Aggression After Traumatic Brain Injury: Prevalence and Correlates
Prior studies have shown that post-TBI aggression is correlated with depression, frontal lobe lesions, poor pre-TBI psychosocial functioning, and a history of alcohol and substance abuse. 2 , 6 – 8 However, most of these studies focused on subjects who were several months to years postinjury. There is only one published study of aggression in the first 3 months after injury, which reported that severe TBI patients had cognitive and behavioral problems, but not emotional problems, when compared with mild to moderate TBI patients. 7 In the current study, we present preliminary cross-sectional findings on the prevalence and that correlates of aggression within 3 months of TBI in participants who are not delirious. We hypothesized that aggression would be common after TBI, aggression would include both verbal aggression and physical aggression, and correlates of aggression would include major depression, substance abuse, and adult/childhood behavior problems. 2 , 3
We sought to examine aggression in the first 3 months of TBI and characterize its severity and association with psychiatric diagnoses in adults with a first-time closed-head injury. This study is part of a larger ongoing study to determine prevalence and risk factors associated with the development of psychiatric disorders after TBI. The results presented here are thus preliminary.
METHODS
We performed an observational prospective study of the prevalence of aggression in the 3 months following TBI in a cohort of participants recruited within 3 months of trauma. We assessed the prevalence and subgrouping of aggression symptoms. We then examined the correlates of post-TBI aggression in a nested case-control design.
Participants and Procedures
A total of 107 patients with first-time closed-head injuries were recruited within 3 months of trauma from the acute trauma unit of the Johns Hopkins Hospital and the Brain Injury (rehabilitation) Unit of Kernan Hospital at the University of Maryland. Evaluations were completed only on participants who were able to give informed consent; we evaluated the ability of participants to give informed consent based on their treating physicians’ opinions and based on the abilities of the participants to accurately summarize the study and their roles in it. All participants received two study evaluations within 3 months of the TBI. The first evaluation (V0) assessed lifetime history of psychiatric problems and pre-TBI psychosocial functioning in those participants who were able to provide written informed consent within the first 2 weeks of trauma. The second evaluation (V1) of these participants was done approximately 3 months postinjury to assess psychiatric problems and psychosocial functioning after traumatic brain injury. However, for participants who were unable to give consent within the first 2 weeks, both pre-TBI and post-TBI status were assessed at the time they were able to provide informed consent, but within 3 months after the TBI (i.e., for these participants, V0 and V1 data were collected at a single visit corresponding to the V1 visit). Information from a collateral informant was collected whenever possible on both pre-TBI and post-TBI status on all psychosocial measures.
Of the 107, 40 participants did not return for the follow-up visit and were therefore excluded from this analysis. Of the 40 excluded, 25 could not be contacted because of incorrect address and telephone numbers, eight refused to participate, five had unstable medical problems, one was incarcerated, and one had anoxic brain injury.
For the purposes of the study, TBI was defined as having at least one of the following : (a) clear history of loss of consciousness; (b) Glasgow Coma Scale score less than 15; and/or (c) evidence of trauma (contusion or hemorrhage) on computerized tomography (CT) scans done as part of clinical workup. Other inclusion criteria included the ability to provide consent personally, age at least 18 years old, and admission to the hospital for evaluation of head trauma. Exclusion criteria included prior TBI, an open-head injury (e.g., a displaced skull fracture or a gunshot wound), or a history of any other type of brain illness (e.g., stroke, seizure, or encephalitis). The study was approved by the institutional review board of both universities.
Measures
All measures were administered by a neuropsychiatrist (VR) except the cognitive tests, which were administered by the study research coordinators (JS and MB).
Aggression
The Overt Aggression Scale 9 was used to assess verbal and physical aggressive behavior. This scale has two sections. The first assesses four types of aggressive behavior: verbal aggression, physical aggression against objects, physical aggression against self, and physical aggression against others. The severity of each subtype can be rated using a weighted score: verbal aggression (1–4 points), physical aggression toward objects (2–5 points), physical aggression toward self (3–6 points), and physical aggression toward others (3–6 points), with a range of 0–21 for the total Overt Aggression Scale score (higher scores indicate more severe aggression). The second section rates interventions provided by staff at the time of the incident. As the focus of the study is to assess prevalence and correlates of aggression after TBI, only the first section of the scale was administered at V1. The Overt Aggression Scale has been validated in adult and pediatric inpatients with neuropsychiatric illness and violent behavior. 10 – 12 Other researchers have also noted significant correlation between the Overt Aggression Scale and the Aberrant Behavior Checklist Community Scale irritability subscale in a study of outpatient youths with aggression. 13
Psychiatric Diagnoses
Axis I psychiatric diagnosis was determined using the Structured Clinical Interview for DSM-IV axis I disorders (SCID-I)–Clinician Version. 14 Diagnoses such as impulse control disorder and intermittent explosive disorder, which are probably relevant to TBI aggression, were not obtained because the SCID-I does not contain these diagnoses.
Severity of TBI
The severity of TBI was determined by the Glasgow Coma Scale (GCS), the most widely used instrument for quantifying TBI severity. The GCS is administered by the trauma staff or the emergency room personnel in their initial evaluation and has a range of 3–15. GCS scores of 3–8 are considered severe TBI, 9–12 moderate TBI, and 13–15 mild TBI. 15 All those determined to have mild TBI, as defined by GCS, also met the mild TBI criteria of the American Congress of Rehabilitation. 16 The American Congress of Rehabilitation Medicine (ACRM) defines mild TBI as traumatically induced physiological disruption of brain manifested by at least one of the following : (a) any period of loss of consciousness; (b) any loss of memory for events immediately before or after the accident; (c) any alteration in mental state at the time of the accident (e.g., feeling dazed, disoriented, or confused); and (d) focal neurological deficit(s) that may or may not be transient. However, the duration of posttraumatic amnesia should not be greater than 24 hours, the duration of loss of consciousness should not exceed 30 minutes, and the initial Glasgow Coma Scale (GCS) score should range from 13–15 after 30 minutes postinjury.
Medical Comorbidity
Medical comorbidity was assessed using the General Medical Health Rating scale. 17 This rating, which ranges from 1 (poor health) to 4 (excellent health), provides a global assessment of a person’s medical problems and medications. Data on the number of medications used by each patient were collected, but not the type of medications.
Psychosocial Functioning
Participants’ pre- and post-TBI psychosocial functioning was assessed using the Social Functioning Exam and the Social Ties Checklist. 18 Both these scales have been used in prior TBI studies. 19 , 20 Scores on the Social Functioning Exam and the Social Ties Checklist range from 0 (greatest satisfaction) to 1 (least satisfaction). The reliability and validity of these instruments have been demonstrated in patients with brain injury. 21
Family History of Psychiatric Illness
Family history of psychiatric illness was assessed using the Family History Screen instrument. 22
Behavior and Legal Problems
Pre- and post-TBI behavior and legal problems were also analyzed. Childhood behavioral problems were defined by the presence of two or more of the following: suspension from school, expulsion from school, setting fires, cruelty to animals, and/or destroying property. Adult behavioral problems were defined as being fired from work, fights, or violent behavior that interfered with interpersonal relationships and occupational or social life. Legal problems were defined as the presence of one or more of the following: arrests, incarcerations, jailed, or being placed on parole or probation. Both pre- and post-TBI legal status were included in the assessment.
Cognitive Tests
Neuropsychological tests were administered to all study participants at V1. The battery consisted of the Mini-Mental State Examination (MMSE) 23 ; the National Adult Reading Test 24 ; verbal fluency (letters “s” and “p”) and category (animals and supermarket) 25 ; Hopkins Verbal Learning Test—Revised 26 ; Brief Visuospatial Memory Test—Revised 27 ; Trail Making test 28 ; Stroop Color and Word Test 29 ; Brief Test of Attention 30 ; and the Wisconsin Card Sorting Test. 31
Neuroimaging
All participants had a head CT scan performed as part of routine clinical care. The CT results were categorized as presence or absence of lesions in different brain regions (i.e., right, left, bilateral frontal, temporal, parietal, occipital, subcortical).
Data Analysis
Participants were categorized as having “aggression” if they endorsed any of the subtype anchor questions on the Overt Aggression Scale. Those who endorsed a screening question for a particular subtype were then asked follow-up questions to quantify the severity of aggression on that subtype.
Descriptive statistics were calculated for all participants and for subgroups stratified by post-TBI aggression status. The significance of group differences (two-tailed) on individual variables was compared using Pearson’s chi-square and Fisher’s exact test for categorical variables and Student’s t test for continuous variables. The criterion for statistical significance was set at p<0.05.
To assess the strength of the relationship between aggression and the demographic and clinical factors, we conducted univariate logistic regression analysis with presence/absence of aggression as the dependent variable. On comparison of the two groups, those variables that were statistically significant and those that trended toward significance were included as independent variables in the univariate regression analyses. Significance levels were set at p<0.05.
RESULTS
A total of 107 participants who met the study inclusion/exclusion criteria were enrolled at V0. Of these, only those who received a first follow-up visit (i.e., only those with data for V1) (n=67) were included in this analysis. Forty participants (n=40) were excluded from the analysis as they did not have a V1 visit 1–3 months postinjury.
There were no significant differences between those with V1 data and those without V1 data in age, gender, severity of TBI, nature of TBI, income, race, marital or living situation, pre-TBI depression, and pre-TBI alcohol or substance abuse problems. The only significant difference was that the majority of participants without V1 data had a normal head CT (80% compared with 53.7%, p=0.007).
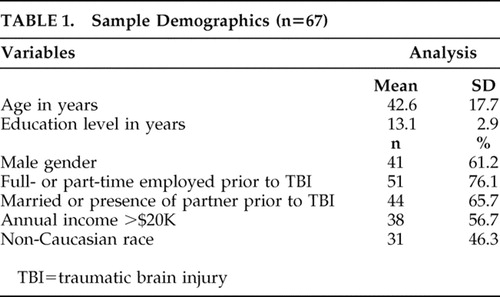
Sample Demographics
Table 1 summarizes the demographic information of the sample. Motor vehicle accident (53.7%) was the most common cause of TBI, followed by falls (22.4%) and assaults (22.4%). Mild TBI (GCS score of 13–15) was seen in 59.7% of the sample, moderate TBI (GCS score 9–12) in 13.4%, and severe TBI (GCS score<9) in 26.9%. All patients were ambulatory and medically stable. Only 9% had a General Medical Health Rating score of 1 (poor health: several unstable medical problems), 21% had a score of 2 (fair health: more than one unstable medical condition and/or several stable but chronic medical problems), 40% had a score of 3 (Good: one unstable medical problem or few stable medical problems) and 30% had a score of 4 (Excellent: no current unstable medical problems).
 |
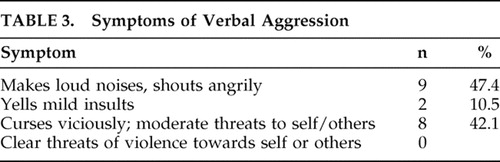
Prevalence and Symptoms of Aggression ( Table 2 and Table 3 )
The prevalence of aggression was found to be 28.4%. Verbal aggression (28.4%) was the most prevalent subtype of reported aggression in the post-TBI period. Only one participant displayed both verbal aggression and physical aggression against objects. No participants displayed aggression against self or others. The two most common symptoms of verbal aggression included angry shouts and vicious cursing with moderate threats of violence. As verbal aggression was the most common type of aggression, subsequent analyses examine solely verbal aggression as an outcome.
 |
 |
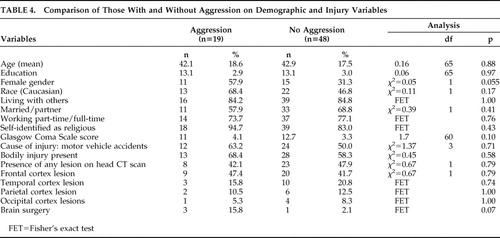
Participants With and Without Aggression on Demographic and Injury Variables
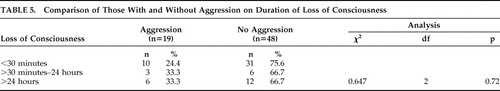
There were no statistically significant differences on demographic and injury variables between participants with and without aggression in the post-TBI period. However, female gender was associated with aggression, trending toward statistical significance (p=0.055) ( Table 4 ). In addition to comparing the two groups on the GCS, the groups were also compared on the duration of loss of consciousness, but no significant differences were noted between those with and without aggression ( Table 5 ). Similarly, there was also no significant difference on the Overt Aggression Scale between those with mild TBI and moderate/severe TBI (t=1.18, df=17, p=0.25)
 |
 |
Participants With and Without Aggression on Clinical Variables
Participants with aggression in the post-TBI period had a higher prevalence of new-onset major depression in the post-TBI period (mood disorder due to general medical condition, major depression-like episode) (p=0.02). No group differences were observed for any other axis I psychiatric diagnosis, including pre-TBI depression and post-TBI major depression, recurrent. Those with aggression were also more likely to have poorer social functioning (p=0.04) and increased dependence in personal and instrumental activities of daily living as assessed by the Lawton Activities of Daily Living Scale 32 (p=0.03) ( Table 6 ). Similarly, there were no differences between those with and without aggression on pre- or post-TBI history of alcohol or substance abuse, pre- or post-TBI legal problems, or pre- or post-TBI history of adult behavior problems and childhood behavior problems.
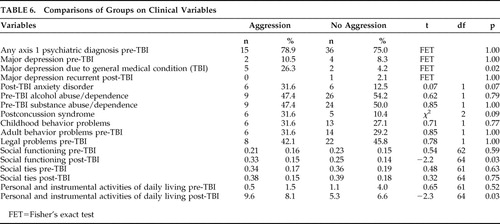 |
There was no significant difference between the two groups on cognitive tests ( Table 7 ) or head CT abnormalities ( Table 4 ).
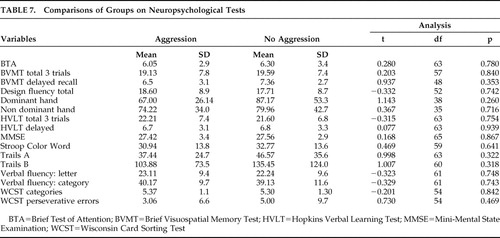 |
To determine if new-onset major depression in the post-TBI period (mood disorder due to general medical condition, major depression-like episode), psychosocial impairment, and increased dependence in activities of daily living were associated with injury-related medical problems, we compared those with and without body injury on these three variables. Body injury was associated with increased dependence in activities of daily living (t=3.29, df=64, p=0.002), but not with psychosocial impairment (t=−0.149, df=64, p=0.88) or mood disorder due to general medical condition, or major depression-like episode (χ 2 =0.56, df=1, p=0.69).
Correlates of Post-TBI Aggression ( Table 8 )
Univariate logistic regression was conducted with presence/absence of aggression as the dependent variable. The presence of psychosocial impairment postinjury was found to increase the odds of aggression in the post-TBI period 62-fold (odds ratio=62.4; 95% CI=1.32–2957.42), diagnosis of mood disorder due to general medical condition, major depression-like episode increased the odds eightfold (odds ratio=8.2; 95% CI=1.43–47.06), and increased dependence on activities of daily living increased the risk by 8% (odds ratio=1.08; 95% CI=1.006–1.164). No other correlates attained statistical significance.
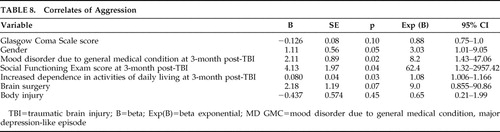 |
DISCUSSION
In a nested case-control study of TBI patients during the first 3 months following injury, we found that verbal aggression was quite prevalent, but physical aggression was virtually absent. Aggression in the post-TBI period was associated with impaired post-TBI psychosocial functioning, new-onset major depression post-TBI, and increased dependence in activities of daily living. Interestingly enough, recurrence of pre-TBI major depression was not a predictor of aggression.
We report several significant negative findings. Only one of the 67 participants exhibited physical aggression, and this was in a milder form (aggression only toward objects). None exhibited physical aggression toward self or others. We observed no significant association between post-TBI aggression and several covariates reported in prior studies, including childhood behavior problems, adult behavior problems, legal charges, pre- or post-TBI substance abuse or dependence, neuropsychological tests or brain lesions.
The prevalence of aggression is similar to what is reported in the literature, 2 , 8 as is the mix of TBI severity, with approximately 60% having mild TBI. 33 On reviewing the literature on the subtypes of aggression after brain injury, several studies have noted an association between brain injury and physical aggression. 34 – 37 Only one person reported a history of physical aggression in our study. Our findings are consistent with anecdotal reports on the predominance of verbal aggression after TBI. 38 , 39 Dyer et al. 39 have also noted a higher prevalence of verbal aggression rather than physical aggression in a sample of TBI patients studied 6 months postinjury. This discrepancy in the literature could be due to differences in the definition of aggression, severity of TBI, and duration since TBI. Another possibility is that TBI aggression occurs in two forms: mild verbal aggression associated with mood disorder but not with preinjury or postinjury behavior problems, and severe physical aggression which can be more complex and associated with significant behavior and legal problems.
We did, however, observe that aggression was strongly associated with new-onset major depressive episodes which increased the risk of aggression by eightfold, but not with recurrent major depression. Possibly new-onset depression after TBI is a phenotypic variant of idiopathic major depression with verbal aggression as a presenting symptom. Several other studies 2 , 8 have also noted a significant association between depression and aggression, but none of them have separated post-TBI depression as new-onset depression and recurrent depression. This is one of the strengths of our study.
Many researchers have also noted a relationship between aggression and premorbid characteristics such as childhood behavior problems, 6 adult behavior difficulties, legal problems 40 and substance abuse. 2 , 41 However, we did not observe these associations in our cohort. All prior studies have examined global aggression, combining both verbal and physical aggression. The lack of an association in our study could be because our study observed only verbal aggression, which is likely to reflect a milder form of aggression than physical aggression. Thus, it is possible that mild (verbal) aggression is associated with post-TBI depression, while more severe (physical) aggression may be associated with adult and childhood behavioral problems and substance abuse. Our finding of the relationship between female gender and aggression (that trended toward statistical significance) was unexpected but not surprising, given our findings of a positive relationship between aggression and depressive syndrome. It is well known that females are at higher risk of developing major depression, 42 though this has not been established for TBI cohorts.
Our study also found a significant association between verbal aggression and post-TBI social impairment. Baguley et al. 8 have also noted a significant association between poor satisfaction with life and aggression 6 and 24 months post-TBI. This is not surprising as one might predict that aggression would be interfering with relationships and reintegration into the community. However, the increased risk of aggression in persons with poor social functioning is quite remarkable (62-fold increased), even higher than that of depression. This finding has significant therapeutic implications for the immediate post-TBI period, suggesting the particular importance of improving psychosocial support, strengthening social connections, and providing adequate resources via individual, group, and family therapy in reducing aggression. Studies have shown that psychosocial support and positive interpersonal relationships play an important role in reducing aggression. 43 An alternative explanation for our results is that early diagnosis and appropriate treatment of aggression might lead to better social and interpersonal functioning.
The significant association between aggression and increased dependence in activities of daily living has been reported before. 44 One possibility is that persons with TBI are resistant to assistance provided by their caregiver, perceiving assistance as an invasion of personal space. It is also possible that aggression may be associated with lack of motivation in the context of post-TBI depression with concomitant lack of interest in self-care and help from care providers. Alternatively, aggression may only be a confounder, as our results showed that those with bodily injuries were more likely to have increased dependency in activities of daily living.
The literature suggests potential brain mechanisms for post-TBI aggression. Tateno et al. 2 found frontal lesions to be associated with aggression and suggest that frontal lobe injury can cause damage to the ascending serotonergic pathways, which can contribute to the pathophysiology of both depression and violent behavior. As systematic analyses of neuroimaging data were not part of our study, we are unable to comment on this possibility. However, we found no significant difference between those with and without aggression on CT head scans, performed as part of clinical workup. Similarly, we also did not find any group differences on neuropsychological tests, and more specifically, on tests particularly sensitive to the frontal functioning system. Starkstein and Robinson 45 have pointed out that TBI aggression is probably secondary to loss of balance between inhibitory pathways in the prefrontal cortex and excitatory limbic structures that mediate mood. These mechanisms could explain our finding of an association between aggression and new-onset depression in the immediate post-TBI period. More sensitive neuroimaging tools such as functional MRI or diffusion tensor imaging scans may shed light on the brain mechanisms underlying TBI aggression.
Our findings do not prove that the aggression in the post-TBI period observed in our subjects was caused by the TBI nor that any association was due to the biological effects of brain injury. For example, bodily injury was associated with increased dependence in activities of daily living which was further associated with aggression. It is possible that bodily injury could be an important mediator of aggression in the population, but the small sample size did not allow for systematic assessment of mediators and interactions. Similarly, the lack of association between TBI severity and aggression suggests the possibility that factors other than TBI are responsible for aggression; conversely, it could also suggest that TBI itself (rather than TBI severity) is an important risk factor for aggression. The lack of control groups with exposure to non-TBI bodily injuries and/or no injury is a limitation in addressing these issues. Other limitations of the study include the assessment of a narrowly defined cohort of TBI patients. Our study sample included only those with first-time closed-head injury, clear history of loss of consciousness, and those who were hospitalized. These strict inclusion/exclusion criteria may limit generalizability. Further, medication data were not available, precluding any study of the association between medications and aggression. Antipsychotics, benzodiazepines, antidepressants, and opioid analgesics are often used in the acute TBI period and might be effective treatments for severe agitation, which could account for the absence of physical aggression in our cohort. Systematic analyses of brain imaging data were also not performed, and thus we were unable to assess the association of exact lesion location and aggression. Finally, aggression was assessed only once in the acute TBI period by self-reports, which are often associated with underreporting and minimization of symptoms. However, whenever collateral informants were available, the subjects’ history was always corroborated.
To the best of our knowledge, this is the second published study examining aggression in nondelirious patients within the first 3 months of TBI. Our finding of a significant association between poor psychosocial functioning and verbal aggression underscores the point that psychosocial support is an important aspect of emotional recovery and therefore should be an integral part of rehabilitation. Our preliminary finding that new-onset major depression after TBI, but not recurrent major depression, is associated with post-TBI aggression is novel and may have substantial implications for the phenomenology and treatment of post-TBI aggression.
CONCLUSION
Aggression in the acute TBI period is common. It is predominantly verbal, characterized by anger and threats of violence rather than physical aggression. Significant correlates include impaired psychosocial functioning and new-onset major depression. Implications of the study include early screening for aggression, evaluation for depression and consideration of psychosocial support in patients with aggression, and education of care providers regarding the frequency of post-TBI aggression. Future studies should focus on effective interventions to reduce aggression and improve psychosocial functioning.
1. Brooke MM, Questad KA, Patterson DR, et al: Agitation and restlessness after closed head injury: a prospective study of 100 consecutive admissions. Arch Phys Med Rehabil 1992; 73:320–323Google Scholar
2. Tateno A, Jorge RE, Robinson RG: Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci 2003; 15:155–160Google Scholar
3. Kim E, Lauterbach EC, Reeve A, et al: Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research). J Neuropsychiatry Clin Neurosci 2007; 19:106–127Google Scholar
4. Garno JL, Gunawardane N, Goldberg JF: Predictors of trait aggression in bipolar disorder. Bipolar Disord 2008; 10:285–292Google Scholar
5. Hall KM, Karzmark P, Stevens M, et al: Family stressors in traumatic brain injury: a two-year follow-up. Arch Phys Med Rehabil 1994; 75:876–884Google Scholar
6. Greve KW, Sherwin E, Stanford MW, et al: Personality and neurocognitive correlates of impulsive aggression in long-term survivors of severe traumatic brain injury. Brain Inj 2001; 15:255–262Google Scholar
7. Rapoport M, McCauley S, Levin H, et al: The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 2002; 15:123–132Google Scholar
8. Baguley IJ, Cooper J, Felmingham K: Aggressive behavior following traumatic brain injury: how common is common? J Head Trauma Rehabil 2006; 21:45–56Google Scholar
9. Yudofsky SC, Silver JM, Jackson W, et al: The Overt Aggression Scale for the Objective Rating of Verbal and Physical Aggression. Am J Psychiatry 1986; 143:35–39Google Scholar
10. Silver JM, Yudofsky SC: The Overt Aggression Scale: overview and guiding principles. J Neuropsychiatry Clin Neurosci 1991; 3:S22–29Google Scholar
11. McNiel DE, Binder RL: Clinical assessment of the risk of violence among psychiatric inpatients. Am J Psychiatry 1991; 148:1317–1321Google Scholar
12. Kafantaris V, Lee DO, Magee H, et al: Assessment of children with the Overt Aggression Scale. J Neuropsychiatry Clin Neurosci 1996; 8:186–193Google Scholar
13. Hellings JA, Nickel EJ, Weckbaugh M, et al: The Overt Aggression Scale for rating aggression in outpatient youth with autistic disorder: preliminary findings. J Neuropsychiatry Clin Neurosci 2005; 17:29–35Google Scholar
14. First MB, Spitzer RL, Gibbon M, et al: Structured Clinical Interview for DSM-IV—Clinical Version (SCID-CV) (User’s Guide and Interview). Washington, DC, American Psychiatric Press, 1997Google Scholar
15. Teasdale G, Jennett B: Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 2:81–84Google Scholar
16. Kay T, Harrington DE, Adams R, et al: Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8:86–87Google Scholar
17. Lyketsos CG, Galik E, Steele C, et al: The General Medical Health Rating: a bedside global rating of medical comorbidity in patients with dementia. J Am Geriatr Soc 1999; 47:487–491Google Scholar
18. Starr LB, Robinson RG, Price TR: Reliability, validity, and clinical utility of the Social Functioning Exam in the assessment of stroke patients. Exp Aging Res 1983; 9:101–106Google Scholar
19. Jorge RE, Robinson RG, Arndt SV, et al: Comparison between acute- and delayed-onset depression following traumatic brain injury. J Neuropsychiatry Clin Neurosci 1993; 5:43–49Google Scholar
20. Jorge RE, Robinson RG, Moser D, et al: Major depression following traumatic brain injury. Arch Gen Psychiatry 2004; 61:42–50Google Scholar
21. Robinson RG, Lipsey LR, Bolla-Wilson K, et al: Mood disorders in left-handed stroke patients. Am J Psychiatry 1985; 142:1424–1429Google Scholar
22. Weissman MM, Wickramaratne P, Adams P, et al: Brief screening for family psychiatric history: the family history screen. Arch Gen Psychiatry 2000; 57:675–682Google Scholar
23. Folstein MF, Folstein SE, McHugh, PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Google Scholar
24. Nelson HE: National Adult Reading Test (NART): Test Manual. Windsor, UK, NFER Nelson, 1982Google Scholar
25. Spreen O, Benton AL: Neurosensory Center Comprehensive Examination for Aphasia (NCCEA). Victoria, University of Victoria Neuropsychological Laboratory, 1969, 1977Google Scholar
26. Brandt J, Benedict RHB: Hopkins Verbal Learning Test–Revised (HVLT-R): Professional Manual. Lutz, Fla, Psychological Assessment Resources, 2001Google Scholar
27. Benedict RHB: Brief Visuospatial Memory Test–Revised (BVMT-R): Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1997Google Scholar
28. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Therapy and Clinical Interpretation. Tucson, Ariz, Neuropsychological Press, 1985Google Scholar
29. Golden JC: Stroop Color and Word Test. Chicago, Stoelting Company, 1978Google Scholar
30. Schretlen D: Brief Test of Attention (BTA): Professional Manual. Odessa, Fla, Psychological Assessment Resources, 1996Google Scholar
31. Grant DA, Berg EA: A behavioral analysis of degree of impairment and ease of shifting to new responses in a Weigl-type card sorting problem. J Experimental Psychol 1948; 39:404–411Google Scholar
32. Lawton MP, Brody EM: Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179–186Google Scholar
33. Centers for Disease Control and Prevention (CDC), National Center for Injury Prevention and Control: Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, Centers for Disease Control and Prevention, 2003Google Scholar
34. Diaz F: Traumatic brain injury and criminal behavior. Med Law 1995; 14:131–140Google Scholar
35. Lewis DO, Pincus JH, Feldman M: Psychiatric, neurological and psychoeducational characteristics of 15 death row inmates in the United States. Am J Psychiatry 1986; 143:838–845Google Scholar
36. Rosenbaum A, Hoge SK, Adelman S: Head injury in partner-abusive men. J Consult Clin Psychol 1994; 62:1187–1193Google Scholar
37. Slaughter B, Fann JR, Ehde D: Traumatic brain injury in a county jail population: prevalence, neuropsychological functioning and psychiatric disorders. Brain Inj 2003; 17:731–741Google Scholar
38. Bowman M: Brain impairment in impulsive violence, in Impulsivity. Edited by Webster CD, Jackson MA. New York, Guilford, 1997, pp 116–142Google Scholar
39. Dyer KF, Bell R, McCann J, et al: Aggression after traumatic brain injury: analyzing socially desirable responses and the nature of aggressive traits. Brain Inj 2006; 20:1163–1173Google Scholar
40. Kreutzer JS, Marwitz JH, Witol AD: Interrelationship between crime, substance abuse, and aggressive behaviors among persons with traumatic brain injury. Brain Inj 1995; 9:757–768Google Scholar
41. Dunlop TW, Udvarhelyi GB, Stedem AFA, et al: Comparison of patients with and without emotional/behavioral deterioration during the first year after traumatic brain injury. J Neuropsychiatry Clin Neurosci 1991; 3:150–156Google Scholar
42. Eaton WW, Shao H, Nestadt G, et al: Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry 2008; 65:513–520Google Scholar
43. Klonoff PS, Lamb DG, Henderson SW, et al: Outcome assessment after milieu-oriented rehabilitation: new considerations. Arch Phys Med Rehabil 1998; 79:684–690Google Scholar
44. Sloane PD, Hoeffer B, Mitchell CM, et al: Effect of person-centered showering and the towel bath on bathing-associated aggression, agitation, and discomfort in nursing home residents with dementia: a randomized, controlled trial. J Am Geriatr Soc 2004; 52:1795–1804Google Scholar
45. Starkstein SE, Robinson RG: Mechanism of disinhibition after brain lesions. J Nerv Ment Dis 1997; 185:108–114Google Scholar



