [C]: Neuroprotection strategies aiming at prevention of secondary neuronal damage (such as reducing cerebral edema and inflammatory reaction, attenuating hemorrhage, and preventing cerebral vasospasm), may need to be executed as soon as the blast-induced volumetric blood surge causes damage to tiny cerebral blood vessels and the BBB.
Chronic Psychiatric Disorders Resulting From a Volumetric Blood Surge Induced by Emotional and Psychological Trauma
Some disorders, such as PTSD and memory and cognitive impairments, are more likely to be a psychological consequence of secondary neuronal damage induced by physical trauma (such as blast-induced TBI, non-blast TBI, hemorrhagic stroke, ischemic stroke, nerve-agent poisoning, prolonged hypoxia, infection, and neurological illness).22–27 These psychiatric disorders can also possibly be induced by emotional and psychological trauma. These emotional and psychological traumatic events include 1) serious accidents; war or terrorist attacks; car, train, or plane crashes; the sudden death of a loved one; captivity as a hostage; serious physical illness diagnosis, medical emergencies, etc.;28–32 2) devastating natural disasters, such as flood, tornado, hurricane, volcanic eruption, earthquake, landslide, etc.;33–35 3) violent attacks, such as rape, sexual abuse, physical and emotional abuse, bullying, domestic violence, indoctrination, etc.;37–43 and 4) other psychosocial stressors, such as marital separation, divorce, family argument, dispute at work, loss of job, unemployment or underemployment, serious financial problems, poverty, retirement, socioeconomic deprivation, alcohol and drug use, etc.44–49 The mechanisms underlying the psychiatric disorders induced by the emotional and psychological traumatic events may also involve a volumetric blood surge created by a sudden rise in cardiovascular pressure.
When exposed to an emotional and psychological traumatic event, the victim’s heart will beat faster and stronger than usual, and blood pressure will dramatically rise because of the increased cardiac output, which may create a volumetric blood surge in the cardiovascular system. The volumetric blood surge induced by a sudden increase in blood pressure will move quickly through blood vessels to organs and tissues (including the brain). Unlike the peripheral tissues (such as lung, liver, and stomach) that have good tolerance to volumetric blood surge, a sudden rush of blood to the brain will dramatically increase cerebral perfusion pressure, owing to the limited space inside blood vessels in the brain. The extremely rapid increase in cerebrovascular pressure may cause damage to both very small cerebral blood vessels and the BBB in the brain, because cerebral blood vessels and the BBB are vulnerable to sudden fluctuations in perfusion pressure. Therefore, psychiatric disorders induced by emotional and psychological trauma may potentially also be a result of secondary neuronal damage caused by volumetric blood surge in the brain (Figure 2).
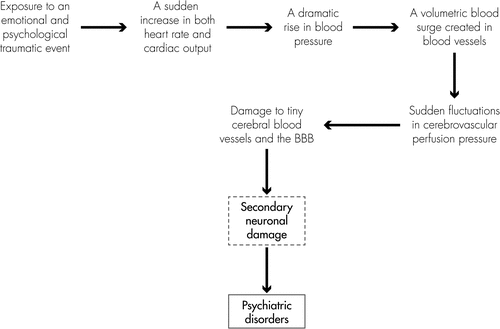
Because chronic disability after brain damage is largely attributable to mental sequelae, rather than focal motor or sensory neurological deficits in humans,50 psychiatric disorders should be the long-term prominent clinical manifestations of the victims of brain damage induced by either physical trauma or emotional and psychological trauma. These chronic psychiatric consequences are serious public health problems caused by various types of brain damage, which result in the loss of many years of productive life and incur large healthcare costs.
Medical Imaging Techniques for the Diagnosis of Blast-Induced Brain Injuries
Blast-induced brain injuries (TBI and PTSD) are typically difficult to detect by using traditional neuroimaging techniques (i.e., computed tomography [CT], magnetic resonance imaging [MRI], and angiography), because the damage to tiny cerebral blood vessels and the BBB might be the initial injuries induced by blast shock-wave in the brain.1 Much of neuronal damage can be seen only under a microscope. Changes in both cerebral blood vessels and cerebral blood flow and vasogenic cerebral edema should be the most common pathophysiological characteristics of cerebrovascular insults and BBB damage.51–53 Focused clinical diagnostic techniques that are able to sensitively detect tiny cerebral blood vessels, blood flow, and edema may be helpful for diagnosis of blast-induced brain injuries.
Using single-photon emission computerized tomography (SPECT) that is able detect the level of perfusion or blood flow inside the brain, decreased levels of blood flow in the temporal lobes that contain the cortex and the limbic system (the amygdala, hippocampus, gyrus cinguli, thalamus, and kleinhirn) were detected in a soldier who was exposed to a blast in Afghanistan and was diagnosed with both mild TBI and PTSD.54 Diffusion tensor imaging (DTI), an advanced form of MRI that produces in-vivo images of the white-matter brain tissues weighted with the local microstructural characteristics of water diffusion, is sensitive to measurement of the restricted diffusion of water in the brain. Using DTI, marked persistent abnormalities in the middle cerebellar peduncles, cingulum bundles, and the right orbitofrontal white matter were observed in 18 of the 63 U.S. military personnel with blast-induced mild TBI within 90 days after the injury. The persistent abnormalities in the brain may involve diffuse axonal injury.55
Three medical imaging technologies that are currently used in the clinical diagnosis of diseases can be considered for use in diagnosis of blast-induced brain injuries (Figure 1[B]): 1) three-dimensional CT or MR cerebral angiography using nanosized contrast materials (dyes) can be useful for measuring damage to tiny cerebral blood vessels. Cerebral angiography is clinically helpful for detecting and diagnosing acute stroke.56–58 It is used to image the blood vessels of the brain and the blood flowing through the blood vessels and to detect abnormalities in the brain's blood vessels, such as narrowing (vasospasm) or blockage (thrombosis); 2) SPECT may be an ideal diagnostic tool for detection of early changes in cerebral blood flow after blast-induced TBI because it can measure the blood perfusion level relative to the brain's current need.59,60 A decrease in blood flow levels in a region of the brain may suggest an injury to that brain region; 3) diffusion MRI, such as diffusion tensor imaging (DTI) may be beneficial to detect cerebral edema since it measures diffusion rates of water molecules in the brain parenchyma.55,61 A decreased apparent diffusion coefficient obtained by the diffusion MRI represents reduced water diffusion or mobility in the brain, which indicates that edema has formed and neuronal damage has occurred.
Neuroprotection Strategies Aimed at Prevention of Secondary Neuronal Damage
Brain injuries, to-date, are untreatable disorders, because no pharmacological treatment has currently been proven to prevent secondary damage processes.62 However, secondary damage process offers a potential therapeutic window of opportunity,63 in which progressive neural injury and neuronal cell death may be prevented, and the extent of disability may be reduced during the first few hours after initial injury.64 According to the AANS (American Association of Neurological Surgeons) recommendations, there is no medication or “miracle treatment” that can be given to prevent brain damage or promote neuronal healing after TBI. The primary goal in the acute management of the patient with TBI is to prevent any secondary damage to the brain.65 Therefore, neuroprotective strategies intended to halt or mitigate secondary neuronal damage at early stage of injury (2–3 hours after injury) may help block or slow down the development of subsequent neurological and neuropsychiatric impairments (including PTSD, persistent memory and cognitive deficits, nonspecific mental and emotional symptoms, chronic motor deficits, Alzheimer-like dementia, and Parkinson’s disease).
Diffuse cerebral edema, hyperemia, vasospasm, and hemorrhaging are the initial pathophysiological changes in the brain after non-impact, blast-induced brain injuries.66,67 Proper and prompt medical treatment aiming to attenuate these pathophysiological changes (Figure 1[C]) may improve clinical outcome of blast-induced brain injuries, thus leading to a significant decrease in both the direct cost for medical care and indirect cost of lost productivity resulting from blast-induced TBI and PTSD. When an effective treatment is discovered, it may be important to treat the victims exposed to blasts as soon as possible, preferably within 8 hours, and not later than 24 hours after exposure.
Treatment for Cerebral Edema
Osmotherapy, using oral administration or intravenous injection of mannitol or diuretics (such as furosemide, bumetanide, torsemide, and ethacrynic acid) to induce dehydration, is a most common approach to treating cerebral edema and possible elevated intracranial pressure.68,69 Administration of mannitol may be an effective treatment for patients with blast-induced mild brain injuries within 8 hours after exposure to blasts. For patients with blast-induced severe brain injuries, mannitol or diuretics may need to be given intravenously as soon as possible after the injury. If needed, corticosteroids can be administered orally or intravenously, in addition to osmotherapy, to reduce brain swelling.70
Treatment for Intracranial Hemorrhage
Medical therapy of intracranial hemorrhage is principally focused on minimizing brain damage and stabilizing the patient’s condition. Certain medications, including painkillers, corticosteroids, mannitol, or diuretics to reduce swelling, and anticonvulsants to control seizures, may be prescribed.71,72 Blood products or intravenous fluids may be administered if needed.73 Normotonic, rather than hypotonic fluids will be used to maintain brain perfusion without exacerbating brain edema.74 Antihypertensive therapy aimed at maintaining blood pressure to a mean arterial pressure (MAP) less than 130 mm Hg can be applied to patients with intracranial hemorrhage within 3 hours of onset.75 Patients may be treated with recombinant factor VIIa (rFVIIa) within 4 hours after the onset of intracerebral hemorrhage, to limit the bleeding and formation of a hematoma. However, VIIa is reported to increase the risk of thromboembolism, and may not result in improved clinical outcomes.76 Frozen plasma, vitamin K, protamine, or platelet transfusions can be given in cases of coagulopathy.77
Treatment for Cerebral Vasospasm
Medical therapy of cerebral vasospasm will aim at limiting or reducing delayed ischemic injury due to cerebral vasospasm. Nimodipine, a dihydropyridine calcium-channel blocker, has shown good results in preventing cerebral vasospasm caused by subarachnoid hemorrhaging.78 Nimodipine can be administered orally (60 mg, every 4 hours) or intravenously (intravenous infusion at a rate of 1–2 mg/hour) based on the patient's clinical status and medication needs. Also, the condition can be treated with hypertensive hypervolemic hemodilution (HHH) therapy,79 which combines intravenous medications and large volumes of intravenous fluids to elevate cerebral blood perfusion pressure, increase cerebral blood volume, and thin the blood, thus providing blood to the affected regions of the brain. If the HHH therapy is unsuccessful, balloon angioplasty may be used to open tight, spastic vessels.80
Anti-Inflammatory Treatment
Anti-inflammatory treatment refers to an attempted remediation that reduces or suppresses inflammation. Current anti-inflammatory drugs include steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), immune-selective anti-inflammatory derivatives (ImSAIDs), and herbs. Steroidal anti-inflammatory drugs, such as glucocorticoids, are effective in reducing inflammation or brain-tumor-associated brain swelling by binding to glucocorticoid receptors in the brain.81 NSAIDs such as aspirin, ibuprofen, naproxen, and other specific COX-inhibitors, reduce inflammation by inhibiting both the COX-1 and COX-2 enzymes that synthesize prostaglandins to create inflammation.82 ImSAIDs are a class of peptides that are reported to have anti-inflammatory effects. ImSAIDs can attenuate the amplified inflammatory response by limiting the activation and migration of inflammatory cells.83 Some herbs that contain helenalin or salicylic acid, such as harpagophytum, hyssop, ginger, turmeric, arnica Montana, and willow bark, may have anti-inflammatory effects. Cannabichromene, found in the cannabis plant, has shown the ability to reduce inflammation.84,85 These anti-inflammatory drugs may be administered or given orally within 8 hours after the injury, depending on the patient's clinical condition. However, recent clinical trials have shown that these anti-inflammatory agents may not be effective for some conditions, such as penetrating head injury and spontaneous intracerebral hemorrhage.
Conclusions
A volumetric blood surge may be created when the kinetic energy of a blast shock-wave is transferred into hydraulic energy in the cardiovascular system to cause a rapid physical movement or displacement of blood. It may move rapidly through blood vessels to the cranial cavity, causing damage to tiny cerebral blood vessels and the BBB and triggering a secondary cascade of neuronal damage in the brain. The volumetric blood surge may be a major contributor not only to blast-induced TBI and PTSD, but also to some psychiatric disorders induced by emotional and psychological trauma. Three-dimensional CT or MR cerebral angiography, SPECT and diffusion MRI may help detect tiny cerebrovascular insults, changes in blood flow, and cerebral edema in the brains of victims exposed to blasts. Neuroprotective strategies for victims exposed to blasts, which include but not limited to reducing cerebral edema and inflammatory reaction, attenuating hemorrhage, and preventing cerebral vasospasm, may need to be executed within 8 hours after exposure to blasts.
1 : Non-impact, blast-induced mild TBI and PTSD: concepts and caveats. Brain Inj 2011; 25:641–650Crossref, Medline, Google Scholar
2 : Shell-shock and mild traumatic brain injury: a historical review. Am J Psychiatry 2007; 164:1641–1645Crossref, Medline, Google Scholar
3 : Improvement in cerebral function with treatment of posttraumatic stress disorder. Ann N Y Acad Sci 2010; 1208:142–149Crossref, Medline, Google Scholar
4 : A signature wound of war: mild traumatic brain injury. J Psychosoc Nurs Ment Health Serv 2010; 48:22–28Crossref, Medline, Google Scholar
5 : Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) military mental health issues. Information on the wars’ signature wounds: posttraumatic stress disorder and traumatic brain injury. Pa Nurse 2010; 65:4–11, quiz 12–13Medline, Google Scholar
6 : Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil 2006; 21:398–402Crossref, Medline, Google Scholar
7 Hoge CW, Castro CA, Messer SC, et al: Combat duty in Iraq and Afghanistan: mental health problems and barriers to care. US Army Med Dep J 2008; Jul–Sep:7–1Google Scholar
8 : Traumatic brain injuries sustained in the Afghanistan and Iraq wars. J Trauma Nurs 2008; 15:94–99, quiz 100–101Crossref, Medline, Google Scholar
9 : Trauma exposure, branch of service, and physical injury in relation to mental health among U.S. veterans returning from Iraq and Afghanistan. Mil Med 2009; 174:773–778Crossref, Medline, Google Scholar
10 : Psychiatric diagnoses, comorbidity, and functioning in National Guard troops deployed to Iraq. J Psychiatr Res 2011; 45:126–132Crossref, Medline, Google Scholar
11 : Overlap of mild TBI and mental health conditions in returning OIF/OEF service members and veterans. J Rehabil Res Dev 2008; 45:xi–xviMedline, Google Scholar
12 : Posttraumatic stress symptoms in OIF/OEF service members with blast-related and non-blast-related mild TBI. NeuroRehabilitation 2010; 26:223–231Medline, Google Scholar
13 : Posttraumatic stress disorder and traumatic brain injury: can they co-exist? Clin Psychol Rev 2001; 21:931–948Crossref, Medline, Google Scholar
14 Summerall EL: Traumatic Brain Injury and PTSD. (National Center for PTSD, U.S. Department of Veterans Affairs. January 1, 2007; accessed July 15, 2011, at http://www.ptsd.va.gov/professional/pages/traumatic-brain-injury-ptsd.aspGoogle Scholar
15 : Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med 2009; 76:111–118Crossref, Medline, Google Scholar
16 : Blast Shock Wave Mitigation Using the Hydraulic Energy Redirection and Release Technology. PLoS ONE 2012; 7(6):e39353 doi:10.1371/journal.pone.0039353Crossref, Medline, Google Scholar
17 : Mechanism and characteristics of the remote effects of projectiles. J Trauma (China) 1990; 6:16–20Google Scholar
18 Stuhmiller JH: Blast injury: translating research into operational medicine. Army Medical Research and Materiel Command. BI-QP-JHS-CH10, April, 2008; accessed June 7, 2010, at http://www.bordeninstitute.army.mil/other_pub/blast/Blast_monograph.pdfGoogle Scholar
19 : Traumatic Brain Injury: Methods for Clinical and Forensic Neuropsychiatric Assessment, 2nd Edition. Boca Raton, FL, CRC, 2007, pp 26–32Crossref, Google Scholar
20 : Dose–response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience 2000; 101:289–295Crossref, Medline, Google Scholar
21 : Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma 2000; 17:915–925Crossref, Medline, Google Scholar
22 : From human immunodeficiency virus (HIV) infection of the brain to dementia. Genitourin Med 1997; 73:343–347Medline, Google Scholar
23 : From neuronal and vascular impairment to dementia. Pharmacopsychiatry 1999; 32(Suppl 1):17–24Crossref, Medline, Google Scholar
24 : Cognitive impairments following traumatic brain injury: etiologies and interventions. Crit Care Nurs Clin North Am 2000; 12:447–456Crossref, Medline, Google Scholar
25 : Pathology and pathways of Alzheimer’s disease with an update on new developments in treatment. J Am Geriatr Soc 2003; 51(Suppl Dementia):S314–S320Crossref, Medline, Google Scholar
26 : Long-term psychiatric disorders after traumatic brain injury. Eur J Anaesthesiol Suppl 2008; 42:123–130Crossref, Medline, Google Scholar
27 : Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res 2011; 221:261–270Crossref, Medline, Google Scholar
28 : Symptoms of stress disorder and depression among trauma counselors after an airline disaster. Psychiatr Serv 1996; 47:424–426Crossref, Medline, Google Scholar
29 : The spectrum of human reactions to terrorist attacks with weapons of mass destruction: early management considerations. Prehosp Disaster Med 2003; 18:253–257Crossref, Medline, Google Scholar
30 : Emotional impact of exposure to terrorism among young-old and old-old Israeli citizens. Am J Geriatr Psychiatry 2005; 13:705–712Crossref, Medline, Google Scholar
31 : Psychological interventions following terrorist attacks. Br Med Bull 2008; 88:7–22Crossref, Medline, Google Scholar
32 : Post-trauma symptoms following indirect exposure to the September 11th terrorist attacks: the predictive role of dispositional coping. J Anxiety Disord 2009; 23:915–922Crossref, Medline, Google Scholar
33 : When disaster strikes, acute stress disorder may follow. J Trauma Stress 1995; 8:29–46Crossref, Medline, Google Scholar
34 : Psychological reactions in Icelandic earthquake survivors. Scand J Psychol 2004; 45:3–13Crossref, Medline, Google Scholar
35 : Psychological effects of earthquakes in children: prospects for brief behavioral treatment. World J Pediatr 2008; 4:165–172Crossref, Medline, Google Scholar
36 : Tsunami-exposed tourist survivors: signs of recovery in a 3-year perspective. J Nerv Ment Dis 2011; 199:162–169Crossref, Medline, Google Scholar
37 : Indoctrination procedures for personnel. Am J Ment Defic 1952; 56:547–550Medline, Google Scholar
38 : Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. J Clin Child Psychol 2001; 30:349–363Crossref, Medline, Google Scholar
39 : The aftermath of violence: children, disaster, and posttraumatic stress disorder. J Pediatr Health Care 2002; 16:235–244Crossref, Medline, Google Scholar
40 : Childhood maltreatment in adult female psychiatric outpatients with eating disorders. Eat Behav 2006; 7:404–409Crossref, Medline, Google Scholar
41 : Major depressive disorder in persons exposed to trauma: relationship between emotional intelligence and social support. J Am Psychiatr Nurses Assoc 2011; 17:237–245Crossref, Medline, Google Scholar
42 : Lifetime prevalence of gender-based violence in women and the relationship with mental disorders and psychosocial function. JAMA 2011; 306:513–521Medline, Google Scholar
43 : The relationship between mental disorders and different types of crime. Crim Behav Ment Health 2011; 21:307–320Crossref, Medline, Google Scholar
44 : Managing anxiety and depression in alcohol and drug dependence. Addict Behav 1998; 23:919–931Crossref, Medline, Google Scholar
45 : Personal and workgroup incivility: impact on work and health outcomes. J Appl Psychol 2008; 93:95–107Crossref, Medline, Google Scholar
46 : Emotion dysregulation and negative affect: association with psychiatric symptoms. J Clin Psychiatry 2011; 72:685–691Crossref, Medline, Google Scholar
47 : A multi-national study of mental disorders, marriage, and divorce. Acta Psychiatr Scand 2011; 124:474–486Crossref, Medline, Google Scholar
48 : Attributable risk of psychiatric and socio-economic factors for suicide from individual-level, population-based studies: a systematic review. Soc Sci Med 2011; 72:608–616Crossref, Medline, Google Scholar
49 : Impact of employment status and work-related factors on risk of completed suicide: a case–control psychological autopsy study. Psychiatry Res 2011; 190:265–270Crossref, Medline, Google Scholar
50 : Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 1981; 44:285–293Crossref, Medline, Google Scholar
51 : Cerebral vascular volume after repeated ischemic insults in the gerbil: comparison with changes in CBF and brain edema. J Cereb Blood Flow Metab 1989; 9:163–170Crossref, Medline, Google Scholar
52 : Mediators of vascular and parenchymal mechanisms in secondary brain damage. Acta Neurochir Suppl (Wien) 1993; 57:64–72Medline, Google Scholar
53 : Elements of cerebral microvascular ischaemia. Brain Res Brain Res Rev 2001; 36:23–34Crossref, Medline, Google Scholar
54 Hall KM: Army tries new brain scans to hunt blast effects. May 23, 2011, Associated Press; accessed July 20, 2011, at http://www.cnsnews.com/node/89398Google Scholar
55 : Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011; 364:2091–2100Crossref, Medline, Google Scholar
56 : MR imaging of middle cerebral artery stenosis and occlusion: value of MR angiography. AJNR Am J Neuroradiol 1994; 15:335–341Medline, Google Scholar
57 : Mild carotid artery atherosclerosis: assessment by three-dimensional time-of-flight magnetic resonance angiography, with reference to intravascular ultrasound imaging and contrast angiography. Stroke 1999; 30:827–833Crossref, Medline, Google Scholar
58 : C-arm flat detector computed tomography: the technique and its applications in interventional neuro-radiology. Neuroradiology 2010; 52:319–327Crossref, Medline, Google Scholar
59 : Brain single photon emission computed tomography: newer activation and intervention studies. Semin Nucl Med 1991; 21:40–57Crossref, Medline, Google Scholar
60 : Neuropsychological dysfunction in systemic lupus erythematosus is not associated with changes in cerebral blood flow. J Neurol 2001; 248:595–602Crossref, Medline, Google Scholar
61 : Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma 2010; 27:683–694Crossref, Medline, Google Scholar
62 : Does it matter how head injured patients are resuscitated? in Neurotrauma: Evidence-Based Answers To Common Questions. Edited by Valadka AB, Andrews BT. New York, Thieme, 2005, pp 3–4Google Scholar
63 : Traumatic subarachnoid hemorrhage: our current understanding and its evolution over the past half century. Neurol Res 2006; 28:445–452Crossref, Medline, Google Scholar
64 : Pathophysiology and treatment of intracranial hypertention, in Intensive Care in Neurosurgery. Edited by Andrews BT. Thieme Medical, New York, 2003, pp 52–53Google Scholar
65 American Association of Neurological Surgeons: Traumatic Brain Injury. AANS. March, 2011; accessed September 30, 2011, at http://aans.org/en/Patient%20Information/Conditions%20and%20Treatments/Traumatic%20Brain%20Injury.aspxGoogle Scholar
66 : Explosive blast neurotrauma. J Neurotrauma 2009; 26:815–825Crossref, Medline, Google Scholar
67 : Computational biology: modeling of primary blast effects on the central nervous system. Neuroimage 2009; 47(Suppl 2):T10–T20Crossref, Medline, Google Scholar
68 : Treatment options for large hemispheric stroke. Neurology 2001; 57(Suppl 2):S61–S68Crossref, Medline, Google Scholar
69 : Osmotherapy in neurocritical care. Curr Neurol Neurosci Rep 2007; 7:513–521Crossref, Medline, Google Scholar
70 : Treatment of intracranial hypertension. Curr Opin Crit Care 2008; 14:129–134Crossref, Medline, Google Scholar
71 : Pharmacologic management of brain edema. Curr Treat Options Neurol 2009; 11:64–73Crossref, Medline, Google Scholar
72 : Preventing and treating posttraumatic seizures: the human experience. Epilepsia 2009; 50(Suppl 2):10–13Crossref, Medline, Google Scholar
73 : Exploring neuroprotective drug therapies for intracerebral hemorrhage. J Pharmacol Sci 2010; 114:366–378Crossref, Medline, Google Scholar
74 : Changes in tonicity of perfusion medium cause prolonged opening of calcium channels of the rat chromaffin cells to evoke explosive secretion of catecholamines. J Neurosci 1986; 6:2625–2634Crossref, Medline, Google Scholar
75 : Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH): rationale and design. Neurocrit Care 2007; 6:56–66Crossref, Medline, Google Scholar
76 : Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med 2011; 154:529–540Crossref, Medline, Google Scholar
77 : Anticoagulant-associated intracerebral hemorrhage. Semin Neurol 2010; 30:565–572Crossref, Medline, Google Scholar
78 : Role of intra-arterial therapy for cerebral vasospasm secondary to aneurysmal subarachnoid hemorrhage. Pharmacotherapy 2010; 30:405–417Crossref, Medline, Google Scholar
79 : Hypertensive, hypervolemic, hemodilutional therapy for aneurysmal subarachnoid hemorrhage. Is it efficacious? Yes. Crit Care Clin 1996; 12:697–707Crossref, Medline, Google Scholar
80 : Transluminal balloon angioplasty for symptomatic distal vasospasm refractory to medical therapy in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery 2011; 69:95–101, discussion 102Crossref, Medline, Google Scholar
81 : Glucocorticoid therapy in neurologic critical care. Crit Care Med 2005; 33:1214–1224Crossref, Medline, Google Scholar
82 : Non-steroidal anti-inflammatory drugs and cyclooxygenase in Alzheimer’s disease. Curr Drug Targets 2003; 4:461–468Crossref, Medline, Google Scholar
83 : Recent patents on novel P2X(7) receptor antagonists and their potential for reducing central nervous system inflammation. Recent Patents CNS Drug Discov 2010; 5:35–45Crossref, Medline, Google Scholar
84 : Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol Res 2005; 27:287–301Crossref, Medline, Google Scholar
85 : Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases: a focus on traditional medicines and flavonoids. Neurosignals 2005; 14:23–33Crossref, Medline, Google Scholar



