Neurobehavioral Correlates of Perceived Mental and Motor Slowness in HIV Infection and AIDS
Abstract
The authors assessed 72 human immunodeficiency virus (HIV)–infected patients with a self-rating slowness scale (SRSS) concerning mental and motor slowness in their activities of daily living. In order to understand the relationship between complaints of slowness and predictor variables, the investigators developed a preliminary model using multiple regression analysis. Reports of slowness on the SRSS were independently associated with self-reported cognitive and neurological symptoms and with peripheral neurological syndromes (e.g., neuropathy, myopathy). Lesser contributions to self-perceived mental and motor slowness were found for neuropsychological measures of information processing speed, severity of the infection, depression, HIV encephalopathy, and sociodemographic factors (e.g., age, education). The relationship among the predictor variables showed that complaints of slowness reflect neurological, psychiatric/psychological, and cognitive symptomatology of the HIV infection.
Human immunodeficiency virus (HIV) globally affects the central nervous system, although it appears to have a predilection for subcortical structures, especially the frontal-subcortical systems (e.g., the basal ganglia).1–5 Damage to these structures can cause specific motor and cognitive abnormalities, including poor fine-motor precision, hypophonia, memory deficits, and difficulties performing complex cognitive tasks.6–10 These symptoms are also common among patients with neurological disorders with cortical-subcortical involvement, such as Huntington's disease and Parkinson's disease (PD).11–14 Damage to these systems can also cause significant neuropsychiatric syndromes, including depression, obsessive-compulsive disorder, mania, and apathy.11,15,16 One aspect of subcortical neurological disease is the presence of motor and cognitive retardation (including deficits in mental speed and agility, spontaneity of action, spontaneity of speech for comment or question, initiative, and enthusiasm).12–14
Studies of correlation between HIV-seropositive (HIV+) subjects' perceptions of their own cognitive problems and their actual clinical status have been conducted to detect subclinical neurological or cognitive deficits.17–23 These studies are particularly important in early stages of the HIV infection, when specific complaints may be related to early cerebral dysfunction that eventually may respond to antiviral medication. Stern et al.17 reported a correlation between complains of neurological/cognitive problems and neuropsychological performance in asymptomatic HIV+ patients. By contrast, other studies have found complaints to be correlated with psychiatric symptoms, but not with neuropsychological performance.18,19,21,22 In some cases motor complaints were correlated with abnormal motor performance, but not with psychiatric symptomatology.20 Therefore, it has been suggested that there may be two groups of HIV-infected individuals who complain of problems: those whose subjective cognitive and motor complaints reflect true cognitive impairment, and those whose complaints reflect affective disorders.20
Clearly, self-reported cognitive and motor deficits in HIV infection are subject to multiple influences, including psychiatric and medical conditions and patients' own perceptions of their medical problems. However, the most common approach to the study of self-reported symptoms has been the correlation of complaints—neurological, psychiatric, cognitive, and concerning performance in activities of daily living—with actual psychiatric syndromes or cognitive measures.17–23 Consequently, how the disease as a whole (including effects of medication) affects reports of medical problems has not been examined in detail. Most research has centered on asymptomatic patients.17,19,20,23 Few studies included patients with acquired immunodeficiency syndrome (AIDS),18,21,22 and only one of them examined the effects of severity of the infection on the association between psychiatric complaints and actual psychiatric syndromes.21 Furthermore, as noted above, motor and cognitive retardation appear to be the core of the neuropsychological characteristics of the HIV infection. Although some studies have included items that investigate this aspect of the disease in their research questionnaires for self-reported symptoms,17,20,22 none of them have examined the issue of slowness in isolation.
In this study, we examined the relationship between complaints of mental and motor slowness to medical and cognitive and psychiatric disorders, using a self-rating scale designed to assess psychomotor retardation in the subjects' activities of daily living. We hypothesized that subjective complaints of psychomotor retardation would correlate with objective clinical signs of central nervous system involvement.
METHODS
The neuropsychological and psychiatric characteristics of 72 HIV-infected individuals who completed the self-reported slowness scale were examined. These patients were part of a larger cohort that is being followed every 6 months (the Allegheny County Neuropsychiatric Survey).24 Each patient was assessed with detailed neuropsychological, neurological, and psychosocial examinations. In addition, each had a complete physical examination, medical history, and laboratory testing focusing on HIV/AIDS-related conditions. Centers for Disease Control and Prevention (CDC) criteria for AIDS25 were the presence or history of one or more AIDS-defining illnesses and/or a CD4 cell count of less than 200/mm3. Demographic characteristics and CDC staging of the subjects are shown in Table 1.
Each subject was examined for specific DSM-III-R disorders26 including major depression, generalized anxiety disorder (GAD), adjustment disorder with depressed and/or anxious mood, alcohol abuse/dependence, and drug abuse/dependence. These disorders were assessed with the appropriate sections of the Structured Clinical Interview for DSM-III-R.27 Interviewers were trained to assess mental health in physically ill subjects. In addition, we reviewed all medications taken by the patient before or during the presence of psychiatric symptoms and eliminated medication-induced symptomatology for purposes of determining diagnosis. Details of the psychiatric examination have been published previously.28
Each subject was tested by a trained examiner with experience in assessing physically ill, cognitively impaired adults. Each subject was assessed with a battery that included components of the Wechsler Adult Intelligence Scale–Revised,29 Wechsler Memory Scale–Revised,30 Controlled Oral Word Association,31 Category Test,32 National Adult Reading Test,33 Word Verbal Free Recall Task,34 Trail Making Test,35 and Rotary Pursuit Learning Test.36 Details of the neuropsychological battery are described elsewhere.24 Because different patients completed the slowness questionnaire at different visits to our clinic, we have included in this study the neuropsychological measures common to all patients. Fifty patients had the neuropsychological assessment at study entry and 22 at the 6-month follow-up examination.
A medical history and physical and neurological exams were conducted by a neurologist with experience in HIV/AIDS or by a specially trained nurse. The semistructured exam included a review of systems and covered the entire central nervous system, with particular emphasis on signs and symptoms relevant to HIV-associated neurological conditions. Systemic signs included oral/throat mucosa lesions (including oral hairy leukoplakia), eyes/skin lesions (including Kaposi's sarcoma [KS]), adenomegaly, abnormal chest, lung, heart, and extremities, enlarged liver and spleen, and signs of systemic infections (such as pneumonia, upper respiratory tract infection, or genital infections). Neurological signs included abnormal cranial nerves, abnormal speech, abnormal motor tone, abnormal movements, motor deficits, sensory deficits, stereoagnosia, agraphesthesia, clonus, abnormal deep tendon reflexes (DTRs), abnormal plantar response, release signs, abnormal cerebellar testing, and abnormal gait.
On the basis of the medical history and exam, we further investigated the relationship between slowness and the presence of four debilitating conditions: KS, chronic diarrhea, wasting syndrome, and systemic infections (such as histoplasmosis, Pneumocystis carinii pneumonia, esophageal and lung candidiasis, upper respiratory tract bacterial infection, toxoplasmosis, and cytomegalovirus), current or within the last 6 months. In addition, we investigated the relationship between slowness and the following neurological syndromes commonly associated with HIV infection: 1) neuropathy: includes history of numbness or burning sensations, with documented sensory and/or motor deficits in the neurological examination; DTRs could be diminished or absent, with or without trophic changes; 2) myopathy: includes a gradually progressive muscle weakness, symmetric and with a predominantly proximal pattern; DTRs could be diminished or absent; 3) encephalopathy: includes the presence of neurological signs indicative of generalized CNS involvement (such as release signs, abnormal cerebellar exam, generalized hyperreflexia, or extrapyramidal signs), with or without cognitive deficits. None of these patients exhibited HIV-myelopathy or HIV-associated inflammatory demyelinating polyradiculoneuropathy.
Self-reported symptoms, which were elicited by the examiner as part of the review of symptoms, included 1) medical symptoms: episodes of fever, night sweats, loss of weight, fatigue, oral/genital herpes, oral/esophageal candidiasis, diarrhea, frequent bruises, shortness of breath, and persistent coughing; 2) cognitive symptoms: memory deficits, word-finding difficulty, difficulty writing, reading, speaking, and doing mental calculations, episodes of disorientation, and impaired judgment; 3) neurological symptoms: episodes of syncope, hyper/hyposomnia, loss of libido, impotence, tinnitus, vertigo, dysphagia, dysarthria, dysphonia, disequilibrium, inability to walk/run, frequent falls, seizures, head trauma, blurred vision, diplopia, abnormal movements, and focal motor and sensory deficits. We calculated a total score indicating the total number of symptoms in each area.
The subjects completed a self-rating slowness scale (SRSS) that included the most frequent activities of daily living.37 The subjects were asked to rate the slowness of their own performance during the 6-month period prior to the study visit. The SRSS lists 11 common behaviors, 5 of which are focused on motor slowness and 6 on mental slowness. Each question was rated from 0 (normal) to 5 (extremely slow). The maximum possible score was 55, corresponding to the maximum severity of subjective slowness, and results are presented as an overall score. Content validity was evaluated with a principal component factor analysis in which two factors were extracted. The eigenvalues were 6.83 (62.1% variance) for the first factor and 1.06 (9.6% variance) for the second factor; a single-factor solution was thought to best account for the data. Details of the SRSS have been described elsewhere.37
RESULTS
Complaints of Slowness and Psychiatric, Neuropsychological, and Medical Findings
Sixty-four (89%) of the patients reported at least one symptom of psychomotor slowing on the SRSS (Table 2). The SRSS total score ranged from 1 to 42 (mean±SD=12.2±11.3), and differed as a function of the clinical CDC staging (F=3.09, df=2, P=0.05). Mean SRSS total scores for the different CDC stages were as follows: A, 8.8; B, 11.7; C, 16.6. Post hoc comparisons indicated that patients in stage CDC-C reported significantly more slowing than patients in stage A (Tukey's highly significant difference test, P<0.05). Patients with AIDS had higher slowness scores than patients without AIDS (t=–3.16, df=70, P=0.002). The CD4+ cell count did not correlate with SRSS scores (r=–0.02, P=0.86).
Results for psychiatric symptoms and substance abuse are detailed in Table 3. Patients with major depression had significantly higher SRSS scores than those without depression (t=–2.07, df=70, P=0.04). By contrast, no statistical differences were observed in patients with and without GAD (t=–0.68, df=70, P=0.48), although only one patient met criteria for GAD. The SRSS score was not statistically different between patients with and without alcohol abuse (t=–0.94, df=70, P=0.34) or other substance abuse (sedatives, cannabis, cocaine, opiates, hallucinogenic, polypharmacy, other drugs; t=0.81, df=70, P=0.42), or intravenous drug use (t=–0.86, df=70, P=0.39).
Of the neuropsychological test scores (Table 4), immediate recall of the WMS-R drawings and the measures of verbal free recall had both significant and moderate correlation with SRSS scores. Smaller but statistically significant correlations were found between SRSS scores and word generation, Digit Symbol, Trail Making B, and Rotor Pursuit scores.
The number of systemic, neurological, and cognitive complaints (symptoms) reported during the examination correlated significantly with the SRSS score (Table 5).
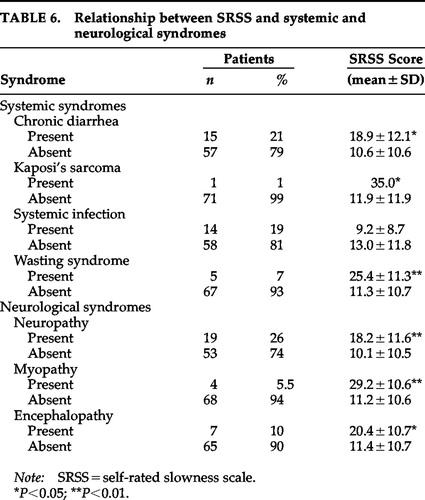
Results for systemic and neurological syndromes appear in Table 6. Patients with chronic diarrhea (t=2.53, df=70, P=0.01), KS (t=–2.06, df=70, P=0.04), wasting syndrome (t=–2.81, df=70, P=0.006), neuropathy (t=–2.77, df=70, P=0.007), myopathy (t=–3.28, df=70, P=0.002), or encephalopathy (t=–2.04, df=70, P=0.04) had higher SRSS scores than those without the syndromes. However, the SRSS score was not statistically different between patients with and without systemic infections (t=1.11, df=70, P=0.27).
Bivariate analyses of past use of antiretroviral medications (including acyclovir, ganciclovir, saquinavir, alpha- and beta-interferon, zidovudine, stavudine, dideoxycitidine [ddC], dideoxyinosine [ddI], foscarnet, recombinant CD4, ribavirin, and vidarabine), and nonviral medication (such as antibiotic or antifungal medication) showed no association with the SRSS score. Similarly, the total number of current antiretroviral (r=–0.13, P=0.36) and nonviral (r=0.19, P=0.22) medications taken per patient did not correlate with the SRSS score.
Multiple Regression Analysis
The results described above demonstrate a number of reliable bivariate associations between various predictor variables and the SRSS score. However, some of these predictors were themselves correlated, and thus we undertook a multiple regression analysis in order to understand the contribution of a variable of interest to predicting the SRSS score.38–40
To aid in the analysis, we first created composite variables based on the neuropsychological and neurological assessment. We used the standardized scores (z-scores) of the Digit Symbol and Trail Making A tasks to create a composite variable reflecting mental processing speed (Speed). This choice was based on a previous assessment on a larger group of patients using the same neuropsychological assessment, where we found that Speed was a significant predictor of cognitive processing.24 We used the presence of either neuropathy or myopathy to create a variable reflecting peripheral motor deficit (PMD).
In order to understand these data we made assumptions about the sequence of events that would result in higher scores on the SRSS. Thus, the presence of symptoms of slowed mentation and physical skills (higher SRSS scores) would be predicted by the presence of peripheral neurological signs and symptoms and the presence of cognitive signs and symptoms. We further assumed that cognitive symptoms were themselves the result of decreasing cognitive efficiency as marked by the Speed composite variable.
The first stage of the analysis was to regress the SRSS score on all of the variables shown in Figure 1. The resulting multiple regression model (F=7.70, df=9,62, P<0.001) indicated that only the PMD (t=2.6, df=64, P=0.01), neurological symptoms (t=4.14, df=64, P=<0.001), and cognitive symptoms (t=2.12, df=64, P=0.04) significantly and independently accounted for variance in the slowness score. The standardized regression coefficients for each of these relationships are shown in Figure 1.
We next regressed each of these three predictor variables—peripheral motor, cognitive, and neurological symptoms—on all of the remaining variables. In the case of the PMD (t=1.93, df=59, P=0.05) and neurological symptoms (t=2.08, df=59, P=0.04), only the CDC stage significantly predicted these factors. By contrast, Speed predicted cognitive symptoms, and Speed itself, as also noted in a separate report,24 was predicted by age (t=–0.31, df=66, P=0.003), education (t=3.1, df=66, P=0.003), and the presence of major depression (t=–1.99, df=66, P=0.05). Further, Speed was also predicted by the presence of encephalopathy (t=3.76, df=66, P<0.001), which was defined as the presence of neurological signs and symptoms consistent with cortical dysfunction, and to a lesser extent by CDC stage (β=–0.17, P=0.09).
The results of this analysis therefore suggest that there are multiple factors that result in individuals rating themselves as “slow.” These factors are related to both increased reported symptoms and their presence observed in physical, neurological, and cognitive dysfunction (see Figure 1).
DISCUSSION
In this study, we simultaneously examined the relationship of subjects' perceptions of their mental and motor slowness and the presence of HIV-related neurological and medical syndromes, mood-related disorders, neuropsychological features, and medication as well as complaints of neurological, cognitive, and systemic problems. By contrast, previous studies tended to focus on individual correlations between complaints of symptoms and either neurological, psychiatric, or cognitive manifestations of the HIV infection.17–23 We found that SRSS scores correlated with severity of HIV infection, major depression, cognitive and neurological complaints, and measures of memory and information-processing speed. In addition, the SRSS score also correlated with neurological syndromes (myopathy, neuropathy, encephalopathy) and systemic syndromes (KS, wasting syndrome). Using this information, we examined the predictors of perceived slowness with a multiple regression analysis. Moreover, we developed an exploratory model to help us understand how these variables and the interrelationship among them may affect the subjects' complaints—specifically, complaints of perceived mental and motor slowness.
Two interrelated dimensions appear to affect SRSS scores. The first includes complaints of cognitive and neurological disturbances and peripheral neurological syndromes, where a close relationship exists between psychological symptoms and actual neurological symptomatology. The second includes variables that independently exert influence on the first dimension, such as age, education, severity of the disease, depression, and encephalopathy acting through mental processing speed. These findings suggest a close relationship between CNS involvement, disease severity, mood-related disorders, sociodemographic factors, and complaints in general, especially those of psychomotor retardation.
When HIV+ patients report specific cognitive deficits, this may more accurately reflect overall slowed processing speed. Mapou et al.20 reported that HIV+ patients who complained of language difficulties actually manifested deficits in simple reaction time tasks rather than in language functions. Moreover, in a separate study, we found that impaired mental processing speed was an independent predictor of frontal, memory, verbal fluency, and spatial performance scores.24 It thus appears that reports of mental slowness reflect slowed psychomotor speed and that this in turn has its effect on other aspects of cognition.
The neuropsychological assessment had two limitations. First, in order to present a homogeneous cognitive battery, we limited the number of tests, although they covered the most relevant HIV cognitive manifestations. Second, a group of patients had the SRSS and cognitive testing at follow-up examination; therefore, a learning effect of neuropsychological information may have influenced these patients' performance. However, the SRSS scores correlated with wordlist learning and nonverbal memory and with measures that had large information processing speed components (word generation, Digit Symbol, and Trail Making Test). This pattern is consistent with the idea that frontal-subcortical system dysfunction is a core phenomenology of psychomotor slowness in HIV.6–10 A similar pattern of memory and slow mentation has been reported in PD patients with bradyphrenia, who are more impaired on memory, reaction time, and continuous performance tests than are PD patients without bradyphrenia.14
The presence of peripheral neurological deficits was a significant predictor of self-reported psychomotor retardation. Motor complaints correlate with measures of fine motor control, and HIV+ patients with motor complaints are neuropsychologically worse than those without complaints.18 Psychomotor retardation, as measured by complaints of symptoms or by actual neuropsychological testing, appears to be involved in the whole spectrum of the neuropsychiatric manifestation of the disease, especially as the infection becomes more severe.
The relationship between slowness and depression appears to be attenuated when other cognitive, neurological, and medical signs and symptoms are taken into account in HIV+ individuals. As noted in our model, although depression can contribute to the feelings of slowness, it appears to exert its major influence through the effect that mood-related disorders have on cognitive function. Indeed, Perkins et al.21 found that complaints of fatigue and motor slowing, as symptoms of major depression in asymptomatic HIV+ patients, were independent of dysphoric mood and other symptoms of major depression.
Our findings indicate the importance of complaints of psychomotor slowness in HIV and the need to further examine their presence in larger HIV cohorts. They are associated with severity of the disease, and they occur in the context of specific HIV-related neuropsychiatric symptomatology, including major depression, peripheral neurological deficits, and cognitive impairment.

FIGURE 1. Associations (standardized regression coefficients) between slowness score and predictor variables. Self-reported slowness is significantly and independently associated with peripheral motor deficits (e.g., neuropathy, myopathy), and complaints of cognitive and neurological symptoms. Sociodemographic factors (e.g., age, education), major depression (MDD), and HIV encephalopathy exert their influence on slowness through impaired mental processing speed (Speed). “CDC” (Centers for Disease Control and Prevention) indicates severity of the disease.
 |
 |
 |
 |
 |
 |
1. Kure K, Weidenheim KM, Lyman WD, et al: Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Acta Neuropathol 1990; 80:393–400Crossref, Medline, Google Scholar
2. Everall I, Luthert P, Lantos P: Neuronal loss in the frontal cortex in HIV infection. Lancet 1991; 337:1119–1121Crossref, Medline, Google Scholar
3. Ketzler S, Weis S, Haug H, et al: Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol 1990; 80:92–94Crossref, Medline, Google Scholar
4. Wiley CA, Masliah E, Morey M, et al: Neocortical damage during HIV infection. Ann Neurol 1991; 29:651–657Crossref, Medline, Google Scholar
5. Masliah E, Achim CL, Ge N, et al: Spectrum of human immunodeficiency virus–associated neocortical damage. Ann Neurol 1992; 32:321–329Crossref, Medline, Google Scholar
6. Grant I, Atkinson JH, Hesselink JR, et al: Evidence for early central nervous system involvement in the acquired immunodeficiency virus (HIV) infections. Ann Intern Med 1987; 107:828–836Crossref, Medline, Google Scholar
7. Wilkie FL, Eisdorfer C, Morgan R, et al: Cognition in early human immunodeficiency virus infection. Arch Neurol 1990; 47:433–440Crossref, Medline, Google Scholar
8. Villa G, Monteleone D, Marra C, et al: Neuropsychological abnormalities in AIDS and asymptomatic HIV seropositive patients. J Neurol Neurosurg Psychiatry 1993; 56:878–884Crossref, Medline, Google Scholar
9. Franzblau A, Letz R, Hershman D, et al: Quantitative neurologic and neurobehavioral testing of persons infected with human immunodeficiency virus type-1. Arch Neurol 1991; 48:263–268Crossref, Medline, Google Scholar
10. Heaton R, Grant I, Butters N, et al: The HNRC 500-neuropsychology of HIV infection at different stages of the disease. J Int Neuropsychol Soc 1995; 1:231–251Crossref, Medline, Google Scholar
11. Cummings JL: Frontal-subcortical circuits and human behavior. Arch Neurol 1993; 50:873–880Crossref, Medline, Google Scholar
12. Lees AJ: The concept of bradyphrenia. Rev Neurol 1994; 150:823–826Medline, Google Scholar
13. Rogers D, Lees AJ, Smith E, et al: Bradyphrenia in Parkinson's disease and psychomotor retardation in depressive illness: an experimental study. Brain 1987; 110:761–776Crossref, Medline, Google Scholar
14. Mayeaux R, Stern Y, Sano M, et al: Clinical and biochemical correlates of bradyphrenia in Parkinson's disease. Neurology 1987; 37:1130–1134Crossref, Medline, Google Scholar
15. Hymas N, Lee AJ, Bolton D, et al: The neurology of obsessional slowness. Brain 1991; 114:2203–2233Crossref, Medline, Google Scholar
16. Widlöcher DJ: Psychomotor retardation: clinical, theoretical, and psychometric aspects. Psychiatr Clin North Am 1983; 6:27–40Crossref, Medline, Google Scholar
17. Stern Y, Marder K, Bell K, et al: Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus infection, III: neurologic and neuropsychological findings. Arch Neurol 1991; 48:131–138Crossref, Google Scholar
18. Wilkins JW, Robertson KR, Snyder CR, et al: Implications of self-reported cognitive and motor dysfunction in HIV-positive patients. Am J Psychiatry 1991; 148:641–643Crossref, Medline, Google Scholar
19. van Gorp WG, Satz P, Hinkin C, et al: Metacognition in HIV-1 seropositive asymptomatic individuals: self-ratings versus objective neuropsychological performance. J Clin Exp Neuropsychol 1991; 13:812–819Crossref, Medline, Google Scholar
20. Mapou RL, Law WA, Martin A, et al: Neuropsychological performance, mood, and complaints of cognitive and motor difficulties in individuals infected with the human immunodeficiency virus. J Neuropsychiatry Clin Neurosci 1993; 5:86–93Link, Google Scholar
21. Perkins DO, Leserman J, Stern RA, et al: Somatic symptoms and HIV infections: relationship to depressive symptoms and indicators of HIV disease. Am J Psychiatry 1995; 152:1776–1781Crossref, Medline, Google Scholar
22. Moore LH, van Gorp WG, Hinkin CH, et al: Subjective complaints versus actual cognitive deficits in predominantly symptomatic HIV-1 seropositive individuals. J Neuropsychiatry Clin Neurosci 1997; 9:37–44Link, Google Scholar
23. Beason-Hazen S, Nasrallah HA, Bornstein RA, et al: Self-report of symptoms and neuropsychological performance in asymptomatic HIV-positive individuals. J Neuropsychiatry Clin Neurosci 1994; 6:43–49Link, Google Scholar
24. Becker JT, Sanchez J, Dew MA, et al: Neuropsychological abnormalities among HIV-infected individuals in a community-based sample. Neuropsychology 1997; 11:592–601Crossref, Medline, Google Scholar
25. Centers for Disease Control and Prevention: 1993 revised classification system for HIV infection and expanded case definition for AIDS among adolescents and adults. MMWR 1992; 41:1–19Google Scholar
26. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised. Washington, DC, American Psychiatric Association, 1987Google Scholar
27. Spitzer RL, Williams JB, Gibbon M, et al: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
28. Dew MA, Becker G, Sanchez G, et al: Prevalence and predictors of depressive, anxiety, and substance abuse disorders in HIV-infected and uninfected men: a longitudinal evaluation. Psychol Med 1997; 27:395–409Crossref, Medline, Google Scholar
29. Wechsler D: Wechsler Adult Intelligence Scale–Revised. New York, The Psychological Corporation, 1981Google Scholar
30. Wechsler D: Wechsler Memory Scale–Revised Manual. New York, Harcourt Brace Jovanovich, 1987Google Scholar
31. DeFillipis NA, McCampbell E: The Booklet Category Research and Clinical Form Manual. Odessa, FL, Psychological Assessment Resources, 1991Google Scholar
32. Benton AL, Hamsher KD: Multilingual Aphasia Examination Manual–Revised. Iowa City, IA, University of Iowa, 1978Google Scholar
33. Nelson HE: National Adult Reading Test: Test Manual. Windsor, UK, NFER-Nelson, 1982Google Scholar
34. Glanzer M, Cunitz AR: Two storage mechanisms in free recall. Journal of Verbal Learning and Verbal Behavior 1966; 5:351–360Crossref, Google Scholar
35. Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
36. Heindel WC, Butters N, Salmon DP: Impaired learning of a motor skill in patients with Huntington's disease. Behav Neurosci 1988; 102:141–147Crossref, Medline, Google Scholar
37. Giconi J, Lopez OL, Becker JT, et al: A self-report rating scale for motor and mental slowness: preliminary results. The Clinical Neuropsychologist (in press)Google Scholar
38. Cohen J, Cohen P: Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ, Lawrence Erlbaum, 1975Google Scholar
39. Kenney DA: Correlation and Causality. New York, Wiley, 1979Google Scholar
40. Judd CM, Kenny DA: Estimating the Effects of Social Interventions. Cambridge, UK, Cambridge University Press, 1981Google Scholar



