Recent Neuroimaging Techniques in Mild Traumatic Brain Injury
Although it is clear that most patients suffer some acute cognitive difficulties, the nature and course of postacute cognitive recovery remains an area of intense controversy. Most patients recover fully from mild TBI, but 7% to 33% 9 – 11 have persistent problems. Frequently, complaints involve a constellation of physical, emotional, and cognitive symptoms collectively known as postconcussion syndrome, often without demonstrable structural changes to the brain 12 or neuropsychological dysfunction. 13 , 14 Clinical neuroimaging findings are normal in the majority of mild TBI cases. For example, Borg et al. 15 report that 5% of individuals who have a GCS score of 15, 20% with a GCS score of 14, and 30% with a GCS score of 13 have abnormal findings on clinical computed tomography (CT). Thus, a large majority of mild TBI patients, both symptomatic and asymptomatic, have normal CT scans. Similarly, although magnetic resonance imaging (MRI) is more sensitive than CT in mild TBI patients 12 , 16 , 17 and MRI findings have been correlated with neuropsychological performance in mild TBI, 17 many symptomatic patients have normal MRI scans. Indeed, 43% to 68% of mild TBI patients have normal structural scans on MRI. 18 , 19 This may be either because there is no structural brain damage in those symptomatic patients with normal scans or because current technology is unable to detect it. 20 – 22 Certainly, microscopic diffuse axonal injury, reported as present in autopsy studies of mild TBI, 23 – 25 is largely undetectable using traditional neuroimaging techniques. 23 , 26
Alternatively, others contend that persisting symptoms are the result of psychological mechanisms, such as poor coping styles, 27 , 28 emotional reactions to an adverse event, 29 or expectations of symptoms that may occur following a mild TBI. 30 Though postconcussion syndrome has been recognized for at least the last few hundred years, 5 the debate over the persistence of symptoms following mild TBI in a minority of individuals has led to postconcussion syndrome being a particularly controversial diagnosis in the medical-legal arena.
When clinical neuroimaging findings are present following a mild TBI, the classification changes to “complicated mild TBI,” which has a 6-month outcome more similar to moderate TBI. 31 Therefore, it is in the symptomatic mild TBI patients with negative clinical neuroimaging that a search for more sensitive imaging techniques or biological markers continues. Newer and experimental neuroimaging techniques provide promise in this regard and may also be useful in demonstrating the physiological mechanisms of rehabilitation treatment effects. 32 These include structural or chemical techniques, such as diffusion tensor imaging (DTI), magnetization transfer imaging (MTI), and magnetic resonance spectroscopy, and functional techniques such as functional MRI (fMRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT). This article critically reviews the existing literature of these newer neuroimaging techniques in individuals with mild TBI.
Criteria for Literature Review
The following criteria were employed to evaluate the findings from many different technologies and to suggest directions for future research:
1. The Technique Should Be Sensitive to Brain Injury
There must be evidence of convergent validity either with a concurrent history of brain injury or with other tests of brain injury, such as neurological examination, neuropathological examination, or conventional MRI and/or CT findings. Studies conducted with moderately to severely injured patients suggest that this criterion has been met for the aforementioned techniques. One purpose of this review will be to evaluate the extent to which this criterion has been met for patients with mild TBI. This criterion will be met if there are imaging abnormalities in patients with a known history of mild TBI relative to comparison subjects.
In meeting this criterion, positive neuroimaging findings should also correspond to known pathology associated with mild TBI. Animal models suggest that there can be structural or functional changes in mild TBI with increased neurofilament reactivity within 30 minutes of injury 33 and subsequent impaired anterograde axoplasmic transport with swelling of the axonal cylinder and disconnection of the axon from its target. 34 The extent of diffuse axonal injury (DAI) is correlated with recovery rate in monkeys. 35 Povlishock and Becker 36 have demonstrated that mild injury can elicit axonal swelling that may persist unchanged, degenerate, or undergo a regenerative response, suggesting some variability in recovery prognosis for mild TBI. Early indication of DAI in mild TBI, however, primarily manifests as misalignment of the cytoskeletal network 33 , 37 observed microscopically. Autopsy evaluations in humans have revealed that the extent of DAI can be identified through postmortem microscopic evaluation. 23 – 25 Autopsy of mild TBI patients who died of unrelated causes suggests that the corpus callosum is the most frequent site of DAI, 38 with other frequently reported areas being the brainstem and lobar white matter. 23 Clearly, finding a new technology with more sensitive identification of these injuries and processes in vivo would be useful, both to clinicians and to patients, who often search for objective markers of their symptoms.
2. The Technique Provides Incremental Validity Above and Beyond That Provided by Conventional Structural MRI and/or CT Scans
If experimental neuroimaging techniques add nothing to current clinical assessment protocols, they simply add to health care costs. This criterion is met if a given neuroimaging technology more fully detects or demonstrates damage in patients whose conventional MRI/CT scans are normal. Alternatively, the technique may detect damage in brain regions that appear normal on conventional scans.
3. Ideally, the Technique Should Correlate With Clinical Examination or Symptom Presentation, or Have Predictive Validity
Findings of the neuroimaging technique should be related to functional status as measured in some objective manner (e.g., neurological examination or neuropsychological testing). This establishes that an identified neuroimaging anomaly is not simply an incidental finding. The significance of an unusual pattern of metabolism on PET in an individual who had a mild TBI is unclear if the patient is asymptomatic and presents normally on clinical examination. From a clinical standpoint, correlation with function is important to interpreting the meaning of a positive neuroimaging finding.
The first step in meeting this criterion is to demonstrate correlations between abnormal neuroimaging findings and measures of functioning. A clinically useful second step would be to demonstrate correlations between abnormal neuroimaging findings and measures of future functioning. For example, prospective studies in which acute neuroimaging findings predict persistent symptoms beyond the typical 3-month recovery period 39 would be valuable.
Structural and Chemical Neuroimaging Findings
Diffusion Tensor Imaging
Diffusion tensor imaging (DTI) is a relatively new MRI application that capitalizes on the diffusion of water molecules for imaging the brain. While diffusion-weighted MR imaging measures the diffusion of water molecules in a particular direction, DTI takes this a step further by imaging diffusion in a number of different directions—typically six. This allows for the calculation of a matrix or tensor which represents diffusion in three dimensions. 40 In the white matter, water diffusion is higher along fiber tracts than across them, which allows for directional measurement of diffusion and, hence, measurement of structural integrity. The most robust DTI parameter, 41 fractional anisotropy, provides a measure of tissue microstructure by quantifying the extent to which diffusion occurs in one particular direction within each voxel. Fractional anisotropy is correlated with measures of fine motor speed and verbal fluency in normal aging. 42 , 43
Abnormalities in white matter are detected via DTI in patients with severe head injuries 11 months to 9 years following injury. 44 , 45 Only one study 46 has been conducted with mild TBI patients. This study examined five patients within 24 hours of injury and 10 comparison participants and then retested two of the five patients at 1 month postinjury. Various regions of interest (ROI) were selected, and in each case only from white matter that appeared normal on conventional MR images (i.e., normal-appearing white matter or “NAWM” hereafter). Mild TBI patients demonstrated reduced directional diffusion (fractional anisotropy) in white matter relative to comparison participants, both within 24 hours and at 1 month postinjury. More abnormalities were noted in the internal capsule and the corpus callosum relative to the external capsule. These findings are consistent with animal models of mild TBI. Furthermore, the study found recovery in some areas in two patients who were rescanned 30 days after injury. Based on one study, then, DTI has demonstrated sensitivity to mild TBI at least up to 1 month postinjury. This study also meets the incremental validity criterion as it demonstrated reduced fractional anisotropy in NAWM on conventional MRI. However, this study did not address whether DTI abnormalities in mild TBI are related to clinical variables and/or outcome. Cognitive recovery in head injury correlates with restoration of white matter integrity assessed with xenon-enhanced CT measures of local cerebral perfusion. 47 As such, DTI may be in a unique position to predict recovery in patients with TBI. This will be particularly relevant to mild TBI which results primarily in axonal injury, often within the context of normal clinical CT/MRI scans. The potential ability of DTI to identify subclinical DAI neuropathology explains the excitement about this modality.
Magnetization Transfer Imaging
Magnetization transfer imaging (MTI) is another technique that increases the contrast between tissues by exploiting the exchange of protons between water and macromolecules. When a radio frequency pulse is applied, it selectively saturates those protons that are bound to macromolecules. 48 This technique provides information about tissue changes not detected with conventional T1- and T2-weighted MR images. The magnetization transfer ratio (MTR) represents a quantitative measure of the structural integrity of tissue, with reductions in MTR suggestive of neuropathology. Only a few studies have been conducted with TBI patients. Results suggest that MTI is able to detect abnormalities not seen on traditional MRI scans among TBI patients of mixed severity, though there is poor correlation with outcome to date.
Bagley et al. 49 scanned 28 patients, 21 of whom were symptomatic, of varying severity 1 to 29 days postinjury. Of the five patients with mild TBI (GCS=13 to 15), only one had abnormal MTR findings (in temporal and occipital lobe white matter and the internal capsule). As this patient with mild TBI and abnormal MTR was placed in a long-term care facility 6 months postinjury, it may be that only asymptomatic patients have normal MTR values. Consistent with this hypothesis, these authors conducted another study focusing specifically on 13 patients with persisting cognitive complaints following mild TBI, 50 most of whom were “within months” of injury. Twelve of these patients had normal MRI scans. These investigators found significantly lowered MTR in patients relative to comparison subjects in the splenium of the corpus callosum consistent with autopsy data showing the corpus callosum as the most frequent site of DAI following mild TBI. 38 Regional MTR values correlated with only two of the 25 neuropsychological measures administered. Specifically, MTR values in the splenium were moderately correlated with verbal recognition memory (r=0.59), while MTR values in the pons, although not abnormally low, were moderately correlated (r=0.58) with visual attention span. Finally, Sinson et al. 51 studied 30 patients with a mean admitting GCS score of 11, who were a median of 41 days postinjury. Five of the six patients who had abnormal MTRs had mild TBI (GCS=14 or 15). Their abnormalities were detected in the splenium, internal capsule, pons, and white matter of the temporal and occipital lobes. It is curious that MTR anomalies were more prevalent in mild TBI than in moderate TBI. However, of note is an autopsy study of severe brain injury 52 suggesting the need for intact pathways to cause injury at a distal location; specifically, more damaged frontal cortex related to less damage in the thalamic reticular nucleus.
In summary, with mild TBI patients, MTI largely detects abnormalities within the first month or two following mild TBI. A promising aspect of these findings is that demonstrated areas of abnormalities are consistent with known DAI neuropathology in mild TBI (i.e., lobar white matter, corpus callosum). Further study is needed to demonstrate associations with clinical variables.
Magnetic Resonance Spectroscopy
In contrast to neuroimaging techniques offering structural information about brain integrity, magnetic resonance spectroscopy (MRS) offers in vivo neurochemical information by detecting signals from individual solutes in body tissues. This technique can be used to assess metabolic irregularities following brain injury. MRS is based on measuring magnetic signals from certain nuclei (mostly 1H or 31P) in response to radiofrequency pulses. The main metabolites measured by 1H MRS are: N -acetylaspartate, which is a quantitative marker of neuronal health 53 and is significantly decreased in demyelinated areas in multiple sclerosis; choline, which is a marker of inflammation 54 and is elevated in cell proliferation; 55 myo-inositol, which is a glial marker; 56 lactate, which is an indirect indicator of ischemic and hypoxic conditions; 55 , 57 and creatine and phosphocreatine, which are related to energy metabolism. 58 Data from MRS studies are often expressed as changes in the ratio of N -acetylaspartate to creatine or choline. Reporting metabolite ratios allows investigators to control for reductions in metabolites that may be due to variations in cellular density. 59
Studies conducted with moderately to severely injured patients 1 to 2 months postinjury suggest that MRS is sensitive to injury and correlates with neuropsychological functioning and functional outcomes. 60 – 62 Longitudinal investigations suggest that initially reduced white matter N -acetylaspartate returns to near-normal levels by 2 months in mild TBI patients 63 and by 6 months in moderately to severely injured TBI patients, 61 while gray matter N -acetylaspartate continues to be abnormally low for at least 6 months postinjury 61 in moderate to severe brain injury. However, the study conducted with mild TBI patients sampled from pericontusional areas in white/gray matter that were evident on CT (making these complicated mild TBI patients), while the study conducted with moderate to severe TBI patients sampled only from normal-appearing occipital lobe gray and white matter. Therefore, the recovery rate of white matter N -acetylaspartate for uncomplicated mild TBI may be faster than suggested by these data.
Garnett et al. 64 studied a range of injury severity including mild TBI (defined as either GCS=13 to 15 or PTA <24 hours). The eight mild TBI participants were an average of 8 days postinjury and half had normal MRI findings on T2-weighted images. Significant elevations in choline/creatine ratios were detected in mild TBI patients relative to comparison subjects in NAWM of the frontal lobes. Although N -acetylaspartate/creatine ratios were significantly reduced in moderately and severely injured patients, this ratio was not significantly reduced in mild TBI patients. Interestingly, lactate was not apparent in the spectra of any participants, regardless of severity.
In mild TBI cases (GCS=14 or 15) with normal clinical MRI scans, Cecil et al. 65 reported N -acetylaspartate/creatine ratios in the splenium of the corpus callosum significantly below comparison subjects in 11 of 16 patients. It is not known whether these abnormalities correlate with clinical variables. Also, given the wide range of time since injury in these patients (i.e., 9 days to 4.5 years), it unclear if the low N -acetylaspartate/creatine ratio values were present only in the acute injury patients or also in the more chronic patients. Govindaraju et al. 66 sampled tissue appearing normal on MRI clinical scans in a study of mild TBI patients (GCS=13 to 15, LOC <30 minutes) who were an average of 13.3 days postinjury. They found no focal abnormalities in metabolites in 15 of the 16 subjects tested, although group data differed significantly from comparison subject data in several regions. Specifically, increased choline/creatine ratios in occipital lobe gray matter, decreased N -acetylaspartate/creatine ratios in parietal white matter regions, and decreased N -acetylaspartate/choline ratios in occipital regions were noted in the patient group. Metabolite ratios did not correlate significantly with clinical measures either acutely or at discharge. In addition, most of these patients (10 out of 16) had abnormal CT scans, calling into question the incremental validity of the neuroimaging data.
Son et al., 63 in an investigation of seven mild TBI patients (GCS=13 to 15) with abnormal CT scans, found low N -acetylaspartate/creatine and elevated lactate/creatine ratios in pericontusional areas (mostly temporal lobe) relative to comparison subjects within 7 days of injury. Two months later, there were no significant differences between mild TBI patients and comparison subjects in N -acetylaspartate/creatine ratios, while lactate/creatine ratios were undetectable in two patients and significantly reduced in three others. Clinical correlates or outcomes of these early abnormalities were not examined, although the researchers indicated an “uneventful” course in all patients, with all seven returning to preinjury activities.
In summary, MRS offers promise with regard to the sensitivity criterion, although metabolite abnormalities were inconsistent across studies. Specifically, mild TBI resulted in increased lactate acutely in one study 63 but not in another. 64 Similarly, there were significantly reduced N -acetylaspartate/creatine ratios in the splenium of the corpus callosum, 65 in parietal white matter, 66 and in white/gray matter of pericontused areas of the temporal lobes, 63 but not in white matter of the frontal lobes. 64 Finally, two studies found elevated choline/creatine ratios, one in the white matter of the frontal lobes 64 and one in occipital lobe gray matter. 66 Metabolite abnormalities were not apparent in other sampled brain regions, such as the brain stem or cerebellum. 66 Also, the incremental validity of MRS is unclear as most studies were conducted at least in part with patients who had abnormal clinical scans. Further research is also needed to address relationships with functional correlates. The one study that addressed this issue found no relationship between metabolite ratios and outcomes. 66
MRS research, like many of these newer techniques, suffers from disparate acquisition protocols across research groups. In addition, the use of ratios may be problematic in TBI. Creatine, for instance, is used to standardize other brain metabolites because it is relatively invariant and uniform in normal brain tissue. 67 However, it is not known if creatine is stable in TBI. Problems arise if creatine is affected similarly to the metabolite of interest. Indeed, there is suggestion from other literatures that creatine may be reduced in hypermetabolic and raised in hypometabolic states. 68 , 69 As metabolism may be compromised in mild TBI, 71 it is questionable to assume invariance of creatine in mild TBI.
Magnetic Source Imaging
Magnetic source imaging (MSI) utilizes magnetoencephalograpic technology to acquire electrophysiological data from the brain and combines it with structural data from conventional MRI technology. Only one MSI study has been conducted with mild TBI patients (GCS=13 to 15, LOC<20 minutes) who were 2 to 38 months postinjury. 72 One group (N=10) had normal clinical scans and no postconcussive complaints while the other group (N=20) consisted of patients with continued postconcussive complaints, 10% of whom had abnormal clinical scans. Using MSI, abnormalities were generally detected in symptomatic patients but not in asymptomatic patients. Abnormal low-frequency magnetic activity and magnetic slowing considered in combination were most specific to postconcussive symptoms. Only one patient from the group with mild TBI but no postconcussive complaints had abnormal magnetic slowing. MSI appears to be sensitive in mild TBI patients with postconcussion symptoms, but not necessarily in all mild TBI patients. Incremental validity was also present; the authors reported that MSI was three times more sensitive than MR imaging alone. Functional correlates were not examined.
Functional Neuroimaging Findings
Functional MRI
Functional MRI (fMRI) is a widely used neuroimaging technique for measuring brain functioning. The assumption behind blood-oxygen-level-dependent (BOLD) fMRI, which is most commonly utilized, is that an increase in neuronal activity results in an increase in local blood flow, leading to reduced concentrations of deoxyhemoglobin, a product of oxygen consumption. This reduction of deoxyhemoglobin leads to a smaller local magnetic field gradient, which results in a greater T2 image and an increase in MRI signal. However, the relationship between the signal change in T2 weighted images and vascular flow differences are not fully understood and may be nonlinear. There is an initial hypo-oxygenation response to stimulation that is highly localized and then followed by several seconds of widely dispersed hyperoxygenation. Studies of working memory in moderate to severe TBI suggest blood flow abnormalities relative to comparison subjects, particularly in the frontal lobes. 72 – 74
McAllister et al. 75 , 76 studied working memory in mild TBI patients with normal structural scans who were approximately 1 month postinjury. In the first study, 75 12 mild TBI patients (GCS=13 to 15, LOC<30 minutes) were recruited from emergency room records and tested between 6 to 35 days postinjury. These patients had poor memory, trouble concentrating, and difficulty with their jobs, but did not express greater levels of anxiety or depression relative to comparison subjects. Mild TBI patients had poorer performance on neuropsychological measures of simple reaction time and sustained attention, but not on a variety of other measures, including psychomotor speed, executive functions, and memory. In the scanner, they were asked to complete an auditory “n-back” task that entailed successive levels of working memory tested through presentation of a series of letters.
During the 1-back condition, for example, participants were asked to discern whether a letter presented aurally represented a target letter presented visually a moment before. During the 2-back condition, they had to decide whether the letter heard matched the letter seen two letters prior. Both patients and comparison subjects activated bilateral frontal and parietal regions in response to increasing demands on working memory but produced different brain activation patterns in response to different processing loads. Whereas comparison subjects primarily showed increases in activation from 0-back to 1-back, mild TBI patients primarily showed increases in activation from 1-back to 2-back. However, the mild TBI patients and comparison subjects showed comparable overall levels of activation on the 2-back task and comparable performance on the n -back task. The authors suggest that, rather than neuronal loss, mild TBI patients may have decreased ability to allocate or modulate resources according to processing load. 75
In a follow-up study, 76 these researchers added a 3-back condition with 18 mild TBI patients, six of whom had participated in the prior study. All 18 mild TBI subjects had normal clinical (or structural) MRI scans. Mild TBI participants again showed more cognitive symptoms than comparison subjects and again had poorer performance than comparison subjects on attention measures. Their scores again were comparable to those of comparison subjects on all n -back conditions and activated similar regions (bilateral frontal and parietal regions) in response to increasing demands on working memory.
Consistent with the prior study, the pattern of activation differed between mild TBI and comparison subjects. Mild TBI subjects had higher levels of activation than comparison subjects going from 1-back to 2-back, but less activation than comparison subjects going from 2-back to 3-back. Activation levels between 0-back to 1-back were not reported. This study again suggests subtle differences in brain functioning during increased working memory load. Rather than simply demonstrating additional activation with each increase in task difficulty, the observation was one of variable activation of mild TBI subjects compared to comparison subjects.
Finally, Jantzen et al. 77 conducted a prospective fMRI study of mild TBI using four concussed football players (no LOC but transient confusion) and four player comparison subjects. The scores of both groups on tests of sensorimotor coordination, working memory, memory, and mental calculations did not reliably change from preinjury to within 1 week postinjury. At baseline, the cognitive tasks elicited the expected brain activation patterns in frontal, parietal, and cerebellar regions. Within 1 week following injury to the concussed group, both groups showed increased activation during the cognitive tasks. However, the concussed players demonstrated much larger increases in supplementary motor, bilateral premotor cortex, superior and inferior parietal regions, and bilateral cerebellar regions.
These studies meet the sensitivity, incremental validity, and first step of the functional correlate criteria. However, the finding in McAllister et al.’s second study 76 revealed an anomalous pattern that is inconsistent with the first McAllister et al. study. 75 In the second McAllister et al. study, 76 comparision subjects actually showed significantly greater increases in activation than the mild TBI patients as the n -back task became more demanding. Future research is needed to clarify these differences. Future studies also will need to address a number of methodological considerations, including the difficulty inherent in “controlling” for mood in TBI patients and the susceptibility of fMRI to artifact in regions commonly affected by TBI (i.e., the frontal lobes; see Hillary et al. 78 for a detailed discussion of these and other issues related to conducting fMRI research with TBI patients). In addition, it is unclear how long these anomalous patterns persist given that all studies to date have examined mild TBI participants within the first month postinjury. These issues aside, fMRI offers some of the most promising findings to date.
Positron Emission Tomography
Positron emission tomography (PET) is a diagnostic imaging technique for measuring regional brain metabolism. Glucose is brought to the brain via the bloodstream; accordingly, the rates of regional cerebral blood flow (rCBF) within various brain regions are regulated depending on the changing demands of these regions. Radionuclides used in PET scanning are typically isotopes with short half lives. These isotopes are incorporated into compounds normally used by the body, such as glucose or water, and then are injected into the body to trace where they become distributed.
Following severe TBI, brain cells exhibit a metabolic state called hyperglycolysis in which glucose metabolism is increased above normal levels. The initial brief response of hyperglycolysis is followed by a relatively prolonged period of metabolic depression that is followed by recovery. One study suggests that this recovery occurs at approximately 1 month regardless of severity. 79 This triphasic metabolic pattern has been demonstrated in animal models of TBI 80 and in research with humans using PET technology. 79 , 81 – 84 Nonetheless, the studies summarized below suggest that metabolic abnormalities may persist chronically in at least some mild TBI patients.
Humayun et al. 85 studied three mild TBI patients (GCS=13 to 15, LOC<20 minutes, normal CT and MRI) who were 3 to 12 months postinjury and three matched comparison subjects. All three patients had deficits on neuropsychological testing in attention/concentration and memory abilities. None had a history of prior head injury. Mild TBI patients performed more poorly on a computer-based vigilance task during the scan, but not statistically so given the small number of participants. There were no significant group differences in global metabolic rate. However, decreased glucose metabolism was found in the left posterior lateral temporal cortex, right anterior frontal cortex, and left caudate nucleus of mild TBI patients, while increased metabolism was noted in left and right medial temporal cortices.
Ruff et al. 86 studied nine mild TBI patients (LOC<20 minutes) who were an average of 18 months postinjury (range=1 to 37 months), presented with significant neuropsychological deficits, and were consequently referred for PET scans. The authors reported that CT and/or MRI findings in these patients were “generally negative.” Both mild TBI patients and comparison subjects performed a continuous performance test during scanning. Unfortunately, results were not presented quantitatively. According to the authors, hypoactivity was more prevalent in frontal areas in the mild TBI participants.
Gross et al. 70 studied 20 patients with mild-to-moderate TBI who were being treated for TBI-related complaints (e.g., irritability and decreased attention) an average of 43 months postinjury. Based on loss of consciousness (<30 minutes), 17 of the 20 patients were mildly injured. All but two had normal MRI/CT scans. Both mild TBI patients and comparison subjects (N=31) performed a continuous performance test during scanning. Eighty-two ROIs were collapsed to temporal, frontal, and parietal regions. All 20 patients demonstrated some type of abnormality in at least one region. Although both hypo- and hyper-metabolism were found in the same regions across different mild TBI patients relative to comparison subjects, there was a significant correlation between the overall number of subjective complaints and number of PET metabolic abnormalities. There was also a significant relationship between attention/concentration complaints and temporal lobe abnormalities on PET within the mild TBI patients. Finally, there was an overall significant correlation between metabolism abnormalities in the three brain regions of mild TBI patients and performance on neuropsychological measures of memory and executive functions.
Chen et al. 87 examined differences between five mild TBI patients (GCS=13 to 15, LOC<30 minutes, normal CT/MRI) and five comparison subjects in rCBF, measured via H 215 O during a spatial working memory task. Mild TBI patients were an average of 16.6 months postinjury and presented with persisting complaints. They did not differ from comparison subjects, however, on a checklist measure of depression or anxiety and none of the patients was involved in litigation. Mild TBI patients performed more poorly on neuropsychological measures, particularly recognition memory and psychomotor speed, and expressed more postconcussive symptoms. Though there were no rCBF differences between patients and comparison subjects during the resting scan, there was a smaller percentage increase for mild TBI patients in the right inferior frontal gyrus during the spatial working memory task on which the groups performed comparably.
In summary, the results of PET studies conducted with mild TBI patients in the chronic stage are very inconsistent, with findings ranging from no global abnormalities but significant regional hypometabolism, 70 , 85 , 86 no overall abnormalities in metabolism except during a working memory task, 87 and both hypo- and hyper-metabolism in the same regions across different mild TBI patients. 70 As all of these studies were conducted at least 3 months postinjury, the inconsistency is troubling. In addition, although mild TBI patients typically perform comparably to comparison subjects on attention tasks, both hypometabolism and hypermetabolism are noted regionally in association with these tasks. The Chen et al. 87 study is an exception but utilized H 215 O methodology and showed frontal rCBF changes with the spatial working memory task in mild TBI subjects. PET abnormalities generally are associated with neuropsychological performance, 70 though these comparisons are sometimes made qualitatively. 86 PET studies offer promise, having demonstrated sensitivity to mild TBI, incremental validity, and functional correlates. Nonetheless, more studies conducted exclusively with patients with mild head injury are needed to understand the clinical correlates of hyper- versus hypometabolism.
Single Photon Emission Computed Tomography
Single photon emission computed tomography (SPECT) has been used as a less expensive and more readily available alternative to PET, with its primary application being the gross localization of rCBF. Unlike PET studies, however, SPECT studies typically measure the patient’s brain at rest. Measuring blood flow is considered an indirect gauge of brain metabolism. Also unlike PET, most applications of SPECT imaging require comparisons between an ROI and another brain region presumably free of injury. This methodological requirement is problematic for TBI work, given the potential diffuse nature of the injuries. It should further be noted that blood flow is not necessarily equivalent to metabolism. Although in healthy individuals the two are highly correlated, this relationship is less clear following TBI. 79 Indeed, some data suggest discordance between blood flow and metabolism following TBI. 88 , 89 as has been demonstrated in the PET literature. 90 These caveats aside, SPECT offers promise with regard to meeting the criteria of sensitivity, incremental validity, and clinical correlates.
In patients within 2 days of injury, Audenaert et al. 91 found evidence of focal abnormalities in frontal and temporal regions using cobalt-57-SPECT in mild TBI patients (GCS=15, no loss of consciousness) with no evidence of pathology on CT or EEG. Clearly, incremental validity above and beyond CT is established, although no comparison subjects were studied. As such, there is no way to evaluate the extent to which these findings are specific to brain injury. These investigators found an association with neuropsychological testing in seven of eight patients, although this correlation was made qualitatively. In contrast, Hofman et al. 18 found no consistent relationship between neuropsychological tests and 99mTcHMPAO SPECT abnormalities in patients 2 to 5 days postinjury. In these mild TBI patients (GCS=14 to 15, LOC<20 minutes), SPECT again showed incremental validity over conventional MR images, identifying abnormalities in frontal, parietal, and thalamic regions. Most abnormalities represented hypoperfusion, although, again, there was no comparison with comparison subjects.
Finally, in 20 mildly to moderately injured patients who were 1 to 9 days postinjury, Nedd et al. 92 reported incremental sensitivity over CT scans in terms of both identifying more lesions (87.5% versus 37.5%) and greater surface area of involvement using 99mTcHMPAO SPECT. Most abnormalities reflected hypoperfusion compared with comparison subjects, although three cases exhibited hyperperfusion. This discrepancy could be due to the variability in certain patient characteristics of the group (i.e., differences between those measured at 1 versus 9 days postinjury). No attempt was made to correlate these SPECT findings with functional data.
More studies have examined patients in the postacute and chronic stages. As in the acute stages, SPECT demonstrates increased sensitivity beyond conventional CT/MRI. Umile et al. 93 found that four symptomatic mild TBI (GCS=13 to15, LOC<30 minutes) patients had decreased perfusion on SPECT (primarily frontal-temporal) an average of 19 months postinjury. However, these abnormalities were assessed subjectively, without benefit of a comparison group. All of the patients endorsed symptoms of depression and were impaired on neuropsychological tests of attention, memory, and information processing. Expected relationships between abnormalities within eight ROIs and neuropsychological tests were not demonstrated. Similarly, Varney and Bushnell, 94 in a sample of 14 mild TBI patients (GCS=13 to 15, LOC<30 minutes) with normal CT/MRI who were at least 5 years postinjury and chronically unemployed (despite a preinjury history of responsible employment), demonstrated 99mTcHMPAO SPECT hypoperfusion relative to comparison subjects primarily in anterior mesial temporal lobe. However, given that this sample is not representative of typical mild TBI (i.e., chronically disabled), it is difficult to draw conclusions. Finally, Bonne et al., 95 in a symptomatic group of 28 mild TBI patients (GCS=13 to 15, LOC<20 minutes) an average of 5.2 years postinjury with normal CT/MRI scans, found 99mTcHMPAO SPECT abnormalities (hypoperfusion) relative to comparison subjects primarily in medial and lower temporal and frontal regions. These authors reported meaningful relationships between hypoperfusion in frontal, left posterior and subcortical regions and corresponding neuropsychological tests. However, these relationships were reported qualitatively in a dichotomous fashion with no indication of the strength of association.
Other studies conducted with patients in the chronic stages have tended to use a mixture of patients with both normal and abnormal CT/MRI scans. For instance, Gray et al. 96 utilized a sample of 20 mild TBI patients (GCS=13 to 15, LOC<20 minutes), 25% of whom had abnormal CT scans at least 6 months postinjury. Using 99mTcHMPAO SPECT, hypoperfusion (relative to comparison subjects) was found in 60% of patients, with an overall concordance between CT and SPECT of 55%. Ichise et al. 97 drew a sample of 15 mild TBI patients (GCS=13 to 15, LOC<20 minutes) from the same rehabilitation practice, for whom initial CT scans were available for about half of the sample (88% normal). These patients were again at least 6 months postinjury and were compared with normal comparison subjects. 99mTcHMPAO SPECT revealed relative hypoperfusion primarily in frontal and temporal regions. SPECT was more sensitive than CT/MR images. An anterior-posterior gradient was calculated by summing the activity in four anterior ROIs and dividing by the sum of four posterior ROIs. Whereas volumetric brain volume, as measured using structural T1-weighted MR images was not sensitive to mild TBI, the anterior-posterior SPECT gradient was sensitive to mild TBI and correlated with neuropsychological tests of attention, memory, and executive function in the whole group (both mildly and severely injured patients).
In a larger sample of 43 mild TBI patients (LOC<20 minutes) who were 1 to 65 months postinjury, Kant et al. 98 found 99mTcHMPAO SPECT hypoperfusion in 53% of their sample, with only 12% having documented abnormalities on CT/MR scans. Most SPECT abnormalities were in frontal and temporal regions. Neither memory and executive deficits nor self-reported depressive symptoms were significantly related to these abnormalities. As these analyses were conducted using dichotomous data (i.e., normal versus abnormal), less statistical power was available to detect potential differences. Umile et al., 99 in a sample of 20 mild TBI patients (GCS=13 to15, LOC<30 minutes) who were referred for postconcussive symptoms from 2 weeks to nearly 8 years postinjury, reported inconsistent findings with regard to neuropsychological and PET/SPECT findings. Fourteen patients (70%) had the predicted memory problems coincident with temporal lobe abnormalities, while six patients (30%) had discordant findings (e.g., temporal lobe abnormalities but no memory deficits); these comparisons were made qualitatively.
Jacobs et al. 100 , 101 examined the predictive power of SPECT by following 136 patients with GCS >13 and normal admission CT scans for 1 year postinjury. Clinical outcomes were measured categorically using a neurological exam, postconcussive symptom checklist, and memory and concentration tests. They found initial positive predictive power (i.e., conditional probability of subsequent poor clinical outcome given the presence of an initial abnormal SPECT scan) of SPECT to be 44% at 3 months with improvement to 83% at 12 months, while negative predictive power (i.e., conditional probability of a normal clinical outcome given initial normal SPECT) of the initial scan was 92% at 3 months with improvement to 100% at 12 months. Due to the high number of false positive errors at 3 and 6 months postinjury, these authors recommend caution in attributing an abnormal SPECT finding to posttraumatic sequelae in mild TBI; an abnormal initial SPECT scan postinjury does not preclude good clinical recovery.
However, a normal initial SPECT scan is highly suggestive of good clinical outcome. Indeed, the American Academy of Neurology guidelines recommend the use of SPECT as an investigational tool only, as the clinical significance of positive findings is unknown. 102 The Jacobs et al. study exemplifies one of the best attempts to address the relevant issues with regard to validity issues. It demonstrates the sensitivity of SPECT to mild TBI, its incremental validity by using subjects with normal CT scans, and its relationship with clinical variables. Moreover, this study followed patients prospectively to study predictive validity.
In summary, most SPECT studies, when conducted within the first few weeks postinjury, reveal hypoperfusion associated with mild TBI, 18 , 91 , 92 although relationships to clinical variables were either not demonstrated, 18 reported in a qualitative fashion, 91 or not studied. Studies conducted with more chronic mild TBI patients (i.e., 6 months or more postinjury) also reveal hypoperfusion on SPECT; 93 – 99 patients in all of these studies were symptomatic or presumably symptomatic (e.g., recruited from rehabilitation centers). These studies found inconsistent relationships between both symptom complaints and SPECT findings with neuropsychological testing. Some studies, 95 , 97 , 99 on the other hand, did find a relationship, although the location of metabolic abnormalities detected via SPECT and the site of cerebral dysfunction inferred from neuropsychological testing did not consistently correspond. In the only study to use the anterior-posterior gradient as the comparison with neuropsychological findings, a relationship was observed. 97
Finally, very few of the reviewed SPECT studies used comparison groups, relying instead on clinical judgment. Nonetheless, there is a relatively consistent presence of hypoperfusion in the frontal and temporal regions across studies.
Neuroimaging Conclusions and Caveats
Table 1 presents a summary of studies that reported results separately for mild TBI patients using newer neuroimaging techniques (see Mendez et al. 103 for a review of neuroimaging in sports-related mild TBI). All studies, regardless of technology, demonstrated abnormalities associated with mild TBI in at least some participants. MTI, MRS, fMRI, PET and SPECT all show promise with regard to greater sensitivity than traditional MRI/CT scans. This is sometimes reflected in group differences between mild TBI participants and comparison subjects and other times through demonstration of abnormalities in brain regions appearing normal on MRI. Importantly, most studies that demonstrated incremental validity (by using only participants with normal CT/MRI scans) also correlated neuroimaging abnormalities (at least to some extent) with clinical variables. DTI and MRS in particular require additional work in mild TBI participants to demonstrate clinical utility and abnormalities in the chronic stage.
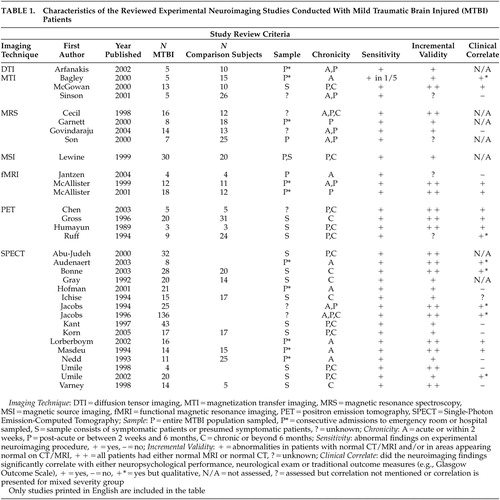 |
As can be seen in Table 1 , the functional studies most closely approach the ideal with regard to the criteria outlined in the introduction, although it remains to be seen whether the abnormalities seen in mild TBI consistently extend beyond the postacute stage. PET studies have consistently found abnormalities that have significant clinical correlates and are demonstrated in those with normal MRI/CT scans, but most studies to date have relied upon symptomatic patients. Similar issues are relevant to SPECT research. The fMRI studies of McAllister et al. 75 , 76 demonstrate recruitment based on prior history of mild TBI without regard to current symptoms. As most functional technologies have demonstrated their utility with regard to sensitivity, incremental validity, and functional correlates, the next step will be to conduct prospective studies with both symptomatic and asymptomatic patients. Jacobs et al. 100 , 101 approach this ideal using SPECT by following patients over time and demonstrating relationships with functional variables. Again, what is needed to strengthen these findings is inclusion of a comparison group, quantification of clinical outcome variables, and inclusion of the full spectrum of mild TBI (rather than just those symptomatic or presenting at a clinic). In addition, given the triphasic metabolic response postinjury, 79 – 84 longitudinal evaluation of postinjury response patterns will be important in determining individual differences in pattern and time course and in developing appropriate normative comparison standards.
The lack of consistency in methodology makes interpretation of the functional neuroimaging literature with regard to mild TBI challenging. For instance, the patient’s mental activity during the actual scan differs across functional studies, with some studies requiring the patient to perform a task and others requiring the patient to do “nothing.” Some studies include patients who require “mild sedation” during the scan, while others do not. Obviously, these differences may be reflected in differential rates of blood flow or metabolism. Also, differences in findings may depend on the type of tracer utilized. 91 There is inconsistency in matching mild TBI and comparison participants on demographic variables.
Further, most studies used relatively small samples. Statistical problems arise when a large number of variables are considered in a small number of patients. Correlating neuroimaging findings with postconcussive symptoms and/or mild cognitive symptoms is prone to reporting bias. For instance, these difficulties could be due to a mild depression. In addition, selection bias is inherent in many of these convenience samples, including confounding by intervening events during postinjury intervals of several years. Issues remain across technologies with regard to the best brain regions and time postinjury to scan and with regard to using a report of initial “normal” clinical neuroimaging as evidence that a given individual in fact had a “normal scan.” 104
Particularly in the area of functional neuroimaging, if these neuroimaging techniques are to become clinically useful, it will be necessary to interpret positive findings. For example, frontal hypometabolism or underactivation may be due to a wide variety of possible neurological or psychiatric conditions besides mild TBI. There is no unique PET or SPECT “profile” that has been clinically validated with TBI. Neuroimaging is not unlike cognitive testing in which those tests that demonstrate abnormalities of brain function may be more sensitive than specific. PET 105 – 107 and SPECT 108 studies of depression and even sad mood induction can result in frontal hypometabolism similar to that seen following mild TBI. It is difficult to have confidence in the specificity of the abnormalities demonstrated in these studies. Indeed, as Deutsch 109 demonstrated, reduction of frontal blood flow is often a result of altered mental activity rather than brain pathology. Further, a recent fMRI study demonstrated that a placebo administered for pain control (i.e., resulting in a “belief or self-expectation” that pain will be reduced) resulted in significant attenuation of activation in response to a painful stimulus in pain-related areas of the cerebrum. Importantly, the magnitude of the self-reported placebo effect was correlated with the fMRI neural change. 110 Additional mild TBI studies will also need to better eliminate inclusion of potentially malingering patients, as fMRI abnormalities in frontal regions have been demonstrated with the mere act of deception. 111 Clearly, mood, beliefs regarding function, and attitudes, irrespective of any underlying brain dysfunction, all can alter functional neuroimaging results. This is particularly relevant in mild TBI given that a significant minority report mood disturbance, subsequently decrease their mental and physical activity levels, and are convinced that they are functionally and cognitively compromised as a result of their mild TBI. Cross-sectional studies conducted with chronic symptomatic patients are therefore difficult to interpret. Following patients prospectively and correlating abnormal imaging findings with outcome will begin to address these issues.
From a clinical perspective, before these newer neuroimaging procedures can be useful in the individual case, normative data are needed. Controlling for age, time since injury, effort, litigation status, scanning technique, and potentially other as yet unknown variables may be important for valid interpretation. Reliability with regard to ratings of what is “normal” versus “abnormal” is inconsistently presented in the literature, and test-retest reliability data are lacking. The subjectivity inherent in making these ratings, particularly in the reviewed SPECT literature, is problematic. As mentioned before, very few of the reviewed SPECT studies used comparison groups, relying instead on clinical judgment. Studies finding greater abnormalities on the left side of the brain are difficult to explain. 101 , 112 Finally, combining structural and functional imaging studies may provide greater sensitivity than any one individual technique. 113
In summary, the reviewed brain imaging technologies detect changes not demonstrated on conventional MRI or CT structural scans. Importantly, these abnormalities are generally correlated with clinical outcomes, although the use of quantified and more varied outcome measures will be helpful in future research. Additional work is needed to assess the duration of these abnormalities, the extent to which they are found prospectively in asymptomatic and symptomatic patients, and the interpretative significance of positive findings. Finally, further study of brain region specificity is needed across technologies.
However, it is important to keep in mind that neuroimaging, like other medical tests and procedures, is typically interpreted in the context of the clinical history and other findings. In etiological conditions, such as mild TBI, where cortical disruptions are likely to be mild and diffuse, medical tests noting abnormalities are likely to be those which are most sensitive but not necessarily specific. As in neuropsychological or any other evaluations of a diffuse process, any attempt to identify or define specific patterns is extremely challenging. When viewed in light of the heterogeneity of this population in terms of the extent of injury (or even the presence of injury given that patient report is often the only evidence of altered consciousness) and the variability in clinical presentation and outcomes, the mixed findings of neuroimaging studies are not surprising. Intraindividual or “within subject” studies, wherein experiments using different imaging modalities are conducted within the same patient, would be a logical first step to ameliorating some of these issues.
1. Sosin DM, Sniezek JE, Thurman DJ: Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj 1996; 10:47–54Google Scholar
2. Kraus JF, Nourjah P: The epidemiology of mild, uncomplicated brain injury. J Trauma 1988; 28:1637–1643Google Scholar
3. American Congress of Rehabilitation Medicine: Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8:86–87Google Scholar
4. Duus BR, Kruse KV, Nielsen KB, et al: Minor head injuries in a Copenhagen district: 1: epidemiology. Ugeskr Laeger 1991; 153:2111–2113Google Scholar
5. Evans RW: The postconcussion syndrome and the sequelae of mild head injury. Neurol Clin 1992; 10:815–847Google Scholar
6. Andersson EH, Bjorklund R, Emanuelson I, et al: Epidemiology of traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand 2003; 107:256–259Google Scholar
7. Thornhill S, Teasdale GM, Murray GD, et al: Disability in young people and adults one year after head injury: prospective cohort study. BMJ 2000; 320:1631–1635Google Scholar
8. Thurman DJ: The epidemiology and economics of head trauma, in Head Trauma: Basic, Preclinical, and Clinical Directions. Edited by Miller L, Hayes R. New York, John Wiley & Sons, 2001, pp 327–347Google Scholar
9. Binder LM, Rohling ML, Larrabee J: A review of mild head trauma, part I: meta-analytic review of neuropsychological studies. J Clin Exp Neuropsychol 1997; 19:421–431Google Scholar
10. Alexander MP: Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995; 45:1253–1260Google Scholar
11. Rimel RW, Giordani B, Barth JT, et al: Disability caused by minor head injury. Neurosurgery 1981; 9:221–228Google Scholar
12. Eisenberg HM, Levin HS: Computed tomography and magnetic resonance imaging in mild to moderate head injury, in Mild Head Injury. Edited by Levin HS, Eisenberg HM, Benton AL. New York, Oxford University Press, 1989, pp 133–141Google Scholar
13. Dikmen S, McLean A, Temkin N: Neuropsychological and psychosocial consequences of minor head injury. J Neurol Neurosurg Psychiatry 1986; 49:1227–1232Google Scholar
14. Levin HS, Mattis S, Ruff RM, et al: Neurobehavioral outcome following minor head injury: a three-center study. J Neurosurg 1987; 66:234–243Google Scholar
15. Borg J, Holm L, Cassidy JD, et al: Diagnostic procedures in mild traumatic brain injury: results of the World Health Organization Collaborating Centre Task Force on mild traumatic brain injury. J Rehabil Med 2004; 43:S61–75Google Scholar
16. Jenkins A, Teasdale G, Hadley MD, et al: Brain lesions detected by magnetic resonance imaging in mild and severe head injuries. Lancet 1986; 2:445–446Google Scholar
17. Levin HS, Amparo E, Eisenberg HM, et al: Magnetic resonance imaging and computerized tomography in relation to the neurobehavioral sequelae of mild and moderate head injuries. J Neurosurg 1987; 66:706–713Google Scholar
18. Hofman PA, Stapert SZ, van Kroonenburgh MJ, et al: MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. Am J Neuroradiol 2001; 22:441–449Google Scholar
19. Hughes DG, Jackson A, Mason DL, et al: Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology 2004; 46:550–558Google Scholar
20. Hayes RL, Dixon CE: Neurochemical changes in mild head injury. Semin Neurol 1994; 14:25–31Google Scholar
21. Miller L: Neuropsychology and pathophysiology of mild head injury and the postconcussion syndrome: clinical and forensic considerations. J Cogn Rehabil 1996; 14:8–23Google Scholar
22. Povlishock JT, Coburn TH: Morphopathological change associated with mild head injury, in Mild Head Injury. Edited by Levin HS, Eisenberg HM, Benton AL. New York, Oxford University Press, 1989, pp 37–53Google Scholar
23. Adams JH, Doyle D, Ford I, et al: Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 1989; 15:49–59Google Scholar
24. Bigler ED: Neuropsychological results and neuropathological findings at autopsy in a case of mild traumatic brain injury. J Int Neuropsychological Soc 2004; 10:794–806Google Scholar
25. Oppenheimer DR: Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry 1968; 31:299–306Google Scholar
26. Mittl RL, Grossman RI, Hiehle JF, et al: Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. Am J Neuroradiol 1994; 15:1583–1589Google Scholar
27. Bohnen L, Jolles J, Twijnstra A, et al: Coping styles, cortisol reactivity, and performance on vigilance tasks of patients with persistant postconcussive symptoms after mild head injury. Int J Neurosci 1992; 64:97–105Google Scholar
28. Marsh HV, Smith MD: Post-concussion syndrome and the coping hypothesis. Brain Inj 1995; 9:553–562Google Scholar
29. Bryant RA, Harvey AG: Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J Nerv Ment Dis 1999; 187:302–305Google Scholar
30. Mittenberg W, DiGiulio DV, Perrin S, et al: Symptoms following mild head injury: expectation as aetiology. J Neurol Neurosurg Psychiatry 1992; 55:200–204Google Scholar
31. Williams DH, Levin HS, Eisenberg HM: Mild head injury classification. Neurosurgery 1990; 27:422–428Google Scholar
32. Laatsch LK, Thulborn KR, Krisky CM, et al: Investigating the neurobiological basis of cognitive rehabilitation therapy with fMRI. Brain Inj 2004; 18:957–974Google Scholar
33. Pettus EH, Christman CW, Giebel ML, et al: Traumatically induced altered membrane permeability: its relationship to traumatically induced reactive axonal change. J Neurotrauma 1994; 11:507–522Google Scholar
34. Povlishock JT, Becker DP, Cheng CL, et al: Axonal change in minor head injury. J Neuropathol Exp Neurol 1983; 42:225–242Google Scholar
35. Gennarelli TA, Thibault LE, Adams JH, et al: Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 1982; 12:564–574Google Scholar
36. Povlishock JT, Becker DP: Fate of reactive axonal swellings induced by head injury. Lab Invest 1985; 52:540–552Google Scholar
37. Povlishock JT, Christman CW: The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma 1995; 12:555–564Google Scholar
38. Blumbergs PC, Scott G, Manavis J, et al: Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma 1995; 12:565–572Google Scholar
39. Belanger HG, Curtiss G, Demery JA, et al: Factors moderating neuropsychological outcome following mild traumatic brain injury: a meta-analysis. J Int Neuropsychol Soc (in press)Google Scholar
40. Basser PJ, Mattiello J, LeBihan D: MR diffusion tensor spectroscopy and imaging. Biophysical J 1994; 66:259–267Google Scholar
41. Pierpaoli C, Basser PJ: Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996; 36:893–906Google Scholar
42. O’Sullivan M, Jones DK, Summers PE, et al: Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001; 57:632–638Google Scholar
43. Sullivan EV, Adalsteinsson E, Hedehus M, et al: Equivalent disruption of regional white matter microstructure in ageing healthy men and women. Neuroreport 2001; 12:99–104Google Scholar
44. Wieshmann UC, Symms MR, Clark CA, et al: Blunt-head trauma associated with widespread water-diffusion changes. Lancet 1999; 353:1242–1243Google Scholar
45. Rugg-Gunn FJ, Symms MR, Barker GJ, et al: Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J Neurol Neurosurg Psychiatry 2001; 70:530–533Google Scholar
46. Arfanakis K, Haughton VM, Carew JD, et al: Diffusion tensor MR imaging in diffuse axonal injury. Am J Neuroradiol 2002; 23:794–802Google Scholar
47. Terayama Y, Meyer JS, Kawamura J, et al: Cognitive recovery correlates with white-matter restitution after head injury. Surg Neurol 1993; 39:177–186Google Scholar
48. Wolff SD, Balaban RS: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 1989; 10:135–144Google Scholar
49. Bagley LJ, McGowan JC, Grossman RI, et al: Magnetization transfer imaging of traumatic brain injury. J Magn Reson Imaging 2000; 11:1–8Google Scholar
50. McGowan JC, Yang JH, Plotkin RC, et al: Magnetization transfer imaging in the detection of injury associated with mild head trauma. Am J Neuroradiol 2000; 21:875–880Google Scholar
51. Sinson G, Bagley LJ, Cecil KM, et al: Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. Am J Neuroradiol 2001; 22:143–151Google Scholar
52. Ross DT, Graham DI, Adams JH: Selective loss of neurons from the thalamic reticular nucleus following severe human head injury. J Neurotrauma 1993; 10:151–165Google Scholar
53. Barker PB, Gillard JH, van Zijl PC, S et al: Acute stroke: evaluation with serial proton MR spectroscopic imaging. Radiology 1994; 192:723–732Google Scholar
54. Brenner RE, Munro PM, Williams SC, et al: The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 1993; 29:737–745Google Scholar
55. Go KG, Kamman RL, Mooyaart EL, et al: Localised proton spectroscopy and spectroscopic imaging in cerebral gliomas, with comparison to positron emission tomography. Neuroradiology 1995; 37:198–206Google Scholar
56. Bitsch A, Bruhn H, Vougioukas V, et al: Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 1999; 20:1619–1627Google Scholar
57. Nakai T, Rhine WD, Enzmann DR, et al: A model for detecting early metabolic changes in neonatal asphyxia by 1H-MRS. J Magn Reson Imaging 1996; 6:445–452Google Scholar
58. Anderson ML, Smith DS, Nioka S, et al: Experimental brain ischaemia: assessment of injury by magnetic resonance spectroscopy and histology. Neurol Res 1990; 12:195–204Google Scholar
59. Howe FA, Barton SJ, Cudlip SA, et al: Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2003; 49:223–232Google Scholar
60. Brooks WM, Stidley CA, Petropoulos H, et al: Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 2000; 17:629–640Google Scholar
61. Friedman SD, Brooks WM, Jung RE, et al: Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology 1999; 52:1384–1391Google Scholar
62. Friedman SD, Brooks WM, Jung RE, et al: Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. Am J Neuroradiol 1998; 19:1879–1885Google Scholar
63. Son BC, Park CK, Choi BG, et al: Metabolic changes in pericontusional oedematous areas in mild head injury evaluated by 1H MRS. Acta Neurochir Suppl 2000; 76:13–16Google Scholar
64. Garnett MR, Blamire AM, Rajagopalan B, et al: Evidence for cellular damage in normal-appearing white matter correlates with injury severity in patients following traumatic brain injury: a magnetic resonance spectroscopy study. Brain 2000; 123(pt 7):1403–1409Google Scholar
65. Cecil KM, Hills EC, Sandel ME, et al: Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J Neurosurg 1998; 88:795–801Google Scholar
66. Govindaraju V, Gauger GE, Manley GT, et al: Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR Am J Neuroradiol 2004; 25:730–737Google Scholar
67. Burtscher IM, Holtas S: Proton MR spectroscopy in clinical routine. J Magn Reson Imaging 2001; 13:560–567Google Scholar
68. Castillo M, Kwock L, Mukherji SK: Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 1996; 17:1–15Google Scholar
69. Wood SJ, Berger G, Velakoulis D, et al: Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull 2003; 29:831–843Google Scholar
70. Gross H, Kling A, Henry G, Herndon C, et al: Local cerebral glucose metabolism in patients with long-term behavioral and cognitive deficits following mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 1996; 8:324–334Google Scholar
71. Lewine JD, Davis JT, Sloan JH, et al: Neuromagnetic assessment of pathophysiologic brain activity induced by minor head trauma. Am J Neuroradiol 1999; 20:857–866Google Scholar
72. Christodoulou C, DeLuca J, Ricker JH, et al: Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J Neurol Neurosurg Psychiatry 2001; 71:161–168Google Scholar
73. Perlstein WM, Carter CS, Noll DC, et al: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Google Scholar
74. Scheibel RS, Pearson DA, Faria LP, et al: An fMRI study of executive functioning after severe diffuse TBI. Brain Inj 2003; 17:919–930Google Scholar
75. McAllister TW, Saykin AJ, Flashman LA, et al: Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 1999; 53:1300–1308Google Scholar
76. McAllister TW, Sparling MB, Flashman LA, et al: Differential working memory load effects after mild traumatic brain injury. Neuroimage 2001; 14:1004–1012Google Scholar
77. Jantzen KJ, Anderson B, Steinberg FL, et al: A prospective functional MR imaging study of mild traumatic brain injury in college football players. Am J Neuroradiol 2004; 25:738–745Google Scholar
78. Hillary FG, Steffener J, Biswal BB, et al: Functional magnetic resonance imaging technology and traumatic brain injury rehabilitation: guidelines for methodological and conceptual pitfalls. J Head Trauma Rehabil 2002; 17:411–430Google Scholar
79. Bergsneider M, Hovda DA, McArthur DL, et al: Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J Head Trauma Rehabil 2001; 16:135–148Google Scholar
80. Hovda DA: Metabolic dysfunction, in Neurotrauma. Edited by Narayan RK, Wilberger JE, Povlishock JT. New York, McGraw-Hill, 1996, pp 1159–1178Google Scholar
81. Bergsneider M, Hovda DA, Shalmon E, et al: Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg 1997; 86:241–251Google Scholar
82. Bergsneider M, Hovda DA, Lee SM, et al: Dissociation of cerebral glucose metabolism and level of consciousness during the period of metabolic depression following human traumatic brain injury. J Neurotrauma 2000; 17:389–401Google Scholar
83. Kelly DF, Martin NA, Kordestani R, et al: Cerebral blood flow as a predictor of outcome following traumatic brain injury. J Neurosurg 1997; 86:633–641Google Scholar
84. Yamaki T, Yoshino E, Fujimoto M, et al: Chronological positron emission tomographic study of severe diffuse brain injury in the chronic stage. J Trauma 1996; 40:50–56Google Scholar
85. Humayun MS, Presty SK, Lafrance ND, et al: Local cerebral glucose abnormalities in mild closed head injured patients with cognitive impairments. Nucl Med Commun 1989; 10:335–344Google Scholar
86. Ruff RM, Crouch JA, Troster AI, et al: Selected cases of poor outcome following a minor brain trauma: comparing neuropsychological and positron emission tomography assessment. Brain Inj 1994; 8:297–308Google Scholar
87. Chen SH, Kareken DA, Fastenau PS, et al: A study of persistent post-concussion symptoms in mild head trauma using positron emission tomography. J Neurol Neurosurg Psychiatry 2003; 74:326–332Google Scholar
88. Obrist WD, Langfitt TW, Jaggi JL, et al: Cerebral blood flow and metabolism in comatose patients with acute head injury. relationship to intracranial hypertension. J Neurosurg 1984; 61:241–253Google Scholar
89. Abu-Judeh HH, Singh M, Masdeu JC, et al: Discordance between FDG uptake and technetium-99m-HMPAO brain perfusion in acute traumatic brain injury. J Nucl Med 1998; 39:1357–1359Google Scholar
90. Mintun MA, Lundstrom BN, Snyder AZ, et al: Blood flow and oxygen delivery to human brain during functional activity: theoretical modeling and experimental data. Proc Natl Acad Sci USA 2001; 98:6859–6864Google Scholar
91. Audenaert K, Jansen HM, Otte A, et al: Imaging of mild traumatic brain injury using 57Co and 99mTc HMPAO SPECT as compared to other diagnostic procedures. Med Sci Monit 2003; 9:MT112–7Google Scholar
92. Nedd K, Sfakianakis G, Ganz W, et al: 99mTc- HMPAO spect of the brain in mild to moderate traumatic brain injury patients: compared with CT—a prospective study. Brain Inj 1993; 7:469–479Google Scholar
93. Umile EM, Plotkin RC, Sandel ME: Functional assessment of mild traumatic brain injury using spect and neuropsychological testing. Brain Inj 1998; 12:577–594Google Scholar
94. Varney NR, Bushnell D: Neurospect findings in patients with posttraumatic anosmia: a quantitative analysis. J Head Trauma Rehabil1998; 13:63–72Google Scholar
95. Bonne O, Gilboa A, Louzoun Y, et al: Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry Res 2003; 124:141–152Google Scholar
96. Gray BG, Ichise M, Chung DG, et al: Technetium-99m-HMPAO SPECT in the evaluation of patients with a remote history of traumatic brain injury: a comparison with x-ray computed tomography. J Nuclear Med 1992; 33:52–58Google Scholar
97. Ichise M, Chung DG, Wang P, et al: Technetium-99m- HMPAO SPECT and MRI in the evaluation of patients with chronic traumatic brain injury: a correlation with neuropsychological performance. J Nucl Med 1994; 35:217–226Google Scholar
98. Kant R, Smith-Seemiller L, Isaac G, et al: Tc- HMPAO SPECT in persistent post-concussion syndrome after mild head injury: comparison with MRI/CT. Brain Injury 1997; 11:115–124Google Scholar
99. Umile EM, Sandel ME, Alavi A, et al: Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch Phys Med Rehabil 2002; 83:1506–1513Google Scholar
100. Jacobs A, Put E, Ingels M, Put T, et al: One-year follow-up of technetium-99m- HMPAO SPECT in mild head injury. J Nucl Med 1996; 37:1605–1609Google Scholar
101. Jacobs A, Put E, Ingels M, et al: Prospective evaluation of technetium-99m-HMPAO SPECT in mild and moderate traumatic brain injury. J Nucl Med 1994; 35:942–947Google Scholar
102. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology: assessment of brain SPECT. Neurology 1996; 46:278–285Google Scholar
103. Mendez CV, Hurley RA, Lassonde M, et al: Mild traumatic brain injury: neuroimaging of sports-related concussion. J Neuropsychiatry Clin Neurosci 2005; 17:297–303Google Scholar
104. Wiedmann KD, Wilson JT, Wyper D, et al: SPECT cerebral blood flow, MR imaging, and neuropsychological findings in traumatic brain injury. Neuropsychology 1989; 3:267–281Google Scholar
105. Dunn RT, Kimbrell TA, Ketter TA, et al: Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry 2002; 51:387–399Google Scholar
106. Ketter TA, Kimbrell TA, George MS, et al: Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry 2001; 49:97–109Google Scholar
107. Jaracz J, Rybakowski J: [Studies of cerebral blood flow in metabolism in depression using positron emission tomography (PET)]. Psychiatria Polska 2002; 36:617–628Google Scholar
108. Kimura M, Shimoda K, Mizumura S, et al: Regional cerebral blood flow in vascular depression assessed by 123I-IMP SPECT. J Nippon Med School 2003; 70:321–326Google Scholar
109. Deutsch G: The nonspecificity of frontal dysfunction in disease and altered states: cortical blood flow evidence. Neuropsychiatry Neuropsychol Behav Neurol 1992; 5:301–307Google Scholar
110. Wager TD, Rilling JK, Smith EE, et al: Placebo-induced changes in fMRI in the anticipation and experience of pain. Science 2004; 303:1162–1167Google Scholar
111. Langleben DD, Schroeder L, Maldjian JA, et al: Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage 2002; 15:727–732Google Scholar
112. Ducours JL, Role C, Guillet J, et al: Cranio-facial trauma and cerebral SPECT studies using N-isopropyl-iodo-amphetamine (123I). Nucl Med Commun 1990; 11:361–367Google Scholar
113. Kesler SR, Adams HF, Bigler ED: SPECT, MR and quantitative MR imaging: correlates with neuropsychological and psychological outcome in traumatic brain injury. Brain Inj 2000; 14:851–857Google Scholar



