Clinical Significance of Delirium With Catatonic Signs in Patients With Neurological Disorders
Abstract
Objective:
According to DSM-5, catatonia and delirium are mutually exclusive clinical syndromes. The investigators explored the co-occurrence of delirium and catatonia (i.e., catatonic delirium) and the clinical significance of this syndrome with a sample of neurological patients.
Methods:
This prospective study with consecutive sampling included patients diagnosed with delirium at the National Institute of Neurology and Neurosurgery of Mexico. DSM-5 criteria for delirium, the Confusion Assessment Method, and the Delirium Rating Scale–Revised-98 were used to select and characterize patients. Catatonia was assessed using the Bush-Francis Catatonia Rating Scale and DSM-5 diagnostic criteria. Logistic regression analysis was performed to identify etiological factors associated with catatonic delirium.
Results:
A total of 264 patients with delirium were included, 61 (23%) of whom fulfilled the criteria for catatonia and delirium simultaneously. Brain tumors, subarachnoid hemorrhage, acute hydrocephalus, and ischemic stroke were associated with delirium without catatonic signs. Catatonic delirium was observed among patients with encephalitis, epilepsy, brain neoplasms, and brain tuberculosis. After multivariate analysis, the association between catatonic delirium and encephalitis (both viral and anti-N-methyl-d-aspartate receptor [NMDAR]) was confirmed.
Conclusions:
Delirium is a common complication of neurological diseases, and it can coexist with catatonia. The recognition of catatonic delirium has clinical significance in terms of etiology, as it was significantly associated with viral and anti-NMDAR encephalitis.
According to DSM-5, catatonia and delirium are mutually exclusive syndromes. DSM-5 indicates that to diagnose catatonia, one must verify that “the disturbance does not occur exclusively during the course of a delirium” (criterion D, p. 120) (1). However, in neuropsychiatric clinical practice, both conditions have been recognized in the same patient during the same clinical care episode (2–5). However, the frequency, clinical features, and etiological significance of their co-occurrence are not fully defined.
Delirium is a neuropsychiatric syndrome characterized by an acute disturbance of consciousness and the attentional matrix (6). Although delirium can have a polymorphic clinical presentation, three core domains (proposed by Franco et al. [7]) define this syndrome: attention and other cognitive deficits, higher-level thinking disturbances (semantic language, thought process, and executive function), and circadian disturbances (sleep-wake cycle and motor activity alterations). By definition, delirium is secondary to pharmacological or medical conditions (8). Delirium is the most common psychiatric syndrome found among patients in general hospitals, and it is associated with a significant mortality rate (9–11). It has been estimated that 15% of neurological emergencies entail a delirium diagnosis (12).
Catatonia has been defined as a motor dysregulation syndrome characterized by altered motor behavior, impaired volition, and vegetative abnormalities. It may be associated with psychiatric disorders (mainly affective and nonaffective psychosis) or with medical, neurological, or substance-induced etiologies (13). In a 20-year retrospective cohort study of catatonia, Smith et al. (14) reported 75 cases associated with a primary psychiatric disorder (mainly affective disorders) and 20 due to neurological disease (mainly acute encephalitis). A 2-year prospective study at the National Institute of Neurology and Neurosurgery of Mexico reported a cohort of 42 patients with neurological conditions: viral and autoimmune encephalitis, postictal states in epilepsy, acute disseminated encephalomyelitis, and brain tuberculosis (15).
Is it possible that delirium and catatonia coexist? While describing psychomotor behavior in delirium, Lipowski (16) noted that “an occasional patient may be mute or even catatonic”. Certainly, these conditions could share not only phenomenological but also etiological factors; both may arise among patients with acute encephalopathy, as evidenced by a diffuse slowing on electroencephalogram (EEG) (16, 17). EEG is the most widely available physiological measure of delirium. Although not a specific finding, these patients often reveal a diffuse background slowing that may reach the delta range as a measure of encephalopathy, and it is helpful in clinical practice to distinguish between primary and secondary psychiatric conditions (18). Similarly, the observation of abnormalities in the EEGs of patients with catatonia is associated with the presence of general medical conditions underlying the development of catatonia (19).
In a study of 205 patients in a general hospital in India assessed by a Consultation-Liaison Psychiatric Service, Grover et al. (3) reported that 32.0% and 12.7% of their patients with delirium also fulfilled the diagnosis for catatonia according to the Bush-Francis Catatonia Rating Scale (BFCRS) and DSM-5 criteria, respectively. Likewise, a study in a U.S. academic medical center with a convenience cohort of 136 critically ill patients found that 31.0% of the sample had both catatonia and delirium (2). The exact frequency of catatonia among patients with delirium requires clarification because of methodological issues, including the use of low thresholds for the diagnosis of catatonia and the use of sedatives in intensive care unit patients, both of which would result in overestimation of the co-occurrence of catatonia and delirium. A more assiduous phenomenological analysis is necessary to avoid an inflation of co-occurrence rates attributable to syndrome-nonspecific symptom overlap. However, even if the frequency of this co-occurrence is lower than previously estimated, current research suggests that DSM-5 criteria for mutual exclusion between catatonia and delirium require critical review and possible reconsideration.
The co-occurrence of delirium and catatonia has also been observed among patients with anti-N-methyl-d-aspartate receptor encephalitis (20). Acute or subacute onset of complex neuropsychiatric symptoms, including catatonia and delirium, should raise suspicion of an underlying cause, particularly autoimmune anti-N-methyl-d-aspartate receptor encephalitis. A prompt diagnosis and treatment of these conditions are associated with reduced mortality and an improved outcome (21).
In the current study, we aim to describe the behavioral phenomenology and etiology of neurological patients with co-occurring delirium and catatonia and to compare them with patients who present exclusively with delirium.
Methods
The study was approved by the Institutional Research Committee of the National Institute of Neurology and Neurosurgery, according to the Declaration of Helsinki statement of ethical principles (22).
Patients
We conducted a prospective observational study that included patients with a diagnosis of delirium who were treated consecutively over 6 years by the Neuropsychiatric Service of the National Institute of Neurology and Neurosurgery of Mexico. All participants were treated as inpatients in the Neurology and Neurosurgery Departments and referred by their consulting neurologists and neurosurgeons to the Neuropsychiatry Service. Trained psychiatrists engaged in a 1-year neuropsychiatry fellowship assessed the patients and administered the measures used in this study.
Patients were evaluated during their first contact with the Neuropsychiatry Service at admission. The psychiatrists administering the study assessment measures were blinded to etiological diagnoses because the etiological significance of the co-occurrence of delirium and catatonia was a primary question for the present investigation. The assessment of catatonia and delirium was performed before administration of psychoactive medications (antipsychotics or benzodiazepines), as is the clinical practice at our institution.
Neurological Diagnoses
Clinical assessments also included neuropsychiatric interviews and physical and neurological exams. Basic laboratory tests and structural neuroimaging, including MRI, were performed for all patients. EEG and cerebral spinal fluid (CSF) analysis were requested when deemed necessary, particularly when seizures or encephalopathy were considered.
Patients for whom autoimmune encephalitis was suspected, according to Graus criteria for possible autoimmune encephalitis and probable anti-N-methyl-d-aspartate receptor encephalitis (23), were also studied by means of [18]-fluorodeoxyglucose positron emission tomography and measurement of antibodies against the NR1 subunit of the NMDA glutamate receptor in CSF. Diagnostic criteria for possible autoimmune encephalitis include a subacute onset (less than 3 months) of working memory deficits, altered mental status, or psychiatric symptoms and at least one of the following: new focal CNS findings, seizures not explained by a previously known seizure disorder, CSF pleocytosis, or MRI features suggestive of encephalitis. Similarly, criteria for probable anti-N-methyl-d-aspartate receptor encephalitis include a rapid onset (more than 3 months) of at least four of the following group of symptoms: psychiatric, behavioral, or cognitive dysfunction; speech dysfunction; seizures; movement disorders; decreased level of consciousness; and autonomic dysfunction or central hypoventilation. An abnormal EEG, CSF pleocytosis, or the presence of CSF oligoclonal bands is required. For both criteria, a reasonable exclusion of alternative causes is necessary (23).
Neurological diagnoses were assigned by the treating physicians from the Neurology and Neurosurgery Departments (not involved in the study assessments), and these diagnoses were recorded in the study records on the patient’s discharge from the clinical service.
Assessment for delirium.
We used DSM-5 diagnostic criteria to diagnose delirium, with support from the Confusion Assessment Method (CAM) diagnostic algorithm. To assess its severity and phenomenology, we used the Delirium Rating Scale–Revised-98 (DRS-R-98). To specify the delirium subtypes, we used Lipowski’s (16) classification as described in DSM-5: each patient was classified as having “hyperactive,” “hypoactive,” or “mixed” delirium (16). To operationalize the delirium subtype classification, we used the two motor items of the DRS-R98 (24).
The DRS-R-98 is a 16-item clinician-rated scale with two sections: a three-item section used for diagnostic purposes (temporal onset of symptoms, fluctuation of symptoms, and physical etiology) and a 13-item severity section. All sources of available information are used to rate the patient. The DRS-R-98 has a maximum total scale score of 46 points (including the three diagnostic items) and a maximum severity score of 39 points. The DRS-R-98 includes not only the measurement of the core symptoms of delirium but also the presence of delusions, hallucinations, and affective lability, among others, that enrich the psychopathological descriptions (25).
The CAM diagnostic algorithm was specifically designed to diagnose delirium on the basis of DSM-III-R diagnostic criteria and includes acute onset and fluctuating course, inattention, disorganized thinking, and altered level of consciousness. Delirium diagnosis is made when a patient meets at least three of these four criteria. It has been shown to have high sensitivity (94%–100%), high specificity (90%–95%), high interrater reliability, and high negative predictive accuracy (90%–100%) (26, 27). Days of delirium were counted according to the CAM diagnostic algorithm. Once CAM diagnostic algorithm was negative for more than 48 hours, delirium was considered resolved.
Lipowski’s (16) proposal refers to the classification of delirium as hyperactive, hypoactive, and mixed, which are the same levels of activity used by DSM-5 as specifiers for delirium. To operationalize this classification, we used the motor items of the DRS-R98.
Assessment for catatonia.
The BFCRS was used to register the presence of catatonia signs and to measure the severity of the catatonic syndrome. The BFCRS is a 23-item scale, with the first 14 items representing the Bush-Francis Catatonia Screening Instrument (BFCSI). It is the most widely used catatonia rating scale, and it is easy to use and has good interrater reliability and validity (28, 29). It is applied by observing behaviors during the evaluation and the neurological exam, with special orders for signs such as echopraxia, mitgehen, and automatic obedience. It is usually scored over the course of at least 5 minutes, but other items, such as autonomic alterations and withdrawal, can be taken from nursing reports over the preceding 24 hours (29). In this study, we used the interval assessment of the BFCRS because we included items present over a preceding period of time (30).
The DRS-R-98 and the BFCRS were scored only after 24 hours of observation, as recommended for both scales.
Catatonic Delirium Definition
To establish a categorical diagnosis of catatonia among patients with delirium, we created a case definition that would allow us to avoid overestimating the comorbid condition, considering that many signs are unspecific and may be part of both constructs. A case was defined by the following criteria:
Patients must fulfill DSM-5 diagnostic criteria for delirium (293.0), except for criterion D, which refers to the exclusion of delirium.
Patients must present at least four signs from DSM-5 diagnostic criteria for catatonia (293.89) or from the BFCRSI. The conventional cutoff points of three or more items from DSM-5 (1) and two or more items from the BFCRSI (29) were not used because Peralta et al. (31) have shown that the use of four or more catatonic signs as a diagnostic criterion for catatonia results in 100% specificity, with a small number of catatonic patients not being identified. In the context of delirium, the best sensitivity and specificity values of the BFCSI are obtained with a cutoff point of four or more (2).
The following catatonic signs were included in our algorithm (with the operative definitions provided by the BFCRS): BF3, mutism; BF4, staring; BF5, catalepsy-posturing; BF6, grimacing; BF7, echopraxia-echolalia; BF8, stereotypy; BF9, mannerism; BF10, verbigeration; BF11, rigidity; BF12, negativism; and BF13, waxy flexibility. Items BF15–BF23 were registered but not included to fulfill the case definition because they are part of the BFCRS’s Severity Section, not part of the Diagnostic Section. Items BF1, excitement (agitation); BF2, stupor-immobility; and BF14, withdrawal, were excluded from our case definition of catatonia because these signs are frequent and well-recognized features of delirium, and their inclusion would lead to an overestimation of the comorbid condition.
Follow-up measures.
During their hospitalization, patients were observed daily by the research team. We used a data collection sheet to prospectively register clinical variables, including days of hospitalization, days with a delirium diagnosis, days with a catatonia diagnosis, and the presence of complications.
Data analyses.
IBM SPSS Statistics (version 21.0) was used to perform descriptive statistics, as well as normality tests and inferential statistics, according to the distribution of numerical variables. To explore the clinical significance of the catatonic syndrome among patients with delirium, we compared patients classified as having delirium and catatonia with patients with delirium alone. Regarding demographic variables and neurological diagnoses, the results of the initial bivariate analysis were considered in multivariate analysis using a logistic regression model to control the confounding effect. The Hosmer-Lemeshow goodness-of-fit test was performed. Variables with a significant association (p<0.05) with the catatonic delirium syndrome were included in the model.
Results
General Characteristics of the Study Sample
We included 264 patients with delirium. Their mean age was 42.81 years (SD=18.53), and 101 (38.3%) were female. Ninety-four patients (35.6%) were diagnosed as having hyperactive delirium, 58 (22.0%) as having hypoactive delirium, and 112 (42.4%) as having mixed delirium. Of the patients, 64.1% were treated in the neurology ward, and 35.9% were attended in the neurosurgery ward.
Frequency of Catatonic Signs
The most frequent features among the total sample (N=264) were excitement (37%), immobility-stupor (30%), mutism (26%), negativism (26%), staring (23%) and posturing-catalepsy (22%). As explained in the Methods section, the following items were considered nonspecific for assessing catatonia in the context of delirium (and were excluded from Figure 1): BF1, excitement; BF2, stupor-immobility; BF14, withdrawal; BF15, impulsivity; BF20, grasp reflex; BF22, combativeness; and BF23, autonomic abnormalities.
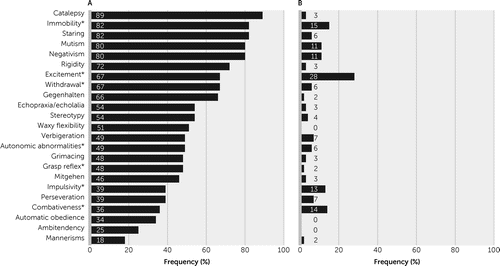
FIGURE 1. Frequency of catatonic signs according to the Bush–Francis Catatonia Rating Scale in patients with catatonia (N=61) and without catatonia (N=203)
a The asterisk denotes the following items that were considered to be nonspecific for the assessment of catatonia in the context of delirium and were excluded from the comorbid catatonia-delirium construct: BF1=excitement; BF2=stupor-immobility; BF14=withdrawal; BF15=impulsivity; BF20=grasp reflex; BF22=combativeness; and BF23=autonomic abnormalities.
Frequency and general features of catatonic delirium.
Two hundred three patients had zero to three catatonic signs. Sixty-one (23.1%) had four or more catatonic signs and fulfilled our definition of catatonic delirium. This classification was used for further analysis to compare patients with and without catatonic syndrome on etiological and outcome variables.
To explore the clinical significance of the catatonic syndrome among patients with delirium, we performed a comparative analysis between patients with and without catatonia. Patients with catatonic delirium were significantly younger (mean age=28.8 years [SD=13.9] vs. 47.0 years [SD=17.6], p<0.001), but there were no significant differences regarding sex (p=0.680) or years of formal education (mean=9.6 years [SD=5.2] vs. 10.5 years [SD=3.7], p=0.177). The group without catatonia had a mean of 1.5 (SD=2.3) positive items on the BFCRS, whereas the group with catatonia had a mean of 12.7 (SD=3.5) positive items (Table 1). This difference was statistically significant. The three types of delirium were present in both groups. The patients with catatonia showed a significantly lower frequency of the hyperactive subtype (21% vs. 39%, p=0.008) and a higher frequency of the hypoactive subtype (19% vs. 31%, p=0.048). There were no differences regarding mixed delirium (47% vs. 40%, p=0.356). In the bivariate analysis, patients with catatonia had both higher DRS-R-98 total scores and DRS-R-98 severity scores (Table 1), although this result was not statistically significant after multivariate analysis (Table 2). Also, multivariate analysis did not confirm hypoactive delirium as being significantly associated with catatonia.
| Variable | Patients without catatonia (N=203) | Patients with catatonia (N=61) | Bivariate analysis | Odds ratio | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |||
| Age (years) (mean±SD) | 47.0 | 17.6 | 28.8 | 13.9 | <0.001b | ||
| DRS-R-98 | |||||||
| Total score | 27.0 | 5.6 | 31.7 | 5.6 | <0.001b | ||
| Severity score | 21.5 | 5.2 | 26.1 | 4.9 | <0.001b | ||
| BFCRS, positive items | 1.5 | 2.3 | 12.7 | 3.5 | <0.001b | ||
| N | % | N | % | ||||
| Female sex | 79 | 38.9 | 22 | 36.1 | 0.688c | 0.88 | 0.48, 1.60 |
| Epilepsy | 35 | 17.2 | 8 | 13.1 | 0.444c | 0.72 | 0.31, 1.65 |
| Ischemic cerebrovascular disease | 19 | 9.4 | 0 | 0 | 0.013c | 0.75 | 0.69, 0.80 |
| Cerebral hemorrhage | 9 | 4.4 | 0 | 0 | 0.094c | 0.76 | 0.71, 0.81 |
| Subarachnoid hemorrhage | 20 | 9.9 | 0 | 0 | 0.011c | 0.75 | 0.69, 0.80 |
| Cerebral venous thrombosis | 4 | 2.0 | 0 | 0 | 0.576d | 0.76 | 0.71, 0.81 |
| Brain neoplasm | 63 | 31.0 | 1 | 1.6 | <0.001c | 0.37 | 0.00, 0.27 |
| Hydrocephalus | 18 | 8.9 | 0 | 0 | 0.016c | 0.75 | 0.70, 0.80 |
| Brain tuberculosis | 6 | 3.0 | 1 | 1.6 | 0.575c | 0.54 | 0.06, 4.63 |
| Bacterial meningo-encephalitis | 7 | 3.4 | 0 | 0 | 0.142c | 0.76 | 0.71, 0.81 |
| Viral encephalitis | 6 | 3.0 | 10 | 16.4 | <0.001c | 6.43 | 2.23, 18.54 |
| anti-NMDAR encephalitis | 20 | 9.9 | 33 | 54.1 | <0.001c | 10.78 | 5.44, 21.35 |
| Autoimmune encephalitis (NMDAR negative antibodies) | 12 | 5.9 | 8 | 13.1 | 0.062c | 2.40 | 0.93, 6.18 |
TABLE 1. Comparative analysis of demographic, etiological, and prognostic characteristics of patients with a delirium diagnosis, with and without catatoniaa
| Variable | Exp(B)b | 95% CI | p |
|---|---|---|---|
| Age (years) | n.a. | 0.029 | |
| Hypoactive delirium | 2.9 | 0.9, 8.8 | 0.053 |
| DRS-R-98 | |||
| Severity score | n.a. | 0.985 | |
| Total score | n.a. | 0.236 | |
| Viral encephalitis | 16.4 | 3.5, 76.1 | <0.001 |
| Anti-NMDAR encephalitis | 10.1 | 3.4, 29.7 | <0.001 |
TABLE 2. Results of the logistic regression model to determine the association of neurological diagnosis with catatonic deliriuma
Etiological significance of catatonic delirium.
The main causes of catatonic delirium were anti-N-methyl-d-aspartate receptor encephalitis (N=33), viral encephalitis (N=10), autoimmune encephalitis with negative CSF NMDAR antibodies (N=8), epilepsy (N=8), brain tumors (N=1), and brain tuberculosis (N=1). Table 1 shows the results of the comparative analysis between patients with delirium with and without catatonia. Patients with catatonic delirium showed higher frequencies of viral encephalitis and anti-N-methyl-d-aspartate receptor encephalitis. We found no significant differences regarding epilepsy, brain hemorrhage, cerebral venous thrombosis, brain tuberculosis, and bacterial meningo-encephalitis. A nonsignificant trend was observed regarding autoimmune encephalitis with a negative determination of NMDAR antibodies. Brain tumors, hydrocephalus, subarachnoid hemorrhage, and ischemic cerebrovascular disease were more frequent in the group with delirium without catatonia (p<0.05). Hence, these conditions were not included in the logistic regression model. Hypoactive delirium was included in the regression model because the initial bivariate analysis showed that it was significantly associated with the catatonic delirium syndrome. The Hosmer-Lemeshow test confirmed the model’s goodness of fit (p=0.254). Table 2 presents the results of multivariate analysis, which confirmed that viral encephalitis and anti-N-methyl-d-aspartate receptor encephalitis are significantly associated with the presentation of catatonic delirium.
Prognostic significance of catatonic delirium.
Patients with catatonic delirium had longer hospital stays (mean=35.3 days [SD=24.2] vs. 26.8 days [SD=20.3], p<0.001) and longer periods of delirium (mean=22.2 days [SD=21.7] vs. 15.8 days [SD=15.8], p<0.030). Catatonic delirium was not associated with an overall higher frequency of complications (75.4% vs. 69.5%, p=0.370). The comparative analysis showed no significant differences between groups regarding pneumonia or other infection, status epilepticus, acute kidney injury, hepatic failure, electrolyte imbalance, or glycemic abnormalities. However, catatonic delirium was associated with a higher frequency during hospitalization of the following events: seizures (47.9% vs. 14.1%, p<0.001) and neuroleptic malignant syndrome (9.8% vs. 1.5%, p=0.002). Electrolytic imbalance was significantly associated with delirium without catatonic features (4.2% vs. 22.9%, p=0.003)
Discussion
The presence of catatonic signs among patients with delirium has been insufficiently studied. Although several researchers have observed this clinical phenomenon in multiple clinical settings (2–5), the current taxonomic approaches to delirium may have the effect of disregarding the possible existence of catatonic signs.
In DSM-5 criteria for catatonia due to general medical condition, criterion D indicates that “one must prove that the disturbance does not occur exclusively during the course of a delirium” (1). This requirement imposes a hierarchical approach in which the presence of delirium precludes the simultaneous diagnosis of catatonia. Thus, catatonic syndrome goes underestimated (4).
To avoid the potentially relevant overlap of delirium and catatonic features (4), we used a case definition that excluded agitation-excitement, immobility-stupor, and withdrawal. From a clinical point of view, several catatonic signs that are not part of the standard construct of delirium (i.e., waxy flexibility, grimacing, stereotypies, mannerisms, gegenhalten, mitgehen) are particularly useful for this distinction. The findings of the present study support the hypothesis that recognizing catatonic delirium may be clinically useful from an etiological perspective and that the mutual exclusivity of delirium and catatonia in DSM-5 requires review and possible reconsideration. Several questions arise and may be helpful in the discussion of both this study’s results and the previous evidence.
Is It Possible to Differentiate Between Delirium Motor Subtypes and Catatonic Delirium?
Both delirium and catatonia exhibit motoric variants (4), so one of the essential questions when considering their co-occurrence is the fact that certain signs may overlap (i.e., they are not specific to either syndrome). Francis and Lopez-Canino proposed that catatonia may account for the motor components of hypoactive delirium (32). Similarly, Grover et al. (3) demonstrated an association between catatonic signs and hypoactive delirium. However, these perspectives on catatonia may overemphasize its motorically hypoactive-stuporous features and ignore the existence of excited catatonia (delirious mania) (33). In a retrospective chart review, Llesuy et al. (34) noted that the presence of agitation increased the likelihood of underdiagnosis of catatonia in general hospitals. More important, fluctuations in psychomotor activity are often seen in both delirium and catatonia (4).
After multivariate analysis, our findings do not support the hypothesis that catatonic delirium is explained by, or fully accounted for by, hypoactive delirium. Catatonic delirium was also observed among patients with hyperactive delirium and mixed delirium. Because this was a referral population, delirious patients who tended to be hypoactive may have been underrepresented in this sample because they are less disruptive and therefore less likely to be referred for care at our institution.
Does Comorbid Catatonia Have Significance for Etiology, Pathophysiology, Prognosis, or Therapeutics Among Patients With Delirium?
Our study revealed a significant association of catatonic delirium with two neurological diagnoses: viral encephalitis and anti-N-methyl-d-aspartate receptor encephalitis. This finding is consistent with recent studies that have highlighted the importance of catatonia in the recognition of patients with this form of autoimmune disease (20, 35). An important link has been observed between catatonic syndrome and immunological mechanisms and conditions (36). However, delirium in patients with anti-N-methyl-d-aspartate receptor encephalitis has received little attention and is probably underestimated (37).
Seizures are a common manifestation of anti-N-methyl-d-aspartate receptor encephalitis, occurring among approximately 70%−80% of cases (38), so the high proportion of these patients in our study may also raise the question of whether features of catatonic delirium were the product of epileptic activity, because postictal states and nonconvulsive status epilepticus have been reported as a cause of catatonic features (39). This issue deserves further research.
Our sample is limited to neurological patients, and thus it must be complemented by studies in general hospitals, geriatric and pediatric settings, and psychiatric wards. One would expect that the clinical phenomenon we describe is not common in general hospitals. Australian and Spanish studies with samples of geriatric patients described catatonia as present in 5.5%−6.3% of the cases being referred to consultation-liaison psychiatry services; the diagnosis was associated with neurological and psychiatric preexisting diagnoses, metabolic conditions, and multifactorial etiologies; some patients presented with additional features of delirium (40, 41). A study from a general hospital in the United States described 54 cases of catatonia and reported that the attribution of catatonia to a psychiatric etiology was associated with significantly less diagnostic workup. In contrast, clinical suspicion of comorbid delirium (in 53% of the cases) was a strong predictor of a more thorough general medical workup, leading to the recognition of sepsis, hyponatremia, lithium toxicity, and renal failure as causes of encephalopathy (42).
In our study, 23.1% of the patients with delirium had four or more catatonic signs, fulfilling our definition of catatonic delirium. In previous studies, the prevalence of catatonia among patients with delirium has been higher. A sample of critically ill patients (N=136) showed a 30% rate of catatonic delirium, and the most significant proportion of those patients had sepsis and acute respiratory distress (2). With a larger sample in a general hospital (N=205), Grover et al. (3) found that catatonia was present among 12.7%–32% of patients with delirium, depending on the diagnostic approach (DSM-5 or BFCRS). Unfortunately, they did not report the medical diagnosis of patients with catatonia and delirium. It is important to consider the possible overestimation of the frequency of the comorbid condition due to nonspecific symptom overlap when a low threshold is used for catatonia diagnosis with the BFCRS.
Diagnosing catatonic signs among patients with delirium has repercussions not only related to the underlying medical conditions but also when symptomatic treatment is necessary (4). The antipsychotics often used to manage agitation during hyperactive delirium can precipitate neuroleptic malignant syndrome in patients with catatonia (43). Benzodiazepines, the first-line treatment for catatonia (44), are known to be deliriogenic, worsening symptoms and lengthening hospital stay among patients with delirium (43). Electroconvulsive therapy (ECT) is considered the most effective treatment for catatonia, even after pharmacotherapy with benzodiazepines has failed (45). However, ECT is not recommended in the management of delirium (43), and this issue continues to be understudied.
In our sample, the presence of catatonic delirium was associated with a longer hospital stay, a more prolonged delirium, a higher incidence of seizures, and neuroleptic malignant syndrome. This severe complication or different forms of antipsychotic intolerance have been reported among patients with anti-N-methyl-d-aspartate receptor encephalitis (35, 46) and also among patients with delirium and catatonia arising from other conditions (41). One would therefore use antipsychotic drugs with great caution among patients with delirium who also have catatonic features.
Regarding the pathophysiological aspects of the comorbid conditions, to our knowledge, no functional neuroimaging studies have addressed patients with coexisting delirium and catatonia. However, although the evidence is still scarce, abnormalities of brain resting-state functional connectivity have been found for both syndromes (47, 48). Delirium has been associated with changes in the default mode network, the salience network, and the frontoparietal control network, which would lead to the altered level of consciousness, reduced awareness of the environment, inattention, and impaired reality testing that facilitates delusions and hallucinations (49). However, in a recent functional MRI study by Parekh et al. (50), patients with catatonia showed reduced connectivity in sensorimotor, salience, frontoparietal, and cerebellar networks. Even though a global disruption of the brain network may be present in both delirium and catatonia, further studies are necessary to reveal whether these neuropsychiatric syndromes share common abnormalities in the functioning of brain networks.
Limitations
Several factors limit the conclusions and generalizability of this study’s findings. Knowing the syndromic diagnosis (delirium or catatonia) before administering the BFCRS, coupled with the notion at our center that catatonia and delirium may co-occur, may have led to overestimation of the frequency of catatonic delirium. Our study did not include a comparison group of patients without delirium and catatonia. Another limitation of this study is the absence of a catatonia-only comparison group. This will be addressed in future designs of this research program. The fact that our study was performed over a 5-year period in a clinical referral cohort might generate a sampling bias that could have increased the reported prevalence of comorbid catatonia and delirium because the referring physician was aware of the authors’ interests. In addition, the failure of some of our analyses to find statistically significant results may reflect a type II statistical error.
Concordance between delirium subtyping methods is low, with agreement found in subtype classification for only 34% of patients. Scales such as the Delirium Motor Subtype Scale specifically address motor subtypes among patients with delirium and correlate with bioelectronic methods (24, 51). Not using a specific scale to classify delirium into its motor subtypes is therefore a limitation of this study. Our study was based in the clinical recognition of the signs of catatonia included in DSM-5 and incorporated into the BFCRS, excluding those that are frequent and well characterized in the delirium construct. However, a question remains: are there specific clusters of coincident signs that occur together and should be required for a more specific diagnosis? This problem is relevant for further research on this topic.
Conclusions
The present study supports the construct of catatonic delirium but not the DSM-5 perspective that delirium and catatonia are mutually exclusive syndromes. Our findings further demonstrate that catatonic delirium informs both etiology and prognosis, including an association with viral and autoimmune encephalitis (mainly anti-N-methyl-d-aspartate receptor encephalitis).
DSM-5 taxonomy classifies catatonia in the psychotic disorders chapter, which could have a misleading effect because not all catatonic states arise from psychosis, and there is clinical evidence of patients with catatonia worsening with the use of antipsychotics; for instance, patients with catatonia and autoimmune encephalitis may develop severe adverse reactions to antipsychotics (35, 47). The precise status of catatonia within psychiatric taxonomy is still controversial, although historical, clinical, and epidemiological reasons exist to separate it from the chapter on psychotic disorders (13). The debate over catatonia and delirium has several implications, not only in terms of diagnosis but also in subjacent medical conditions, pharmacological treatment, and outcome. Despite the current evidence, further studies with rigorous methodological approaches are necessary to reconsider DSM-5 criteria for catatonic disorder due to another medical condition.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Publishing, 2013Crossref, Google Scholar
2 : Delirium and catatonia in critically ill patients: the Delirium and Catatonia Prospective Cohort Investigation. Crit Care Med 2017; 45:1837–1844Crossref, Medline, Google Scholar
3 : Do patients of delirium have catatonic features? an exploratory study. Psychiatry Clin Neurosci 2014; 68:644–651Crossref, Medline, Google Scholar
4 : Catatonia vis-à-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry 2015; 37:554–559Crossref, Medline, Google Scholar
5 : The relationship between catatonic-delirious states and schizophrenia, in the light of a follow-up study (Stauder’s lethal catatonia). Br J Psychiatry 1965; 111:254–257Crossref, Medline, Google Scholar
6 :
7 : Three core domains of delirium validated using exploratory and confirmatory factor analyses. Psychosomatics 2013; 54:227–238Crossref, Medline, Google Scholar
8 : Delirium—a framework to improve acute care for older persons. J Am Geriatr Soc 2018; 66:446–451Crossref, Medline, Google Scholar
9 : The epidemiology of delirium in the community: the Eastern Baltimore Mental Health Survey. Int Psychogeriatr 1991; 3:169–176Crossref, Medline, Google Scholar
10 : Delirium and other organic mental disorders in a general hospital. Gen Hosp Psychiatry 1985; 7:101–106Crossref, Medline, Google Scholar
11 : Delirium in the acute care setting: characteristics, diagnosis and treatment. Crit Care Clin 2008; 24:657–722, viiCrossref, Medline, Google Scholar
12 : Frequency of delirium in a neurological emergency room. J Neuropsychiatry Clin Neurosci 2006; 18:108–112Link, Google Scholar
13 : Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull 2010; 36:314–320Crossref, Medline, Google Scholar
14 : Catatonic disorder due to a general medical or psychiatric condition. J Neuropsychiatry Clin Neurosci 2012; 24:198–207Link, Google Scholar
15 : Catatonia in neurologic and psychiatric patients at a tertiary neurological center. J Neuropsychiatry Clin Neurosci 2016; 28:124–130Link, Google Scholar
16 : Delirium (acute confusional states). JAMA 1987; 258:1789Crossref, Medline, Google Scholar
17 : Frequency of neuropsychiatric disturbances in anti-NMDA receptor encephalitis. Acta Psychiatr Scand 2018; 138:483–485Crossref, Medline, Google Scholar
18 : Delirium: definition, epidemiology, and diagnosis. J Clin Neurophysiol 2013; 30; 438–442Crossref, Medline, Google Scholar
19 : Clinical electroencephalograms in patients with catatonic disorders. Clin Electroencephalogr 1995; 26:60–64Crossref, Medline, Google Scholar
20 : Catatonia in patients with anti-NMDA receptor encephalitis. Psychiatry Clin Neurosci 2019; 73:574–580Crossref, Medline, Google Scholar
21 : Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12:157–165Crossref, Medline, Google Scholar
22 . Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2000; 284:3043–3045Crossref, Medline, Google Scholar
23 : A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15:391–404Crossref, Medline, Google Scholar
24 : Motor subtypes of delirium: past, present and future. Int Rev Psychiatry 2009; 21:59–73Crossref, Medline, Google Scholar
25 : Validation of the Delirium Rating Scale–Revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–242Link, Google Scholar
26 : Clarifying confusion: the confusion assessment method. Ann Intern Med 2013; 113:941Crossref, Google Scholar
27 : Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med 1990; 113:941–948Crossref, Medline, Google Scholar
28 : Measuring catatonia: a systematic review of rating scales. J Affect Disord 2011; 135:1–9Crossref, Medline, Google Scholar
29 : Catatonia, I: rating scale and standardized examination. Acta Psychiatr Scand 1996; 93:129–136Crossref, Medline, Google Scholar
30 : Bush Francis Catatonia Rating Scale: training manual and coding guide, 2020. https://www.urmc.rochester.edu/MediaLibraries/URMCMedia/psychiatry/documents/1-BFCRS-Training-Manual-and-Coding-Guide.pdfGoogle Scholar
31 : DSM-IV catatonia signs and criteria in first-episode, drug-naive, psychotic patients: psychometric validity and response to antipsychotic medication. Schizophr Res 2010; 118:168–175Crossref, Medline, Google Scholar
32 : Delirium with catatonic features: a new subtype? Psychiatr Times 2009; 26(7)Medline, Google Scholar
33 : Delirious mania as a frequent and recognizable neuropsychiatric syndrome in patients with anti-NMDAR encephalitis. Gen Hosp Psychiatry 2020; 64:50–55Crossref, Medline, Google Scholar
34 : Catatonia under-diagnosis in the general hospital. J Neuropsychiatry Clin Neurosci 2013; 30:145–151Link, Google Scholar
35 : Refining the psychiatric syndrome of anti-N-methyl-d-aspartate receptor encephalitis. Acta Psychiatr Scand 2018; 138:401–408Crossref, Medline, Google Scholar
36 : Catatonia and the immune system: a review. Lancet Psychiatry 2019; 6:620–630Crossref, Medline, Google Scholar
37 : Autoimmune encephalopathy for psychiatrists: when to suspect autoimmunity and what to do next. Psychosomatics 2017; 58:228–244Crossref, Medline, Google Scholar
38 : An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol 2019; 18:1045–1057Crossref, Medline, Google Scholar
39 : Ictal catatonia in autoimmune encephalitis. R I Med J 2020; 103:55–58Google Scholar
40 : Prevalence and symptomatology of catatonia in elderly patients referred to a consultation-liaison psychiatry service. Australas Psychiatry 2016; 24:164–167Crossref, Medline, Google Scholar
41 : Prevalence and clinical correlations of catatonia in older adults referred to a liaison psychiatry service in a general hospital. Gen Hosp Psychiatry 2013; 35:512–516Crossref, Medline, Google Scholar
42 : Suspected delirium predicts the thoroughness of catatonia evaluation. J Neuropsychiatry Clin Neurosci 2017; 29:148–154Link, Google Scholar
43 : Delirium. Am J Psychiatry 2019; 176:785–793Crossref, Medline, Google Scholar
44 : A clinical review of the treatment of catatonia. Front Psychiatry 2014; 5:181Crossref, Medline, Google Scholar
45 : Electroconvulsive therapy in catatonic patients: efficacy and predictors of response. World J Psychiatry 2015; 5:182–192Crossref, Medline, Google Scholar
46 : Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm 2016; 3:e280Crossref, Medline, Google Scholar
47 : Resting-state fMRI reveals network disintegration during delirium. Neuroimage Clin 2018; 20:35–41Crossref, Medline, Google Scholar
48 : Brain imaging in catatonia: systematic review and directions for future research. Psychol Med 2020; 50:1585–1597Crossref, Medline, Google Scholar
49 : The network model of delirium. Med Hypotheses 2017; 104:80–85Crossref, Medline, Google Scholar
50 : The fMRI signature of acute catatonic state and its response to benzodiazepines. MedRxiv. https://www.medrxiv.org/content/10.1101/2021.03.23.21253765v1. Accessed Nov 10Google Scholar
51 : A new data-based motor subtype schema for delirium. J Neuropsychiatry Clin Neurosci 2008; 20:185–193Link, Google Scholar



