Depression Does Not Influence Basal Ganglia–Mediated Psychomotor Speed in HIV-1 Infection
Abstract
The authors examined the effects of depressive mood (Hamilton Rating Scale for Depression [Ham-D]) on basal ganglia–mediated psychomotor speed (motor test battery) in 202 HIV-1 seropositive homosexual males with no prior history of antiretroviral treatment. HIV-1 seropositive patients showed a significant slowing of most rapid alternating movements (MRAM) and significantly prolonged contraction times (CT) compared with 66 HIV-1 seronegative male control subjects. Factor analysis of Ham-D scores isolated a factor containing the items depressed mood, suicide, and psychic and somatic anxiety. This factor did not correlate with MRAM or CT. Depression and psychomotor speed are independent in HIV-1infection.
Human immunodeficiency virus, type 1 (HIV-1)–associated cognitive/motor complex (HIV-1-CMC) is the predominant clinical manifestation of HIV-1 in the brain. Defining features of the HIV-1-CMC are 1) “an acquired abnormality in…cognitive abilities”; 2) “acquired abnormality in motor function or performance verified by clinical examination, neuropsychological tests or both”; and 3) “a decline in motivation or emotional control or change in social behavior.” (Janssen et al., 1991,1 p 780). Although the complex or syndrome was already described in an early clinical review,2 a long-lasting scientific debate ensued about possible preclinical manifestations of HIV-1-CMC. At first the debate concerned the question of whether there are any such manifestations. Today it has become widely accepted that HIV-1–associated minor cognitive/motor disorders can be diagnosed.1
Although different test paradigms were used, both European3–5 and American6 studies (Multicenter AIDS Cohort Study [MACS]) have shown that psychomotor slowing in HIV-1 infection is a predictor of dementia, AIDS, and death. Furthermore, different regimens of antiretroviral drugs improve psychomotor speed: zidovudine,7 stavudine,8 and combination antiretroviral therapies.9 A careful interpretation of the results of psychomotor speed tests is therefore essential for an individual prognosis.
The relationship between HIV-1 infection and mood disorder is complex10,11 because both HIV-1 seropositive and seronegative homosexual men are at high risk for major depression.11 Both motor disorders and depressive symptoms are constitutive signs of HIV-1-CMC.1 Depressed patients perform less well on neuropsychological tests covering the domain of memory but are not more likely to show significant cognitive impairment than nondepressed patients.12
The question therefore arises of whether depressive symptoms represent a potentially important confounding factor in the interpretation of psychomotor test results. The MACS has shown that rapid complex cognitive processing is not influenced by depressive symptoms in HIV-1 infection.13 But no study to date has examined the effect of depression on fine basal ganglia motor function in HIV-1 infection. The basal ganglia play a major role in HIV-1 neuropathogenesis.14 Basal ganglia motor function can be quantitatively assessed by our electrophysiological motor test battery both in HIV-1 infection3 and in other well-defined basal ganglia diseases such as Parkinson's disease,15 Wilson's disease,16 and Huntington's chorea.17
METHODS
Patients
We analyzed the data sets of all homosexual male HIV-1 seropositive (HIV+) patients who were naive to any antiretroviral treatment at first presentation in our department. These patients (n=202) formed group 1. None of them had a history of neurological diseases or psychiatric disorders other than those related to the HIV-1 infection. Patients with substance abuse were excluded. Stages of HIV-1 infection were grouped according to the current Centers for Disease Control and Prevention (CDC) classification18 into non-AIDS stages (CDC stages A1, A2, B1, and B2) or AIDS stages (all CDC stages C or 3). Eleven patients fulfilled the clinical criteria for HIV-1-CMC defined by the American Academy of Neurology.1 Nine patients showed symptoms of HIV-1–associated polyneuropathy. However, it has been shown that the results of our test battery are not affected by peripheral nerve slowing.19 Sixty-six HIV-1 seronegative (HIV–) healthy male volunteers served as control subjects (group 2). Complete demographic data are given in Table 1.
Central Motor Testing
Central motor testing was established for HIV+ patients in our department many years ago and includes the analysis of postural tremor of the outstretched hands (tremor peak frequency [TPF]); most rapid alternating movement (MRAM) of index finger; and most rapid isometric contraction (extension) of index finger (MRC), including simple reaction time (RT) and contraction time (CT).
The following is a brief summary of methodological details published earlier:3,7
A lightweight accelerometer taped to the nail of the subject's index finger was used for recording the TPF and the MRAM. For TPF, subjects were asked to hold their arms in a horizontal position with the forearms fully pronated and fingers completely outstretched. Spectrum analysis was performed offline, and the frequency of the dominant peak of the average spectrum was defined as TPF. For MRAM, subjects were told to flex and extend the index finger at the metacarpophalangeal joint as rapidly as possible. For MRC recordings, the index finger was firmly attached to a force transducer. Subjects were asked to extend the finger as soon as possible after hearing a “go” signal. The simple reaction time (RT) as well as the time between the onset of the contraction and the point at which it reached its maximum (CT) were recorded.
Psychometric Testing
To assess premorbid verbal intelligence and current nonverbal intelligence, the Mehrfachwortauswahl test MWT-b20 and the Raven's Progressive Matrices test21 were performed. To assess depressive symptomatology, the Hamilton Rating Scale for Depression (Ham-D)22 was used. The Ham-D consists of a semistructured interview evaluating 21 different items. Central motor and psychometric testing were performed by persons blind to all clinical data.
Statistical Analyses
Statistics were analyzed with the commercially available software package Statview for Windows, Version 4.57 (Abacus Concepts, Inc., Berkeley, CA, 1996). Descriptive statistics were used to characterize subjects and parameters. Psychomotor test results were compared by an unpaired t-test, and P-values were Bonferroni adjusted. For Ham-D items, a factor analysis was performed as a principal components analysis. The number of factors was determined by retaining factors until 75% of the original variance was explained by the factors that had been retained. Orthogonal transformation (according to the original procedure by Hamilton22) was carried out by a variance-maximizing (varimax) rotation. Individual scores for the four Ham-D items with the highest saturation for the first factor (items 1, 3, 10, and 11) were added and analyzed separately as Ham-D factor 1. Individual scores for the Ham-D items 4, 5, and 6 were added and analyzed separately as Ham-D factor 2. Individual Ham-D factor 1 scores, factor 2 scores, and the overall Ham-D score were correlated with all motor test results of both hands, and an analysis of variance (ANOVA) was carried out.
RESULTS
Table 1 provides the demographic data of all patients included in groups 1 and 2. Group 1 is a homogeneous group including only HIV+ homosexual men. Patients with any form of antiretroviral therapy that might have interfered with the test results were excluded. Patients belonged to all CDC stages, 40% being AIDS-defined and 5% clinically demented. Patients and control subjects were not significantly different with regard to age, premorbid verbal IQ (MWT-b), and current nonverbal IQ (Raven).
Results of different psychomotor tests are given for both hands in Table 2. The patient group showed significantly prolonged CTs and lower MRAM frequencies compared with the control group. Demented patients performed most poorly, but the differences remained significant even when the test results of the demented patients were excluded from the statistical analysis.
CT and MRAM values did not significantly correlate with age, premorbid verbal IQ (MWT-b), or current nonverbal IQ (Raven).
Ham-D scores were either 0 or 1 in the control subjects. Figure 1 shows the cumulative scores of all 202 patients for each single Ham-D item. Items most affected were #7, work and interests (204 points); #1, depressed mood (143 points); and #4, initial insomnia (94 points). Items #19, depersonalization; #20, paranoid symptoms; and #21, obsessional symptoms, scored 1, 0, and 0, respectively. Because items #18–21 serve to classify the type of depression, they did not enter into the subsequent analysis.
We performed a factor analysis as described above. The orthogonal solution is given in Table 3. Saturations higher than 0.4 are boldfaced. Factor 1 includes the items #1, depressed mood; #3, suicide; #10, psychic anxiety; and #11, somatic anxiety. Two of these items (# 1 and #3) belong to the “depressive triad.” Ham-D factor 1 constituted the major correlate of depression in our patients, accounting for 23.6% of the overall variance of the data set. Ham-D factor 2 includes items #4, initial insomnia; #5, middle insomnia; and #6, delayed insomnia, and can be interpreted as “insomnia,” accounting for 11.7% of the overall variance. All other factors account for less than 10%. Because the solution of factor analysis was similar whether or not demented patients had been included in the calculations, we did not exclude them from the subsequent analysis. The individual total scores of Ham-D, Ham-D factor 1 score, and Ham-D factor 2 score were analyzed for correlation with all motor test parameters. No significant correlations were found.
Figure 2 illustrates the distribution of individual CT and MRAM measurements for both hands and Ham-D factor 1, showing no correlation between these parameters.
Table 4 shows the results of ANOVA for the mean values of TPF, MRAM, RT, and CT for both hands. Ham-D factor 1 scores were separated into three groups (0, 1–3, or 4 or more points). There were no significant differences between these groups with respect to MRAM and CT, the psychomotor parameters significantly affected in HIV+ subjects. Thus, psychomotor speed evaluated by CT and MRAM and depression evaluated by Ham-D are independent. With regard to TPF, depressed patients tended to show even higher (i.e., better) frequencies than nondepressed patients. RT tended to be prolonged in depressed patients, but this tendency was not significant.
DISCUSSION
This study examined the effect of depressive mood on basal ganglia–mediated psychomotor speed, as recorded with a refined motor test battery used to evaluate defined basal ganglia disease. We chose a homogeneous group of 202 HIV-1 seropositive homosexual males, all naive to any type of antiretroviral therapy. In order to avoid the possible effects of learning, only the results of the first psychomotor test done on each subject were used in the statistical analysis. We were interested in the influence of any type of depressed mood, whether HIV-1–related or not, on psychomotor speed, and therefore we did not differentiate between biological and psychological mood disorder. The Ham-D is an effective tool to quantify depression in HIV-1 infection and is currently also used in therapeutic trials.23,24 Patients in our study showed various types and degrees of depressive symptoms (see Figure 1), but factor analysis revealed that Ham-D factor 1, containing the items depressed mood, suicide, and psychic and somatic anxiety, is dominant. Factor 1 can be understood as the correlate of any type of depression in HIV-1 infection. Ham-D factor 1 did not contain any somatic items, which is important insofar as it has been repeatedly shown that the diagnosis “depressive syndrome” in psychometric testing is often influenced by somatic items.25–29 In HIV-1 infection or AIDS, somatic conditions might exaggerate estimates of depression.
Our findings show that depression as assessed by Ham-D factor 1 or Ham-D total score does not influence psychomotor test results as assessed by MRAM and CT. We therefore conclude that depression does not influence basal ganglia–mediated psychomotor speed in HIV-1 infection. Measuring basal ganglia–mediated psychomotor speed is therefore an effective means for evaluating CNS function or dysfunction and can be used without having to take different forms of depression in HIV-1 into account.
ACKNOWLEDGMENTS
The authors thank Mrs. K. Rascher for helpful linguistic advice.
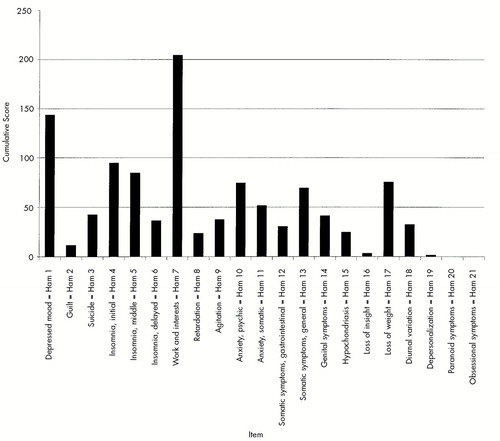
FIGURE 1. Cumulative scores for 202 HIV-1 seropositive patients and HIV-1 seronegative control subjects on 21 Hamilton Rating Scale for Depression (Ham) items
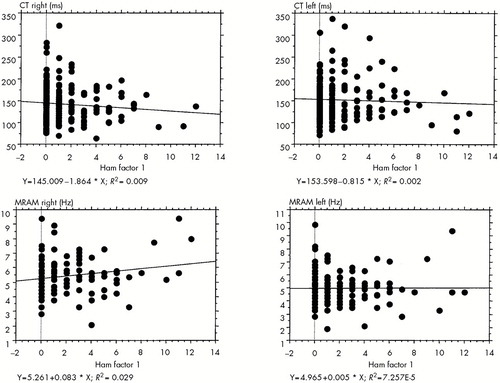
FIGURE 2. Regression plots for psychomotor speed parameters CT (contraction time) and MRAM (most rapid alternating movement) for both hands and Hamilton Rating Scale for Depression (Ham) factor 1
 |
 |
 |
 |
1 Janssen RS, Cornblath DR, Epstein LG, et al: Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus–type 1 (HIV-1) infection. Neurology 1991; 41:778–785Crossref, Medline, Google Scholar
2 Snider W, Simpson D, Nielsen S, et al: Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol 1983; 14:403–418Crossref, Medline, Google Scholar
3 Arendt G, Hefter H, Elsing C, et al: Motor dysfunction in HIV-infected patients without clinically detectable central-nervous deficit. J Neurol 1990; 237:362–368Crossref, Medline, Google Scholar
4 Arendt G, Hefter H, Neuen-Jacob E, et al: Electrophysiological motor testing, MRI findings and clinical course in AIDS patients with dementia. J Neurol 1993; 240:439–445Crossref, Medline, Google Scholar
5 Arendt G, Hefter H, Hilperath F, et al: Motor analysis predicts progression in HIV-associated brain disease. J Neurol Sci 1994; 123:180–185Crossref, Medline, Google Scholar
6 Sacktor NC, Bacellar H, Hoover DR, et al: Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. J Neurovirol 1996; 2:404–410Crossref, Medline, Google Scholar
7 Arendt G, Hefter H, Buescher L, et al: Improvement of motor performance of HIV-positive patients under AZT therapy. Neurology 1992; 42:891–896Crossref, Medline, Google Scholar
8 Arendt G, Giesen HJv, Jablonowski H: Stavudine stops neuro-AIDS in AZT non-responders. 12th World AIDS Conference, 1998, Geneva, Switzerland. Abstract 564/32207Google Scholar
9 Sacktor NC, Lyles RH, Skolasky RL, et al: Combination antiretroviral therapy improves psychomotor speed performance in HIV-seropositive homosexual men. Multicenter AIDS Cohort Study (MACS). Neurology 1999; 52:1640–1647Google Scholar
10 Atkinson JH, Grant I: Mood disorder due to human immunodeficiency virus: yes, no, or maybe? Semin Clin Neuropsychiatry 1997; 2:276–284Google Scholar
11 Perkins DO, Stern RA, Golden RN, et al: Mood disorders in HIV infection: prevalence and risk factors in a nonepicenter of the AIDS epidemic. Am J Psychiatry 1994; 151:233–236Crossref, Medline, Google Scholar
12 Goggin KJ, Zisook S, Heaton RK, et al: Neuropsychological performance of HIV-1 infected men with major depression (HNRC Group, HIV Neurobehavioral Research Center). J Int Neuropsychol Soc 1997; 3:457–464Crossref, Medline, Google Scholar
13 Llorente AM, Miller EN, D'Elia LF, et al: Slowed information processing in HIV-1 disease: the Multicenter AIDS Cohort Study (MACS). J Clin Exp Neuropsychol 1998; 20:60–72Crossref, Medline, Google Scholar
14 Berger JR, Nath A: HIV dementia and the basal ganglia. Intervirology 1997; 40:122–131Crossref, Medline, Google Scholar
15 Hefter H, Hömberg V, Freund HJ: Quantitative analysis of voluntary and involuntary motor phenomena in Parkinson's disease, in Early Diagnosis and Preventive Therapy in Parkinson's Disease, edited by Przuntek H, Riederer P. Vienna and New York, Springer Verlag, 1989, pp 65–73Google Scholar
16 Hefter H, Arendt G, Stremmel W, et al: Motor impairment in Wilson's disease, I: slowness of voluntary limb movements. Acta Neurol Scand 1993; 87:133–147Crossref, Medline, Google Scholar
17 Hefter H, Hömberg V, Lange HW, et al: Impairment of rapid movement in Huntington's disease. Brain 1987; 110:585–612Crossref, Medline, Google Scholar
18 Centers for Disease Control:1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR 1992; 41(RR-17):1–19Google Scholar
19 Logigian EL, Hefter H, Reiners KH, et al: Neurophysiology of fastest voluntary muscle contraction in hereditary neuropathy. Ann Neurol 1990; 27:3–11Crossref, Medline, Google Scholar
20 Lehrl SG, Triebig G, Fischer B: Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 1995; 91:335–345Crossref, Medline, Google Scholar
21 Raven J: The Raven's progressive matrices: change and stability over culture and time. Cognit Psychol 2000; 41:1–48Crossref, Medline, Google Scholar
22 Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–63Crossref, Medline, Google Scholar
23 Elliott AJ, Russo J, Bergam K, et al: Antidepressant efficacy in HIV-seropositive outpatients with major depressive disorder: an open trial of nefazodone. J Clin Psychiatry 1999; 60:226–231Crossref, Medline, Google Scholar
24 Zisook S, Peterkin J, Goggin KJ, et al: Treatment of major depression in HIV-seropositive men. HIV Neurobehavioral Research Center Group. J Clin Psychiatry 1998; 59:217–224Crossref, Medline, Google Scholar
25 Atkinson JH, Grant I: Prevalence of psychiatric disorders among men infected with human immunodeficiency virus. Arch Gen Psychiatry 1988; 45:859–864Crossref, Medline, Google Scholar
26 Drebing CE, Van Gorp WG, Hinkin C, et al: Confounding factors in the measurement of depression in HIV. J Pers Assess 1994; 62:68–83Crossref, Medline, Google Scholar
27 Fell M, Newman S, Herns M, et al: Mood and psychiatric disturbance in HIV and AIDS: changes over time. Br J Psychiatry 1993; 162:604–610Crossref, Medline, Google Scholar
28 Griffin KW, Rabkin JG, Remien RH, et al: Disease severity, physical limitations and depression in HIV-infected men. J Psychosom Res 1998; 44:219–227Crossref, Medline, Google Scholar
29 Maj M: Depressive syndromes and symptoms in subjects with human immunodeficiency virus (HIV) infection. Br J Psychiatry Suppl 1996; 30:117–122Medline, Google Scholar



