EEG Patterns in Persons Exposed to Ionizing Radiation as a Result of the Chernobyl Accident
Abstract
Prospective conventional EEG study was carried out 3–5 and 10–13 years after the Chernobyl accident (1986) in patients who had acute radiation sickness and in emergency workers in 1986 (“liquidators”). Control groups comprised healthy volunteers; veterans of the Afghanistan war with posttraumatic stress disorder; veterans with mild traumatic brain injury; and patients with dyscirculatory encephalopathy. In 3–5 years after irradiation, there were irritated EEG changes with paroxysmal activity shifted to the left frontotemporal region (cortical-limbic overactivation) that were transformed 10–13 years after irradiation toward a low-voltage EEG pattern with excess of fast (beta) and slow (delta) activity together with depression of alpha and theta activity (organic brain damage with inhibition of the cortical-limbic system). Quantitative EEG is likely to be very informative for investigation of dose–effect relationships.
Exposure to ionizing radiation has multiple effects on the brain and behavior. These changes depend largely on the dose received.1 Ionizing radiation influences central nervous system (CNS) functions and behavior both as a result of direct effects on the nervous system and indirectly through CNS reactivity to the radiation damage of other systems.2,3
The problem of the mature human brain's radiovulnerability has been a point at issue ever since the world's first radiobiological experiments, which Russian physiologist-biophysicist Tarkhanov carried out in 1896.4 Edison (1896) showed that “the blind may see a little” if an object is situated between closed eyes and a source of ionizing radiation.5 In 1896–1906 Russian physiologists showed significant neurophysiological abnormalities due to exposure to ionizing radiation6–8 and came to the conclusion that the nervous system is the most radiosensitive tissue.9
At the beginning of the 20th century German pathomorphologists Scholz, Obersteiner, and others found no histological evidence of brain damage after irradiation, which became the basis of the point of view that the brain is radioresistant. It should be stressed that this position completely ignored the data of neurophysiological studies.10
In 1906 French radiotherapeutists Bergonie and Tribondeau formed the foundation of cancer radiotherapy, the principle that “the sensitivity of cells to irradiation is in direct proportion to their reproductive activity and inversely proportional to their degree of differentiation.” Consequently, adult nervous tissue was recognized as an excellent example of a “closed static population,” and because of its fixed postmitotic state, this population was considered as “extremely radioresistant.”11 The problem of radiation effects on the nervous system was conceptualized as “traditionally Russian” and ignored.
H. Davis and P. Davis (1939) were the first authors who used EEG for study of ionizing radiation effects on the brain. These researchers revealed significant brain electrical activity changes in irradiated monkeys.12 Multiple spikes, asymmetry of amplitudes at the temporal areas, and other subcortical (diencephalic) signs were described in 27 atomic bomb survivors in Hiroshima and Nagasaki who had acute radiation symptoms.13 Three of another 30 A-bomb survivors had epileptiform activity on EEG as bilateral spikes and polyspike–slow wave activity.14
In the 1960s Soviet researchers published a lot of information concerning high radiosensitivity of the nervous system.15,16 Total irradiation at doses significantly smaller than 0.01 Gy (1 rad) can irritate CNS, as confirmed by registration of brain electrical activity changes. The revealed changes of CNS were primary relative to vascular and other disturbances provoked by exposure to ionizing radiation.16 Changes of brain biopotentials were found directly at the time of irradiation with a dose rate of 0.13 mGy·s–1 that formed the total absorbed dose of 0.5 mGy only. The dose–effect relationship is described by an S-shaped curve. Doses of ionizing radiation–induced EEG abnormalities were 20–50 times lower than those caused by histologically assessed brain pathology.17 Increased interest in radiation effects on the brain resulted in the organization by the International Atomic Energy Agency (IAEA) of the international symposium “Effects of Ionizing Radiation on the Nervous System,” in 1961 in Vienna.18 The data of Soviet authors about the highest brain radiosensitivity were supported by other researchers: exposure to 2–4 Gy caused spike discharges in hippocampus and an increase of fast low-voltage activity.19,20
Exposure to ionizing radiation in doses up to 1.5–4 Sv (150–400 rem) caused an increase of beta and abnormal slow activity in proportion to dose of irradiation.21 At a period remote from the radiotherapy of tinea capitis in childhood (dose on the brain 1.3 Gy), increased beta power on EEG was recognized as a “radiation trace”; however, the routine EEG pattern was changed moderately.22
Even though the mature CNS is commonly considered to be extremely radioresistant, evidence is dramatically increasing in support of the exceptional radiosensitivity of the brain.23 The limbic system, particularly the hippocampus, looks likely to be the most radiosensitive structure. The hippocampus (paleocortex), as “heart of the limbic system,” is being given increasing consideration for an explanation of the neurophysiological effects of irradiation.2,24,25 A direct activating effect of ionizing radiation on endogenous (pacemaker) generation of nervous impulses in hippocampus was registered at doses of 6–8 mGy.26
Changes in electrical activity of the brain are related to the degree of irradiation, although the relationship is not a straightforward linear one. They occur at thresholds of 0.3 to 1 Gy and increase with the dose absorbed.27 These data suggest that alteration in CNS functioning is likely to occur after relatively low doses of radiation.3
After the Chernobyl accident (April 26, 1986), publications relating neuro- and psychophysiological aftereffects in exposed populations were dramatically increased.28–41 The main methodological limitations of the majority of these studies are as follows: 1) uncertainties in diagnosis of neuropsychiatric disorders; 2) an absence or lack of dosimetrical assessment; 3) an absence of statistical comparison with relevant control groups with similar neuropsychiatric pathology; and 4) a lack of description of conventional EEG phenomena. As a result, the question of whether EEG abnormalities in Chernobyl survivors are due to ionizing radiation, are caused by a complex of radiation and nonradiation (predominantly psychogenic) factors, or developed independently just after the accident is still open to discussion. That is why an absence of clear data about specificity of neurophysiological changes following exposure to ionizing radiation and their correspondence to a relationship “dose effect” does not allow confirmation or rejection of the hypothesis about high radiosensitivity of the human brain.
The object of this study was prospective characterization of brain electrical activity by conventional EEG in Chernobyl accident survivors who had been exposed to ionizing radiation, in order to test the hypotheses that the following would be found: 1) specificity of neurophysiological abnormalities in irradiated patients that can be considered as radiation effects on the brain, and 2) possible dose-related neurophysiological effects of ionizing radiation.
METHODS
Background
Among Chernobyl accident survivors, the most critical group from the radiological point of view is the patients who had acute radiation sickness (ARS)—systemic disease due to momentary (or short-term) whole-body exposure to ionizing radiation at a dose more than 1 Gy. ARS has been diagnosed in 237 Chernobyl accident victims, of whom 29 died as a result of the exposure 7–96 days after the accident,42 but it is an internationally accepted fact that there were 134 patients with verified ARS only.43
The second radiologically critical group includes 126,000 “liquidators” (emergency cleanup workers) who worked during the period from April 26, 1986, to the beginning of 1987. Their average dose of irradiation was 120–180 mSv, but 6%–15% (7,560–18,900) of them were irradiated by more than 250 mSv. At the same time, the effective doses of irradiation for other Chernobyl accident survivors' contingents were significantly lower.44–46
Neuropsychiatric disorders in the exposed population are the dominating medical and social problem in the period remote from the accident. However, etiology of these neuropsychiatric disorders is conceptualized in a variety of ways, ranging from an overestimation of radiation effects even when exposure was close to background level (“postradiation encephalopathy,” “postradiation epilepsy,” “postradiation dementia,” etc.) to diagnosis of psychogenic stress-related “somatoform” and “neurotic” disorders even in those Chernobyl accident survivors who developed ARS.23
“Vegetative-vascular dystonia” (autonomic nervous system dysfunction or dysautonomia) or “neurocirculatory dystonia” were the first and the most common diagnoses for the exposed Chernobyl population that referred to etiologically heterogeneous abnormalities of the diencephalic-limbic-reticular complex. The patients manifested with fatigue, lability of heart rate and blood pressure, sweating, headache, pain in the chest and heart area, back pain, pain in limbs, vertigo, memory and concentration deterioration, weakness, irritability, affective lability, anxiety, sleep disorders, meteotropia, etc. Symptoms of vegetative-vascular dystonia in irradiated patients were often paroxysmal. In 3–5 years after exposure neuropsychiatric symptoms became more severe, and these patients were diagnosed with “dyscirculatory encephalopathy,” a chronic cerebrovascular disorder. However, clear signs of proper vascular diseases (arterial hypertension, cerebral atherosclerosis) were observed in 65% of them only. Further, the neuropsychiatric syndrome in patients at the period more remote from ARS was classified as “postradiation encephalopathy,” and in those exposed to 0.3–1 Gy, “somatogenous-exogenous encephalopathy,” indicating the etiologic role of ionizing radiation.23,35,36
Experts of the International Chernobyl Project (IAEA, 1992) stressed the differences in diagnosis of mental disorders and diseases of the nervous system between the USSR and the Western countries. The terms “neurocirculatory dystonia” and “vegetative-vascular dystonia” are not accepted in the West, where such disorders could be classified as posttraumatic stress disorder (PTSD), anxiety, depression, or somatoform disorders.47 However, such interpretation leads toward an underestimation of health effects of ionizing radiation and an overestimation of the role of psychogenic traumatization following the nuclear accident. The Russian conception of “vegetative-vascular dystonia” in irradiated patients is closer to Penfield's “diencephalic autonomic epilepsy,” with paroxysmal activity focus at the hypothalamus, and the etiologically heterogeneous radiation-psychogenic “diencephalosis” that Konuma described in A-bomb survivors.13,48,49
The differences should be stressed between the diagnostic systems of the former USSR and the Western countries. These differences are beyond the authors' ability to change; however, an attempt to bridge the gap between the East and West has been undertaken in this study.
Patient Selection
We present the background information in detail, noting below a number of limitations and assumptions of the study that should be taken into account in interpretation of the data obtained, and explaining the criteria for patient selection.
| 1. | Accidental character of the exposure. This resulted in an absence of data concerning baseline brain electrical activity before exposure and difficulties with dose of radiation assessment. Consequently, we selected those exposed patients who retrospectively were healthy before the accident and for whom reliable irradiation dose assessment was available. | ||||
| 2. | Clear definition of the disease studied. If diagnosis of ARS patients with ARS verified by international experts does not provoke misinterpretation, the clinical syndrome in cases of liquidators exposed to doses of less than 1 Gy may be confusing for classification. In such cases we used the selection criterion of onset of vegetative-vascular dystonia after irradiation without any physical disease. | ||||
| 3. | Gender distribution. Taking into account that 95% of ARS patients are males and that the most of the liquidators (firemen, reservists, soldiers, officers, miners, etc.) are males also, we selected for the study males only. | ||||
| 4. | Independent control group selection. The main criterion was exposure to background radiation only. The normative group included age- and gender-matched healthy volunteers. Veterans of the Afghanistan war with PTSD were the control group for stress. Veterans of the Afghanistan war with mild traumatic brain injury (MTBI) were the control group for nonspecific brain injury. Nonirradiated age- and gender-matched patients with dyscirculatory encephalopathy were the control group for alternative explanation (due to cerebrovascular disorders) of the genesis of the neuropsychiatric syndrome in irradiated persons. | ||||
| 5. | Dose of irradiation ranges. Taking into account the above description of radiation effects, the subjects selected for this study can be reasonably divided into two groups: I—ARS patients (dose more than 1 Gy), and II—liquidators (0.1–1 Gy). | ||||
| 6. | Periods after the Chernobyl accident for prospective examinations of the selected subjects. Taking into account the above-described clinical course of neuropsychiatric disorders (vegetative-vascular dystonia just after exposure and encephalopathy 3–5 years later), we carried out a two-stage study. The first stage occurred in 1989–1991 (“dysfunction” stage) and the second stage in 1996–1999 (“encephalopathy” stage). It should be noted that the same subjects (both irradiated and control) were examined twice—in 1989–1991 and in 1996–1999, except for the control group of patients with dyscirculatory encephalopathy, who were examined at the second stage only, in 1996–1999, for comparison with irradiated patients at the “encephalopathy” stage. Two-stage examination (in 1989–1991 and 1996–1999) of the same control groups and the same exposed patients aimed to exclude a possible bias due to aging of patient population. | ||||
Patient Population
The Research Centre for Radiation Medicine (RCRM) of the Academy of Medical Sciences (AMS) of Ukraine, in Kiev, is the major referral center for Chernobyl accident survivors. ARS patients were inpatients of the Department of Radiation Pathology; other irradiated patients were inpatients of the Department of Neurology of the Institute for Clinical Radiology of RCRM. Healthy volunteers and nonexposed patients with dyscirculatory encephalopathy were selected from control subjects recruited by the Department of Neurology of the Institute for Clinical Radiology of RCRM. Veterans of the Afghanistan war were recruited from the Hospital for Soldiers of International Military Conflicts (Kiev).
Group I (“ARS patients”) consisted of Chernobyl accident victims (males) who developed verified ARS. Patients were excluded if they had any mental, neurological, and/or physical disease or head trauma before the accident, as well as head trauma, neuroinfections, and dependence on any psychoactive substances (other than tobacco) after the accident. Patients were excluded also if they were older than 65 years at the time of the latest examination. Accordingly, the cohort of ARS patients selected corresponding to the above inclusion and exclusion criteria who were examined in the first stage of the study (1989–1991) consisted of 78 ARS patients. During the next years, 9 of them died of different causes. Additionally, 5 ARS patients were excluded from the study (2 met the exclusion criterion of age more 65 years; 2 moved away; and 1 was refused because of a severe mental disorder, paranoid schizophrenia), resulting in a reduction of the cohort to 64 ARS patients in 1996–1999.
In 1986 all patients from the ARS cohort were diagnosed with vegetative-vascular dystonia (as neuropsychiatric aftermath of ARS). Their leading clinical syndrome in the first stage of examination in 1989–1991 was also vegetative-vascular dystonia. However, their clinical symptoms were progressive, and in the second stage of examination, in 1996–1999, the neuropsychiatric syndrome at the period remote from ARS was classified as postradiation encephalopathy, which was proposed by us as a new ICD-10 diagnostic category, F07.3, “Postradiation organic syndrome” at the XI World Psychiatry Congress in Hamburg, 1999.50 “Postradiation organic syndrome” was classified under the ICD-10 F07 “Personality and behavioural disorders due to brain disease, damage and dysfunction,” which also corresponds to the DSM-IV 310.1 “Personality change due to general medical condition.”
Group I in 1989–1991 included 78 ARS patients, ages 24–60 years at the time of examination (mean±SD: 38.9±8.2 years). Their absorbed dose of whole-body irradiation was 1–6 Gy (2.1±1.1 Gy). Dosimetrical assessment of ARS patients was provided by the Department of Dosimetry and Radiation Hygiene of RCRM, Kiev, and was also supported by the data of cytogenetic dosimetry provided by the Institute of Biophysics, Moscow, in 1986. Group I in 1996–1999 included 64 ARS patients, ages 31–65 years (44.4±8.4 years) at examination.
Group II (“liquidators”) consisted of emergency workers (males) who took part in cleaning up the Chernobyl accident consequences in 1986. Patients were excluded if they had any mental, neurological, and/or physical disease or head trauma before the accident, or if they had head trauma, neuroinfections, or dependence on any psychoactive substances (other than tobacco) after the accident. Patients were excluded also if they were older than 65 years at the time of the latest examination. Accordingly, the group of liquidators selected according to the above inclusion and exclusion criteria and examined in the first stage of the study (1989–1991) consisted of 96 patients. During the next years, 7 of them died from different causes. Additionally, 9 liquidators were excluded from the study during the first stage (5 met exclusion criteria; 4 moved away), resulting in a reduction of the group to 80 liquidators in 1996–1999.
Between 1986 and 1988 all of the patients from the liquidators group were diagnosed with newly revealed vegetative-vascular dystonia. Their leading clinical syndrome in the first stage of examination was also vegetative-vascular dystonia. However, their clinical symptoms were also progressive, and in the second stage of examination the neuropsychiatric syndrome was classified as encephalopathy that in the main met the criteria of ICD-10 diagnostic category F07.0, “organic personality disorder,” which also corresponds to DSM-IV 310.1, “personality change due to general medical condition.”
Group II in 1989–1991 included 96 liquidators, ages 24–58 years at the time of examination (39.8±8.7 years). Their absorbed dose of whole-body irradiation was 0.1–0.99 Gy (0.4±0.2 Gy). Dosimetrical assessment of ARS patients was provided by RCRM. Group II in 1996–1999 included 80 liquidators, ages 30–64 years (45.2±8.6 years) at examination.
The normative group (“normal subjects”) in 1989–1991 included 20 healthy volunteers (males), ages 25–55 years at the time of examination (39.4±6.7 years). Participants were excluded from the normative group if they currently or in the past met ICD-10 criteria for any diseases or dependence on any psychoactive substances (other than tobacco). During the next years, 1 of them died in a car accident. Additionally, 4 healthy volunteers were excluded from the study (2 met exclusion criteria for head trauma or alcohol abuse; 2 moved away), resulting in a reduction of the group to 15 healthy volunteers in 1996–1999 (at examination, 30–61 years old; 43.5±8.9 years). Subjects of the normative group were exposed to background radiation only.
Control group A (veterans of the Afghanistan war with PTSD) consisted of soldiers and officers (males) who had taken part in the military operation of Soviet troops in Afghanistan and experienced a severe psychogenic stress (without head injury) during battle. Upon return from Afghanistan they demonstrated symptoms of posttraumatic stress disorder (ICD-10: F43.1; DSM-IV: 309.81). Patients were excluded if they had current or past mental (other than PTSD), neurological, and/or physical disease or head trauma. Patients were excluded also if they met ICD-10 criteria for current or past dependence on alcohol, marijuana, or any psychoactive substances (other than tobacco). Accordingly, the control group A of veterans selected according to the above inclusion and exclusion criteria and examined in the first stage of the study consisted of 25 veterans of the Afghanistan war with PTSD. During the next years, 1 of them died by suicide. Moreover, 3 veterans were excluded from the study because of head injury and psychoactive substance abuse, resulting in a reduction of control group A to 21 patients in 1996–1999. Control group A in 1989–1991 included 25 soldiers and officers with PTSD, ages 23–52 years (37.8±7.7 years). According to the ICD-10 criteria, if PTSD symptoms persist 2 years or more, the psychopathology should be classified as F62.0—enduring personality changes after catastrophic experience. However, according to the DSM-IV this disorder, independently to the time after the stress event, should be still classified as PTSD (309.81). Consequently, in the second stage of the study veterans of the Afghanistan war with PTSD were diagnosed with enduring personality changes after catastrophic experience (28–58 years old; 44.5±8.7 years). Patients of control group A were exposed to background radiation only.
Control group B (veterans of the Afghanistan war with mild traumatic brain injury) consisted of soldiers and officers (males) who had taken part in the military operation of Soviet troops in Afghanistan and had mild closed head injury during battle. Upon return from Afghanistan they demonstrated signs of postcommotial organic syndrome (ICD-10: F07.2), which also corresponds to the DSM-IV 310.1, “personality change due to a general medical condition.” Patients were excluded if they had current or past mental (other than MTBI), neurological, and/or physical disease. Patients were excluded also if they met ICD-10 criteria for current or past dependence on alcohol, marijuana, or any psychoactive substances (other than tobacco). Accordingly, the control group B of veterans selected according to the above inclusion and exclusion criteria and examined in the first stage of the study consisted of 25 veterans of the Afghanistan war with MTBI. During the next years, 1 of them died in a car accident. Moreover, 2 veterans were excluded from the study because of psychoactive substance abuse, resulting in a reduction of control group B to 22 patients in 1996–1999. Control group B in 1989–1991 included 25 soldiers and officers with consequences of MTBI, ages 24–53 years (38.2±7.4 years), and in 1996–1999, 22 patients (29–59 years old; 44.8±8.1 years). Patients of control group B were exposed to background radiation only.
Control group C (“DEP”) consisted of 20 patients (males) with dyscirculatory encephalopathy (DEP) as a result of arterial hypertension and/or cerebral atherosclerosis without stroke, who in the main met the criteria of ICD-10 diagnostic category F07.0, “organic personality disorder,” which also corresponds to the DSM-IV 310.1, “personality change due to a general medical condition.” These patients, ages 36–64 years (47.9±8.8 years), were examined in the second stage of the study only, in 1996–1999. Patients of control group C were exposed to background radiation only.
Thus, “organic personality disorder” (ICD-10: F07.0) or “personality change due to general medical condition” (DSM-IV: 310.1) following the exposure to ionizing radiation were compared with the same disorders resulting from cerebrovascular pathology and head trauma (groups C, B), as well as with stress aftermath only (group A).
Procedures
Neurophysiological investigations were carried out in the screened neurophysiological laboratory of the Department of Neurology, Institute for Clinical Radiology, RCRM AMS of Ukraine, in the first half of the day during the resting (passive awake) state of a patient. The patients were nonmedicated for 3 or more days.
Conventional EEG recordings were made using silver-silver chloride bridge electrodes (Ag-AgCl) attached by Hel EEG gel. EEG was recorded monopolarly using the International 10–20 System on 16 channels, referenced to linked ears on an EEG machine (ERA-16; ESA O.T.E. Biomedica Italiana). Time constants were set at 0.3 s and filters at 75 Hz. EEG signals were recorded on tape with rate of paper 15 mm·s–1, and 1 cm of altitude, corresponding to 50 μV. EEGs were registered at 1) resting, eyes closed, 1 min; 2) resting, eyes open, 30 s; 3) hyperventilation, eyes closed, 3 min; and 4) resting, after hyperventilation, eyes closed, 1 min. EEG was performed twice—in 1989–1991 and in 1996–1999.
Conventional EEG assessment and interpretation was based on the classical EEG handbook,51 using a syndromological approach to EEG characterization.52
Statistical analysis was performed on a Pentium 133 computer, using STATISTICA 5.0 and MS Excel 97 software. Statistical processing included descriptive statistics, Student's t-test, chi-square tests (criterion χ2), and relative risk (RR).53,54
RESULTS
The First Stage of the Study (1989–1991)
Groups I and II as well as the normative group and control groups A and B were statistically similar in age and gender. At the same time, the dose of irradiation in group I (2.1±1.1 Gy) was significantly higher than in group II (0.4±0.2 Gy; z=13.47; P<0.001). This allowed us to study a dose–effect relationship of EEG.
Conventional EEG phenomenology in the first stage of the study is presented in Table 1. There were no foci of abnormal electrical activity in any group. Irradiated persons were distinguished dramatically by much paroxysmal (epileptiform) activity—spikes and polyspikes, spike-waves, acute waves, and high-amplitude slow waves. RR for paroxysmal activity in ARS patients compared with the PTSD group was 3.3 (χ2=25.98, df=1, P<0.001), and compared with the MTBI group, 1.6 (χ2=9.28, df=1, P<0.01). RR for paroxysmal activity in liquidators compared with the PTSD group was 2.8 (χ2=14.81, df=1, P<0.001), and compared with the MTBI group, 1.4 (χ2=2.96, df=1, P>0.05). There was a tendency (RR=1.2, P>0.05) toward greater paroxysmal activity in group I compared with group II.
Irradiated subjects had no differences, either between groups I and II or between these groups and nonexposed control subjects, in paroxysmal activity bilateral or lateralized to the right hemisphere, except that patients of groups I and II had more bilateral paroxysmal activity than the normative group (P<0.01 and P<0.05, respectively). At the same time, irradiated persons were distinguished dramatically from all control groups by lateralization of paroxysmal activity to the left hemisphere (left temporal and left frontotemporal region): P<0.001 for ARS patients and P<0.05–0.01 for liquidators. There was a tendency (RR=1.5, P>0.05) toward an increase of left-hemisphere lateralized paroxysmal activity in group I compared with group II.
EEG patterns of both groups of irradiated patients differed from those of control groups, showing less dominance of alpha activity (index more than 50%) as well as less organized alpha activity. There were no strong differences between exposed and nonexposed subjects concerning amplitude of alpha activity at this stage of the study. Although irradiated patients had more alpha activity redistributed to the front compared with normal subjects (P<0.01–0.001), it was not significant if compared with control groups A and B. Regarding both diffusive and “sandglass” (almost equal predominance in the frontal and occipital regions with an “isthmus” in the central area) alpha activity distribution, the examined groups were similar at the first stage.
Irradiated patients were distinguished significantly from all control groups by interhemispheric asymmetry (asymmetry index>5%) of alpha activity, especially by depressed alpha activity in the left hemisphere (left parieto-occipital region): P<0.001 for ARS patients and P<0.01 for liquidators. There was a tendency (RR=1.4, P>0.05) toward lower left-hemisphere–lateralized alpha activity in group I compared with group II.
Brain electrical activity in irradiated patients was distinguished dramatically by an excess of fast activity. RR for beta activity with index more than 20% in both ARS patients and liquidators compared with the PTSD group was 4.1 (χ2=22.82, df=1, P<0.001) and compared with the MTBI group, 2.4 (χ2=13.37, df=1, P<0.001). The frequency of beta activity with amplitude more than 20 μV was significantly higher in exposed patients, especially in liquidators (P<0.01–0.001), than in all control groups.
There were no differences between exposed and nonexposed subjects concerning index of theta activity. However, the frequency of low-voltage theta activity with amplitude less than 30 μV was significantly higher in exposed patients, especially in ARS patients (P<0.01–0.001), than in control groups A and B.
Irradiated patients were distinguished significantly by an excess of delta activity. RR for delta activity with index more than 20% in both ARS patients and liquidators compared with the PTSD group was 3.7 (χ2=29.13, df=1, P<0.001), and compared with the MTBI group, 1.5 (χ2=7.23, df=1, P<0.01).
Reactivity of EEG in irradiated patients in regard to changes of alpha activity in “eyes open/eyes closed” conditions at the first stage of the study did not differ from any control groups.
EEG of exposed patients was distinguished dramatically by an excess of paroxysmal activity (instead of just EEG disorganization) following hyperventilation. RR for paroxysmal (epileptiform) activity occurring and/or accelerating after hyperventilation in both ARS patients and liquidators compared with the PTSD group and the MTBI group was 5.25 (χ2=23.37, df=1, P<0.001).
Syndromological assessment of EEG patterns in the first stage of the study is presented in Table 2. Irradiated patients differed significantly in the lack of normal (organized) EEG. RR for an absence of normal EEG pattern 3–5 years after ARS compared with normal subjects was 13.3 (χ2=51.2, df=1, P<0.001); compared with PTSD, 5.3 (χ2=11.24, df=1, P<0.001); and compared with MTBI, 3.3 (χ2=3.99, df=1, P<0.05). RR for an absence of normal EEG pattern 3–5 years after cleanup work at the Chernobyl exclusion zone compared with normal subjects was 8 (χ2=46.08, df=1, P<0.001); compared with PTSD, 3.2 (χ2=7.3, df=1, P<0.01); and compared with MTBI, 2 (χ2=1.68, df=1, P>0.05). There was a tendency (RR=1.7, P>0.05) toward a decreased frequency of normal EEG patterns in group I compared with group II.
In the first stage, irradiated subjects had more disorganized EEG patterns with predominance of alpha activity than normal subjects (P<0.01). Moreover, ARS patients had more low-voltage EEG patterns than all control subjects (P<0.05–0.01), whereas liquidators had more low-voltage EEG patterns than normal subjects (P<0.05) only. There was a tendency (RR=1.5, P>0.05) toward an increased frequency of low-voltage EEG patterns in group I compared with group II.
The Second Stage of the Study (1996–1999)
Conventional EEG phenomenology in the second stage of the study is presented in Table 3. Frequency of paroxysmal (epileptiform) activity in ARS patients in 1996–1999 significantly decreased compared with that in 1989–1991 (24 [38%] vs. 62 [79.5%]; χ2=25.95, df=1, P<0.001). ARS patients still had more lateralized paroxysmal activity to the left hemisphere compared with the right hemisphere (left-sided: 10 [16%]; right-sided: 3 [5%]; χ2=4.2, df=1, P<0.05).
Dominance of alpha activity (index more than 50%) was significantly reduced between the two stages of the study: from 47 (60%) to 16 (25%) in ARS patients (χ2=17.7, df=1, P<0.001) and from 68 (71%) to 41 (51%) in liquidators (χ2=7.1, df=1, P<0.01). ARS patients were distinguished significantly by the reduced dominance of alpha activity compared with all other groups, including liquidators (P<0.01).
In 1996–1999 there was observed a dramatic reduction of alpha activity amplitude in irradiated patients compared with 1989–1991. The number of ARS patients with low amplitude of alpha activity (<30 μV) was significantly increased: from 12 (15%) to 46 (72%) in ARS patients (χ2=46.43, df=1, P<0.001) and from 17 (18%) to 53 (67%) in liquidators (χ2=42.92, df=1, P<0.001). At the second stage of the study, irradiated patients of both groups were distinguished significantly by a predominance of low-voltage alpha activity, as well as asymmetry of alpha activity, from all control groups (P<0.001).
There was a tendency toward an increase of index of low-voltage (<20 μV) beta activity at the period more remote from irradiation (P>0.05). Excess of fast activity at the more remote period was one of the most strongly distinguishing signs of the exposed groups compared with all control groups (P<0.001).
There was a tendency toward a decrease of index of theta activity in 1996–1999 (P>0.05). Decreased theta activity was the most prominent peculiarity of irradiated patients compared with patients with chronic cerebrovascular disorders (DEP; P<0.001).
In 1996–1999, irradiated patients were also distinguished significantly by an excess of delta activity. RR for delta activity with index more than 20% in both ARS patients and liquidators compared with PTSD was 2.5 (χ2=31.49, df=1, P<0.001); compared with MTBI, 1.59 (χ2=17.19, df=1, P<0.001); and compared with DEP, 1.3 (χ2=9.68, df=1, P<0.01).
Reactivity of EEG in irradiated patients for changes of alpha activity in the test “eyes open/eyes closed” was different in the second stage of the study compared with the first stage. High reactivity of EEG observed in 1989–1991 changed to a decrease or even an absence (particularly in ARS patients) of reactivity. Irradiated patients, especially ARS patients, were distinguished significantly from control groups by hyporeactivity of EEG at the period more remote from irradiation (P<0.05–0.001).
The same situation was observed in irradiated patients in regard to hyperventilation effects. EEG of exposed patients at the later period was distinguished dramatically from that in 1989–1991 by an increased number cases with no changes following hyperventilation: in ARS patients, from 11 (14%) in 1989–1991 to 32 (50%) in 1996–1999 (χ2=21.46, df=1, P<0.001); and in liquidators, from 13 (13.5%) in 1989–1991 to 24 (30%) in 1996–1999 (χ2=7.12, df=1, P<0.01). ARS patients had more EEGs that were areactive following hyperventilation than liquidators (P<0.05).
Syndromological assessment of EEG patterns in the second stage of the study is presented in Table 4. In the second stage, no irradiated patient had normal (organized) EEG.
Hypersynchronous EEG pattern in ARS patients was reduced from 17 (22%) in 1989–1991 to 6 (9%) in 1996–1999 (χ2=4, df=1, P<0.05) and in liquidators, from 24 (25%) in 1989–1991 to 10 (13%) in 1996–1999 (χ2=4.37, df=1, P<0.05).
Disorganized EEG pattern with predominance of alpha activity was reduced in ARS patients only: from 25 (32%) in 1989–1991 to 10 (16%) in 1996–1999 (χ2=5.11, df=1, P<0.05).
Disorganized EEG pattern with predominance of slow activity increased a little in 1996–1999 in ARS patients. This pattern was more typical at the later period for ARS patient than for liquidators: 11 (17%) vs. 2 (3%) (χ2=9.34, df=1, P<0.05).
Low-voltage EEG was the most characteristic pattern at the more remote period. In ARS patients, frequency of low-voltage EEG increased from 21 (27%) in 1989–1991 to 37 (58%) in 1996–1999 (χ2=13.88, df=1, P<0.001) and from 17 (18%) in liquidators in 1989–1991 to 37 (46%) in 1996–1999 (χ2=16.71, df=1, P<0.001).
In 1996–1999, RR for low-voltage EEG in ARS patients compared with the PTSD group was 4.14 (χ2=12, df=1, P<0.001); compared with MTBI, 6.44 (χ2=15.7, df=1, P<0.001); compared with DEP, 2.9 (χ2=8.72, df=1, P<0.01); and compared with liquidators, 1.26 (χ2=1.9, df=1, P>0.05),
In 1996–1999, RR for low-voltage EEG in liquidators compared with the PTSD group was 3.28 (χ2=7.1, df=1, P<0.01); compared with the MTBI, 5.11 (χ2=10.1, df=1, P<0.001); and compared with DEP, 2.3 (χ2=4.56, df=1, P<0.05).
According to the data obtained, conventional EEG changes following exposure to ionizing radiation are quite characteristic and include two phases. In the first phase (3–5 years after irradiation), we found irritated EEG changes with paroxysmal activity shifted to the left frontotemporal region (hypersynchronous, disorganized, with predominance of alpha activity and/or slow activity EEG-patterns), which transforms in the second phase (10–13 years after irradiation) toward a low-voltage EEG-pattern with excess of fast (beta) and slow (delta) activity together with depression of alpha and theta activity, and with paroxysmal activity also shifted to the left frontotemporal region (Figures 1, 2, 3, 4).
DISCUSSION
Conventional EEG patterns in persons exposed to ionizing radiation seem to be quite distinctive compared with those in normal subjects and persons with stress aftermath and nonspecific organic brain injuries (traumatic and cerebrovascular). This specificity includes 1) double-phase EEG pattern; 2) lateralized effect—predominant involvement of the left hemisphere, particularly left frontotemporal region; 3) increased beta and delta activity together with decreased alpha and theta activity; and 4) low-voltage EEG as the characteristic pattern of the period more remote from the exposure.
Double-phase EEG pattern following exposure to ionizing radiation has been described in experimental and clinical studies,2,10,15–18 where an “irritated” hypersynchronous or disorganized EEG pattern transformed toward an “inhibited” EEG pattern. A similar double-phase EEG pattern was also described in Chernobyl patients.39,40,55
Predominant involvement of the left hemisphere, particularly the left frontotemporal region, in persons exposed to ionizing radiation was reported in our previous works23,29–36 and in studies of other authors.39,40,55,56 It is very important to note that left frontotemporal-limbic dysfunction is the determining pattern of cerebral disorganization leading to schizophrenia,57 taking into account that we found schizophreniform symptoms in both ARS patients and liquidators.23,28–33,35,36,58,59 We propose that the left (dominant) hemisphere is more vulnerable in right-handed men to whole-body irradiation than the right. Our data are consistent with other types of evidence also indicating a specific vulnerability of the left hemisphere, compared with the right: after unilateral nondominant or bilateral electroconvulsive therapy (ECT), the post-ECT EEG slow activity is lateralized to the left hemisphere.60
Increased beta activity has been described as a “radiation trace” in the period remote from irradiation,22 and an increase of delta activity has been described as a possible marker for assessing absorbed dose of ionizing radiation.27 Increased beta and delta activity and decreased alpha activity were revealed in patients with chronic radiation sickness.21 We found the increase of beta and delta activity together with decrease of alpha and theta activity to be the characteristic EEG pattern following irradiation,23,29–37,59 similarly to other authors.38–41,55,56
The neurophysiological basis of the irritated EEG pattern in the first phase (3–5 years after irradiation) could be outlined as follows. Increased delta activity reflects disinhibition of neurons in deep cortical layers and in the thalamus, normally inhibited by input from the ascending reticular activating system in the midbrain,61 due to inhibition of reticular formation and posterior hypothalamus.52 Increased diffusive beta activity reflects dysfunction of corticocortical and thalamocortical transactions related to specific information processing61 as well as epileptiform phenomenon62 and inhibition of input from the brainstem reticular formation resulting in reinforcement of thalamic and nucleus caudatus impact on cortex.63 Disorganization and hypersynchronization of alpha activity testify to thalamic dysfunction.52 Interhemispheric asymmetry of alpha activity, with its depression in the left hemisphere, testifies to left hemisphere overactivation.64 These findings, together with paroxysmal activity (shifted to the left frontotemporal region), which significantly increased following hyperventilation, could be explained as cortical-limbic overactivation, predominantly in the left, dominant, hemisphere.
In the period more remote from irradiation, we revealed a low-voltage EEG pattern that significantly distinguished exposed patients from control subjects. The differences between the low-voltage and so-called desynchronous EEG patterns should be stressed. Excess of delta activity can be produced by neurons in deep cortical layers and in the thalamus61 as a result of inhibition of reticular formation and posterior hypothalamus.52 Dramatic excess of diffusive beta activity can be produced by dysfunction of corticocortical and thalamocortical transactions61 and increased thalamic and nucleus caudatus input on cortex.63 Reduction of alpha activity testifies to cortical damage and brainstem dysfunction.52 Rhythmic theta activity might originate from direct cortical activation, or from the cortical activation driven by the neuronal impulses from the limbic system.65 Hippocampal theta activity results from activation in the ascending synchronizing system.66 Consequently, significantly reduced theta activity in the period more remote from irradiation could be explained as cortical and limbic, particularly hippocampal, hypofunction.52 Low-voltage EEG with hyporeactivity and even areactivity in the more remote period testifies to organic brain damage67 with metabolic disorders in cortical neurons.52 Thus, this EEG pattern reflects inhibition of the cortical-limbic system.
At 3–5 years after irradiation, ARS patients compared with liquidators had lower amplitude of beta activity, and in the later period they had less paroxysmal and alpha activity as well as more areactive EEG patterns. However, we cannot reveal a statistically significant dose–effect relationship concerning conventional EEG parameters. At the same time, the data obtained seem to be quite promising for further studies involving quantitative EEG analysis. Quantitative EEG and evoked potentials are likely to be very informative for investigation of the neurophysiological basis of low-voltage EEG pattern following irradiation, as we hope to present in further publications.
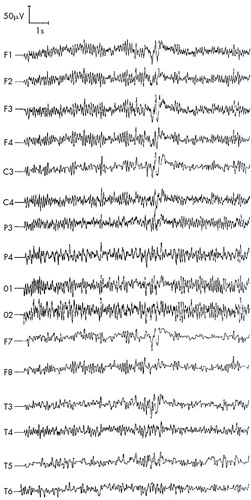
FIGURE 1. ARS patient P (first stage of study, 1990), 42 years old: ARS of second-degree severity, absorbed dose 3 GyResting, eyes closed—disorganized EEG with paroxysmal activity lateralized to the left frontotemporal region.
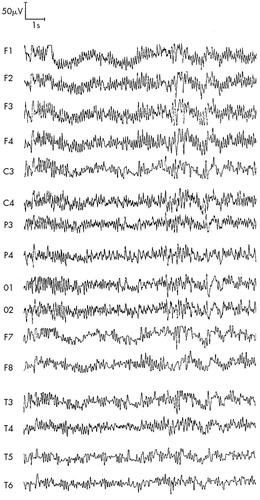
FIGURE 2. ARS patient P (first stage of study, 1990), 42 years old: ARS of second-degree severity, absorbed dose 3 GyHyperventilation, 3 min, eyes closed—an increase of EEG disorganization and paroxysmal activity.
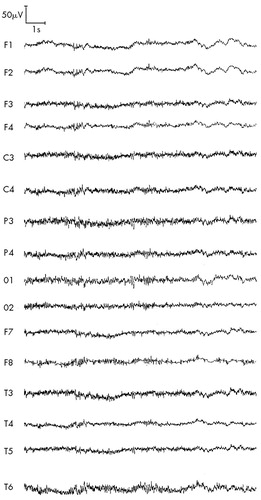
FIGURE 3. ARS patient P (second stage of study, 1997), 49 years old: ARS of second-degree severity, absorbed dose 3 GyResting, eyes closed—low-voltage EEG with excess of beta activity lateralized to the left frontotemporal region.
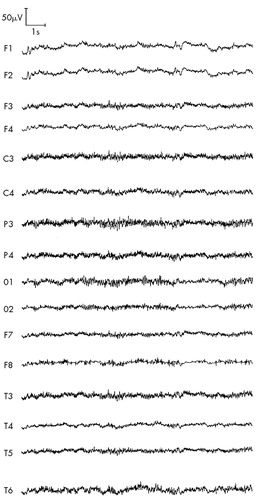
FIGURE 4. ARS patient P (second stage of study, 1997), 49 years old: ARS of second-degree severity, absorbed dose 3 GyHyperventilation, 3 min, eyes closed, no effect of hyperventilation—low-voltage EEG with excess of beta activity lateralized to the left frontotemporal region.
 |
 |
 |
 |
1 Hunt WA: Effects of ionizing radiation on behavior, in Military Radiobiology, edited by Conklin JJ, Walker RI. San Diego, CA, Academic Press, 1987, pp 321-330Google Scholar
2 Kimeldorf DJ, Hunt EL: Ionizing Radiation: Neural Function and Behavior. New York, Academic Press, 1965Google Scholar
3 Mickley GA: Psychological effects of nuclear warfare, in Military Radiobiology, edited by Conklin JJ, Walker RI. San Diego, CA, Academic Press, 1987, pp 303-319Google Scholar
4 Tarkhanov IR: [About physiological action of Röntgen rays on the central nervous system] (Russian). Hospital Newspaper of Botkin, 1896, 33:753, 34:785Google Scholar
5 Edison TA: The blind may see a little. San Francisco Chronicle 19, 1896, p 1Google Scholar
6 London ES: Zur Lehre von den Becquerelstrahlen und ihren physiologischpathologischen bedeutung [On studies of Becquerel's rays and their pathological significance]. Berlin Clinical Weekly 1903, 23, p 523Google Scholar
7 [Zhukovsky MO: About an influence of radium rays upon an excitability of psychomotor centres] (Russian). Review of Psychiatry, Neuropathology and Experimental Psychology (Russia) 1903; 11:801Google Scholar
8 Danysz J: De l'action pathogène des rayons et des emanations emis par le radium sur different tissues et different organismes [On the action of rays and emanations emitted by radium on different tissues and different organisms]. Comptes rendus hebdomadaires des seances de l'Academie de Science (Paris) 1903; 136:461, 137:1296Google Scholar
9 Gorovitz LM: [On Biological Significance of Radium Rays] (Russian). Military Medical Academy, Saint Petersburg, 1906Google Scholar
10 Nemenov MI: [Roentgentherapy Through Exposure of the Nervous System] (Russian). Leningrad, Publishing House Medgiz, 1950Google Scholar
11 Holahan EV Jr: Cellular radiation biology, in Military Radiobiology, edited by Conklin JJ, Walker RI. San Diego, CA, Academic Press, 1987, pp 87-110Google Scholar
12 Davis H, Davis, PA: The electrical activity of the brain its relation to physiological states and to states of impaired consciousness. Research Publications, Association for Research in Nervous Mental Diseases 1939; 19:50Google Scholar
13 Konuma M, Furutani M, Kubo S: [On the diencephalosis as aftereffects in atomic bomb casualties] (Japanese). Japanese Medical Journal 1954; 1544:5-12Google Scholar
14 Izumi C, Hayakawa T: [Electroencephalographic researches of Hiroshima atomic bomb casualty on aftereffects 9 years later (preliminary report)] (Japanese). Folia Psychiatr Neurol Jpn 1955; 9:229-242Google Scholar
15 Lebedinsky AV, Nakhilnitzkaja ZN: [Ionizing Radiation Influence on the Nervous System] (Russian). Moscow, Publishing House Atomizdat, 1960Google Scholar
16 Livanov MN: [Some Problems of Ionizing Radiation Effects on the Nervous System] (Russian). Moscow, Publishing House Medgiz, 1962Google Scholar
17 Grigoriev YuG: [Radiation Damages and Compensation of Disordered Functions] (Russian). Moscow, Publishing House Atomizdat, 1963Google Scholar
18 Effects of Ionizing Radiation on the Nervous System: Proceedings of the International Symposium, Vienna, 1961. Vienna, International Atomic Energy Agency [IAEA], 1962Google Scholar
19 Gangloff H: Acute effects of X-irradiation on brain electrical activity in cats and rabbit, in Effects of Ionizing Radiation on the Nervous System: Proceedings of the International Symposium, Vienna, 1961. Vienna, IAEA, 1962, pp 123-135Google Scholar
20 Haley T: Changes induced in brain activity by low doses of X-irradiation, in Effects of Ionizing Radiation on the Nervous System: Proceedings of the International Symposium, Vienna, 1961. Vienna, IAEA, 1962, pp 171-185Google Scholar
21 Sosnovskaja FM: [Study of brain bioelectrical activity in persons exposed to chronic ionizing radiation] (Russian). Zh Nevropatol Psykhiatr Im SS Korsakova 1971; 71(2):205-209Google Scholar
22 Yaar I, Ron E, Modan B, et al: Long-lasting cerebral functional changes following moderate dose X-radiation treatment to the scalp in childhood: an EEG power spectral study. J Neurol Neurosurg Psychiatry 1982; 45:166-169Crossref, Medline, Google Scholar
23 Nyagu AI, Loganovsky KN: [Neuropsychiatric Effects of Ionizing Radiation] (Russian). Kiev, Publishing House Chernobylinterinform, 1998Google Scholar
24 Davydov BI, Ushakov IB: [Ionizing Radiation and Brain Behavioral and Structural-Functional Patterns: Results of Science and Technics (Radiation Biology, vol 8)] (Russian). Moscow, VINITI, 1987Google Scholar
25 Ushakov IB, Arlashchenko NI, Dolzhanov AY, et al: [Chernobyl: Radiation Psychophysiology and Human Ecology] (Russian). Moscow, SSRTI Aerospace Medicine, 1997Google Scholar
26 Peymer SI, Dudkin AO, Sverdlov AG: [Direct effects of small doses of ionizing radiation on the neurons] (Russian). Reports of the Academy of Sciences of the Union of Soviet Socialist Republics 1985; 284:1481-1484Google Scholar
27 Trocherie S, Court L, Gourmelon P, et al: The value of EEG signal processing in the assessment of the dose of gamma or neutron-gamma radiation absorbed dose, in Le Traitment du Signal en Electrophysiologie Experimentale et Clinique du Systeme Nerveux Central, vol 2, edited by Court L, Trocherie S, Doucet J. Brussels, NATO, 1984, pp 633-644Google Scholar
28 Noshchenko AG, Loganovsky KN: [Bioelectrical activity of the brain and peculiarities of mental disorders in persons involved in the Chernobyl accident] (abstract), in [Biological and Radioecological Aftermath of the Accident at the Chernobyl Nuclear Station: Abstracts of the First International Conference, Zeleny Mys, 1990] (Russian). Moscow, 1990, p 218Google Scholar
29 Noshchenko AG, Loganovskii KN: [The functional brain characteristic of people working within the 30-kilometer area of the Chernobyl Atomic Electric Power Station from the viewpoint of age-related changes] (Russian). Vracheb Delo 1994; 2:16-19Google Scholar
30 Loganovsky KN: Ionizing radiation effects on human brain information processes, in Proceedings of the International Conference on the Mental Health Consequences of the Chernobyl Disaster: Current State and Future Prospects, May 24-28, 1995. Kiev, 1995, pp 52-53Google Scholar
31 Nyagu AI, Noschenko AG, Loganovskii KN: [Late effects of psychogenic and radiation factors of the accident at the Chernobyl nuclear power plant on the functional state of human brain] (Russian). Zh Nevropatol Psykhiatr Im S S Korsakova 1992; 92(4):72-77Google Scholar
32 Nyagu AI, Khalyavka IG, Loganovsky KN, et al: [Psychoneurological characterisation of persons who had acute radiation sickness] (Russian). Problems of Chernobyl Exclusion Zone 1996; 3:175-190Google Scholar
33 Nyagu A, Loganovsky K, Vaschenko E, et al: Psychophysiological effects of chronical irradiation as a result of the Chernobyl disaster, in International Conference “One Decade After Chernobyl”: Book of Extended Synopses. Vienna, IAEA, 1996, pp 347-348Google Scholar
34 Nyagu A, Loganovsky K: Neurophysiological appropriateness of ionizing radiation effects, in Low Doses of Ionizing Radiation: Biological Effects and Regulatory Control, IAEA-TECDOC-976, contributed papers of International Conference, Seville, Spain, November 17-21, 1997. Vienna, IAEA, WHO, UNSCEAR, 1997, pp 261-264Google Scholar
35 Nyagu AI, Loganovsky KN, Chuprovskaja NYu, et al: [Postradiation encephalopathy at the remote period of acute radiation sickness] (Russian). Ukrainian Medical Journal 1997, 2(2):33-44Google Scholar
36 Nyagu AI, Loganovsky KN, Yuryev KL, et al: Psychophysiological aftermath of irradiation. International Journal of Radiation Medicine 1999; 2(2):3-24Google Scholar
37 Yuryev KL: [Pathophysiological mechanisms of movement disturbances in persons irradiated as a result of the Chernobyl accident] (Ukrainian). Ukrainian Medical Journal 1999; 4(12):137-140Google Scholar
38 Danilov VM, Posdeyev VK: [Epileptiform reactions of human brain following long-term exposure to low doses of ionizing radiation] (Russian). Fisiolog Zh Im Sechenova 1994, 80 (6): 88-98Google Scholar
39 Zhavoronkova LA, Kholodova NB: [The assessment of brain functional status by EEG coherence parameters in the late period of exposure to ionizing radiation] (Russian). Zh Vyssh Nerv Deiat Im I P Pavlova 1994; 44(1):159-162Google Scholar
40 Zhavoronkova LA, Kholodova NB, Zubovsky GA, et al: EEG power mapping, dipole source and coherence analysis in Chernobyl patients. Brain Topogr 1995; 8(2):161-168Google Scholar
41 Viatleva OA, Katargina TA, Puchinskaja LM, et al: [Electrophysiological characteristic of functional state in mental disorders in Chernobyl accident clean up participants] (Russian). Zh Nevropatol Psykhiatr Im SS Korsakova 1996, 96(3):41-46Google Scholar
42 Kindselsky LP, Kovalenko AN, Khaliavka IG: [Acute radiation sickness, in Chernobyl Catastrophe] (Russian), edited by Barjakhtar VG. Kiev, Publishing House Naukova Dumka, 1995, pp 447-449Google Scholar
43 Wagemaker G, Bebeshko VG (ed): Diagnosis and Treatment of Patients with Acute Radiation Syndrome, Joint Study Project 3: Final Report. Brussels and Luxembourg, European Commission, 1996Google Scholar
44 Likhtarev I, Chumak V, Repin V: Retrospective reconstruction of individual and collective external gamma doses of population evacuated after the Chernobyl accident. Health Physics 1994; 66:643-652Crossref, Medline, Google Scholar
45 Likhtarev I: Exposure of different population group of Ukraine after the Chernobyl accident and main health-risk assessment. Report for BfS/SKK Seminar “Ten Years After Chernobyl, a Summation.” Munich, March 6-7, 1996Google Scholar
46 National Report of Ukraine: Ten Years after the Chernobyl Disaster (Kiev, Minchernobyl), Vienna, IAEA, 1996Google Scholar
47 International Chernobyl Project: Technical Report: Assessment of Radiological Consequences and Protection Measures. Vienna, IAEA, 1992Google Scholar
48 Konuma M: [About understanding of A-bombs sickness and sequel such as diencepalic distress] (Japanese). Nagasaki Igakkai zasshi [Nagasaki Medical Journal] 1961; 36:706-716Google Scholar
49 Konuma M: [Psychiatric atomic bomb casualties: summary of Psychiatric Department] (Japanese). Hiroshima Medical Journal 1967; 20:231-236Google Scholar
50 Nyagu AI, Loganovsky KN, Zdorenko LL, et al: Postradiation organic syndrome as a new F07.3 diagnostic category for ICD-10, in Abstracts of the XI World Psychiatry Congress, Hamburg, 1999, p 162Google Scholar
51 Niedermeyer E, DaSilva FL (ed): Electroencephalography: Basic Principles, Clinical Application and Related Fields, 3rd edition. Baltimore, Williams and Wilkins, 1993Google Scholar
52 Zhirmunskaya EA: [Clinical Electroencephalography] (Russian). Moscow, Publishing House MAYBE, 1991Google Scholar
53 Kuzma JW: Basic Statistics for the Health Sciences. Palo Alto, CA, Mayfield, 1984Google Scholar
54 Daniel WW: Biostatistics: A Foundation for Analysis in the Health Sciences. New York, Wiley, 1995Google Scholar
55 Zhavoronkova LA, Kholodova, NB, Gogitidze NV: Dynamic assessment of the remote consequences of irradition: clinical-electrophysiological investigation, in Proceedings of the second International Conference “Long-term Health Consequences of the Chernobyl Disaster,” June 1-6, 1998. Kiev, 1998, p 233Google Scholar
56 Chayanov NV, Monosova AG: EEG mapping in Chernobyl disaster suffered persons, in Abstracts of the Third International Congress on Brain Electromagnetic Topography, Amsterdam, June 9-12, 1992Google Scholar
57 Flor-Henry P: Cerebral Basis of Psychopathology. Boston, John Wright, PSG, 1983Google Scholar
58 Loganovsky K: [Clinical and epidemiological aspects of psychiatric aftermath of the Chernobyl disaster] (Russian). Social and Clinical Psychiatry 1999; 1:5-17Google Scholar
59 Loganovsky K: [Neurological and psychiatric syndromes in the remote period of exposure to ionizing radiation] (Russian). Zh Nevrol Psikhiatr Im S S Korsakova 2000; 100(4):15-21Google Scholar
60 Abrams R: Electroconvulsive Therapy. Oxford and New York, Oxford University Press, 1988Google Scholar
61 Hughes JR, John ER: Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci 1999; 11:190-208Link, Google Scholar
62 Brumback RA, Staton RD: Beta activity and electrical seizure phenomena. Electroencephalogr Clin Neurophysiol 1981; 52(3):128-136Google Scholar
63 Traugott NN, Bagrov YaYu, Balonov LYa, et al: Essays on Human Psychophysiology. Leningrad, Nauka, 1968Google Scholar
64 Flor-Henry P: EEG spectral analysis in psychopathology, in The EEG of Mental Activities, edited by Giannitrapani D, Murri L. Basel, Karger, 1988, pp 182-200Google Scholar
65 Futagi Y, Ishihara T, Tsuda K, et al: Theta-rhythms associated with sucking, crying, gazing and handling in infants. Electroencephalogr Clin Neurophysiol 1998; 106:392-399Crossref, Medline, Google Scholar
66 Oddie SD, Bland BH: Hippocampal-formation-theta activity and movement selection. Neurosci Biobehav Rev 1998; 22 :221-231Google Scholar
67 Synek VM: The low-voltage electroencephalogram. Clin Electoencephalogr 1983; 14:102-105Crossref, Medline, Google Scholar



