Delusional Misidentification Syndrome: Phantom Boarder Syndrome Presenting With Intact General Cognition and Functional Brain Imaging Evidence of Bilateral Posterior and Orbitofrontal Hypometabolism
Phantom boarder syndrome is a type of delusional misidentification syndrome in which the patient believes that someone uninvited is residing in his or her home despite evidence to the contrary. Phantom boarder syndrome is typically encountered in elderly patients with major neurocognitive disorders of various etiologies, such as Alzheimer’s disease, vascular dementia, Lewy body dementia, and other forms of dementia due to general medical conditions (1, 2). Phantom boarder syndrome is associated with visual hallucinations, auditory hallucinations, and misidentification phenomenon. Although the syndrome manifests as a result of organic disease, psychosocial factors, such as personal loss, social isolation, and lack of interpersonal relationships also play a major role in the development of the disorder in elderly individuals (3).
The phantom boarder has been described by patients as friends, family members, strangers, and deceased persons or ghosts, and there can be multiple phantom boarders. Some patients report the phantom boarder as an intruder with the intent to harm or rob them, while other patients report that the phantom boarder has no harmful intentions and treats them as guests (1).
To our knowledge, there are no documented cases in the current literature of phantom boarder syndrome manifesting in the absence of dementia. We present a case of a 72-year-old woman with phantom boarder syndrome with intact general cognition.
Case Report
A 72-year-old divorced, affluent Caucasian woman with a graduate-level education was referred to our clinic by the local police department after reporting that a male intruder and his girlfriend and son had been living on the roof of her house for the past 2 years. The patient, who lived alone, described the male intruder as being of Scottish descent and that he wears military clothing and is often accompanied by his girlfriend and son. The patient also reported that the man, his girlfriend, and their son poked her with needles, used cocaine, and stole her jewelry. A police investigation yielded no evidence of any intruders or break-ins, which was corroborated by the patient’s daughter. The police discontinued their responses to the patient’s emergency calls, which prompted her to begin going to sleep with a pistol for self-defense.
During her first clinic visit and all subsequent visits, a Montreal Cognitive Assessment (MoCA) was administered. The raw MoCA scores (range, 23 to 27) were converted to z scores (range, –0.30 to 0.90) based on normative MoCA data generated by the Dallas Heart Study. The Dallas Heart Study analyzed a large, ethnically diverse community-based population (N=2,653) and generated normative MoCA data stratified by age and education level (4). Our patient’s z scores were calculated on the basis of the following parameters of the Dallas Heart Study: age 65–75, >12 years of education, sample size (N=122/2,653), and mean score of 24.0 (SD=3.35) (4).
In order to determine whether our patient met the criteria for mild cognitive impairment, we used the conventional cutoff z score −1.5, which was established by Petersen et al. (5) and reaffirmed by Knopman et al. (6). Knopman et al. conducted the Framingham Heart Study and Mayo Clinic Study, which demonstrated that patients with z scores less than −1.5 had a high risk of progression to dementia (6).
On the basis of the calculated z scores (range, −0.30 to 0.90) compared with the conventional cutoff score (−1.5), our patient did not have mild cognitive impairment and therefore had intact general cognition.
After a complete medical workup, which was within normal limits, and an MRI, which was negative for structural lesions or acute neurological injury, a functional brain imaging study with [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) and computerized tomography (CT) metabolism was conducted and showed extensive hypometabolism within the parieto-temporal cortices that extended to the orbito-frontal lobes bilaterally, which can be seen in advanced Alzheimer’s disease (7, 8). However, our patient’s functional brain imaging did not show hypometabolism in the medial temporal cortices, which is also typical in advanced Alzheimer’s disease (7, 8). Overall, the patient’s functional brain imaging and clinical presentation were not consistent with advanced Alzheimer’s.
Management of phantom boarder syndrome with neurocognitive enhancers and antipsychotic medications has been reported to be effective in the treatment of the neuropsychiatric symptoms (1). In April 2017, our patient was started on memantine hydrochloride extended release/donepezil hydrochloride (7/10 mg/day) as well as risperidone (0.25 mg/day).
Because of our patient’s delusion of a phantom boarder, a Positive and Negative Syndrome Scale (PANSS) was conducted and yielded a baseline index score of 12 (Figure 1) (9). Her PANSS score steadily increased from 16 in August 2017 to 21 in December 2017, which indicated increased severity of the delusion. Interestingly, her PANSS score significantly decreased to 3 in February 2018.
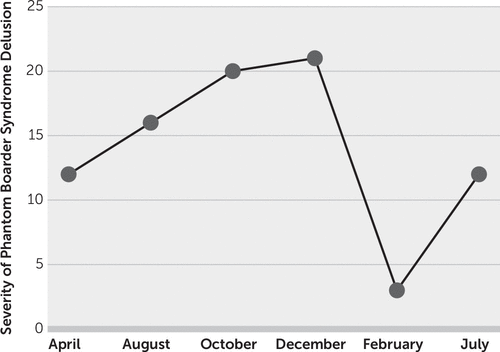
FIGURE 1. Positive and Negative Syndrome Scale (PANSS) scores for a patient with phantom boarder syndromea
a Of the 30 items included in the PANSS, seven constitute a positive (P) subscale (P1, delusions; P2, conceptual disorganization; P3, hallucinatory behavior; P4, excitement; P5, grandiosity; P6, suspiciousness/persecution; and P7, hostility), and an additional seven comprise a negative (N) subscale: (N1, blunted affect; N2, emotional withdrawal; N3, poor rapport; N4, passive/apathetic social withdrawal; N5, difficulty in abstract thinking; N6, lack of spontaneity and flow of conversation; and N7, stereotyped thinking) (9). A composite scale that was scored by subtracting the negative score from the positive score was estimated, yielding a bipolar index that ranged from –42 to +42, which is essentially a difference score reflecting the degree of predominance of one syndrome in relation to the other.
However, the patient’s PANSS score rebounded to 12 in July 2018, and thus she returned to her initial baseline, indicating increased severity of the delusion. These findings were further substantiated during her July 2018 visit in which she indicated that the phantom boarder was bringing large groups of people into her backyard in order to break into her home and rob her. The dosage of memantine hydrochloride/donepezil hydrochloride was increased from 7/10 mg/day to 14/10 mg/day, and the dosage of risperidone was increased from 0.25 mg/day to 0.5 mg/day.
Discussion
Our patient’s functional brain imaging study with 18FDG-PET and CT was compared with a patient with a diagnosis of prodromal Alzheimer’s disease without phantom boarder syndrome and matched for the following characteristics: race and ethnicity, education level, medical conditions, disease duration, antidementia drugs, and diagnosis (Figure 2). Our patient’s brain imaging showed extensive hypometabolism in the parieto-temporal cortices that extended to the frontal lobes bilaterally, particularly affecting the orbital-frontal cortices. The matched comparison patient also showed hypometabolism within the parieto-temporal cortices but not the frontal lobes. Although, our patient showed greater hypometabolism than the comparison patient without phantom boarder syndrome, the comparison patient had clinical symptoms of Alzheimer’s disease with incipient dementia, while our patient did not.
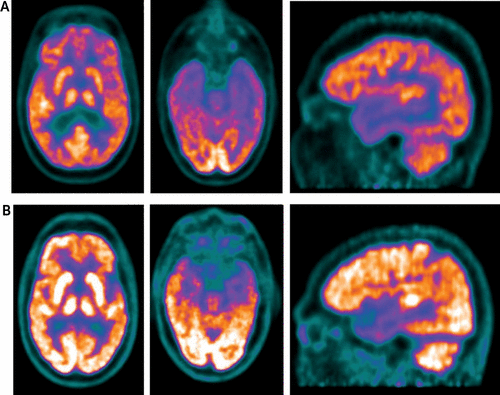
FIGURE 2. Comparison of 18FDG-PET and computerized tomography (CT) imaging of a patient with phantom boarder syndrome and a matched comparison patienta
a Panel A shows [18F]fluorodeoxyglucose positron emission tomography (FDG-PET) and CT images of axial and sagittal views demonstrating marked glucose hypometabolism involving the bilateral parieto-temporal lobes and extending to the frontal lobes bilaterally, including the orbito-frontal cortices, in the patient with phantom boarder syndrome. Panel B shows a moderate degree of hypometabolism in the parieto-temporal cortices and no PET cerebral metabolic rate of glucose reduction in the frontal lobes in a matched comparison patient with dementia but without phantom boarder syndrome.
Sex differences (study patient, female; comparison patient, male) in functional and molecular neuroimaging biomarkers of Alzheimer’s disease have been recently described and reviewed in the literature in the Investigation of Alzheimer’s Predictors in Subjective Memory Complainers (INSIGHT-preAD) study and the Alzheimer Precision Medicine Initiative (APMI) of cognitively intact elderly (10). In the INSIGHT-preAD and APMI studies, significantly reduced glucose metabolism in the posterior cingulate cortex, inferior parietal cortex, and precuneus was found in men compared with women. Additionally, in both studies, the reduced resting-state functional connectivity found in men was in line with the effect observed for glucose metabolism, suggesting that cognitively intact older males might have higher brain reserve capacity compared with cognitively intact females (10).
Studies have hypothesized that delusional misidentification syndromes arise from right hemispheric lesions, which causes dysfunction within the information-processing networks that interlink the left and right hemispheres (11–14). Our patient’s functional brain imaging showed bilateral hemispheric lesions, as demonstrated on 18FDG-PET and CT, with hypometabolism within the parietal-temporal cortices and orbito-frontal lobes (Figure 2). Therefore, the patient had a right hemispheric lesion, which is typical of delusional misidentification syndromes. The patient’s bilateral hemispheric lesion also suggests increased duration of delusion, which correlates with her having phantom boarder syndrome for longer than one year (12). Our patient’s presentation of phantom boarder syndrome supports the hypothesis that delusional misidentification syndromes are predominantly a result of right hemispheric lesions, and this impairs information-processing networks between the left and right hemispheres.
Our patient exhibited phantom boarder syndrome with intact general cognition despite pronounced brain imaging abnormalities on 18FDG-PET and CT. It appears that functional neuroanatomic changes in conjunction with this syndrome can serve as a precursor to early dementia and facilitate early medical intervention to slow the rate of progression.
To our knowledge, this is the first study to capture phantom boarder syndrome on functional brain imaging using 18FDG-PET and CT in a patient with intact cognition and with neuroimaging evidence of extensive decreased cerebral metabolic rate of glucose with hypometabolism in both brain hemispheres. These findings open a new window into the understanding of the pathophysiology of this novel psychopathologic phenomenon.
1 : Phantom boarder symptom in dementia. Int J Geriatr Psychiatry 2003; 18:417–420Crossref, Medline, Google Scholar
2 : Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain 2010; 133:557–567Crossref, Medline, Google Scholar
3 : Perspectives on phantom boarder symptom. Psychogeriatrics 2005; 5:103–107Crossref, Google Scholar
4 : Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology 2011; 77:1272–1275Crossref, Medline, Google Scholar
5 : Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308Crossref, Medline, Google Scholar
6 : Spectrum of cognition short of dementia. Neurology 2015; 85:1712–1721Crossref, Medline, Google Scholar
7 : Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med 2008; 49:390–398Crossref, Medline, Google Scholar
8 : Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci 2011; 34:430–442Crossref, Medline, Google Scholar
9 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
10 : Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in a monocenter cohort of cognitively normal older adults with subjective memory complaints. Alzheimers Dement 2018; 14:1204–1215Crossref, Medline, Google Scholar
11 : Delusional misidentificatoin syndromes: progress and new challenges. J Neuropsychiatry Clin Neurosci 2016; 28:60–161Link, Google Scholar
12 : Lesion-related delusional misidentification syndromes: a comprehensive review of reported cases. J Neuropsychiatry Clin Neurosci 2016; 28:217–222Link, Google Scholar
13 : Delusional misidentifications and duplications: right brain lesions, left brain delusions. Neurology 2009; 72:80–87Crossref, Medline, Google Scholar
14 : Delusions and the right hemisphere: a review of the case for the right hemisphere as a mediator of reality-based belief. J Neuropsychiatry Clin Neurosci 2017; 29:225–235Link, Google Scholar



