Impulse-Control Disorders in Gilles de la Tourette Syndrome
Abstract
Impulse-control disorders (ICDs) are more common in clinic populations with Gilles de la Tourette syndrome (GTS) than in the general population. The clinical phenomenology of ICDs differ between men with GTS (who tend to be externally impulsive) and women with GTS (who tend to be internally impulsive). This article reviews the relevant literature to-date on impulsivity in GTS, with special focus on intermittent, explosive disorder, self-injurious behavior, trichotillomania, and impulsive-compulsive sexual behavior. The medical and legal community should be aware of the full spectrum of organically-based behaviors that may predispose patients with GTS to unwanted legal disciplinary action.
Gilles de la Tourette syndrome (GTS) is a neurodevelopmental disorder characterized by chronic multiple motor tics and one or more vocal/phonic tics, with onset before age 18 years.1 It was originally described by Georges Gilles de la Tourette in 1885, based on the observation of nine patients with childhood-onset tics, accompanied, in some, by involuntary noises and coprophenomena, as well as features that are now associated with attention-deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), poor impulse-control, and other behavioral problems.2–5
Comorbid neuropsychiatric disorders have been shown to occur in up to 90% of patients in both clinic and community settings, the majority of these being ADHD and OCD.6 Antisocial behavior, inappropriate sexual activity, exhibitionism, aggressive behavior, discipline problems, sleep disturbances, and self-injurious behaviors have been reported in a significant proportion of clinic patients with GTS.4,7 It has been suggested that poor impulse-control lies at the root of the many non-obscene, socially inappropriate behaviors in GTS.8 However, it is important to note that both GTS and ICDs are heterogeneous groups of disorders, and different ICDs are not associated with GTS in a consistent pattern.
Impulsivity is defined as the failure to resist an impulse, drive, or temptation that is potentially harmful to oneself or others. It is evidenced behaviorally as carelessness; an underestimated sense of harm; extroversion; impatience, including the inability to delay gratification; and a tendency toward risk-taking and pleasure- and sensation-seeking.9 Impulse-control disorders (ICDs) are currently classified within the DSM-IV-TR1 as an individual category (Table 1). ICDs are characterized by five behavioral stages: an impulse; mounting tension; pleasure on acting; relief from the urge; and, finally, guilt; which may or may not ensue.1 This article will review the available literature that investigates a link between ICDs and GTS.
 |
A literature review was conducted to assess the information currently available on GTS and ICDs. Computerized searches were carried out on the following databases: AMED, BNI, CINAHL, EMBASE, HEALTH BUSINESS ELITE, HMIC, MEDLINE, and PsycINFO. We used the search terms Tourette (4,008 results) OR Tourette's (4,090 results) in the title or abstract in three different literature searches. The first articles identified related to explosive behaviors in GTS, using the following terms: Intermittent Explosive Disorder (IED), Rage Attacks, Aggressive Behaviors, Temper Tantrums. The combined search yielded 43 hits, with 19 unique results (10 original studies).10,11 The second search identified articles related to self-injurious behavior (SIB) in GTS. The following search terms were used: Self-Injurious Behavior, Self-Harm, Self-Mutilation, Self-Injurious Disorder. The combined search yielded 46 hits, with 35 unique results. The third search identified articles related to a variety of other impulsive disorders related to GTS. The following search terms were used: Kleptomania, Pyromania, Pathological Gambling, Sexual Disorder, Internet Usage, Compulsive Buying. The combined search yielded 57 hits, with only 2 relevant original studies. Each literature search was limited to publications from Year 1975 onward.
GTS and Intermittent Explosive Disorder (IED)
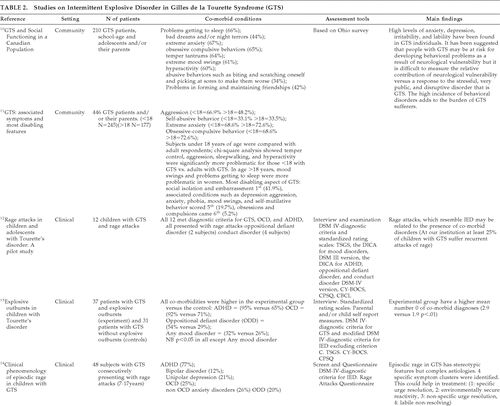
IED is characterized by discrete episodes of failure to resist aggressive impulses, where the degree of aggressiveness expressed during an episode is grossly out of proportion to any precipitating stressor;12 23%–40% of clinically-referred GTS subjects report distressing behavioral symptoms of this type: sudden unpredictable anger, irritability, temper outbursts, and aggression,11,20–22 but because of a lack of systematic evaluation, their prevalence and etiology remain uncertain.
The original studies from the literature review on GTS and IED are summarized in Table 2. A large, community-based study on children with GTS reported temper tantrums to be present in 64%,10 whereas a Danish clinical cohort showed rage attacks in only 34.8%.16 Rage attacks were defined as several discrete episodes of failure to resist aggressive impulses, which result in serious assaultive acts or destruction of property, where the degree of aggressiveness expressed during the episodes is greatly out of proportion to any precipitating psychosocial stressors.16 Interestingly, this study highlighted the increase in frequency of comorbid symptoms, in particular, rage attacks, when ADHD was present, and even more so when OCD-plus-ADHD were present, as shown in Table 3. This concept was also supported by a pilot study of 12 children, which suggests that the rage attacks, resembling IED, are related to the presence of comorbid disorders. When rage attacks were compared in the younger (age 6–17 years) versus older (≥18 years) GTS populations, they appeared to be more problematic in the younger population.11 It is uncertain whether this finding is due to factors associated with a greater maturity in the older group, or learning to avoid provocative stimuli. With regard to the etiology of these attacks, both neurological vulnerability and exposure to a stressful and disruptive environment potentially contribute to developing behavioral problems.10
 |
 |
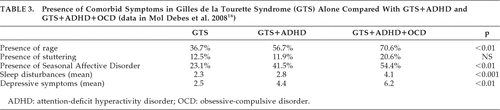 |
In one study looking into what provoked rage attacks in 29 adolescents,17 94.7% reported that their anger was precipitated by being told that they were wrong about something; 78.9% when they failed to have their own way; and 73.7% when there was a change in routine or schedule. This could support the suggestion that impulsivity and compulsivity are interlinked: impulsive individuals have a demand for increased arousal, with a build-up of tension relieved on committing the act. Thus, it could be that engaging in compulsive rituals helps to decrease the dysphoria in a similar way as acting on the impulses.9 Another possible hypothesis is that the sudden explosive, impulsive outbursts are a result of a disruption to routines that are linked to the compulsive disorders associated with GTS. This may also help explain why aggressive outbursts are found to be higher in GTS+ADHD+OCD conditions (70.6%) than in GTS+ADHD (56.7%) or GTS-alone (36.7%).16 This hypothesis does, however, require the substantiation of further studies, possibly including control groups.
GTS and Self-Injurious Behavior (SIB)
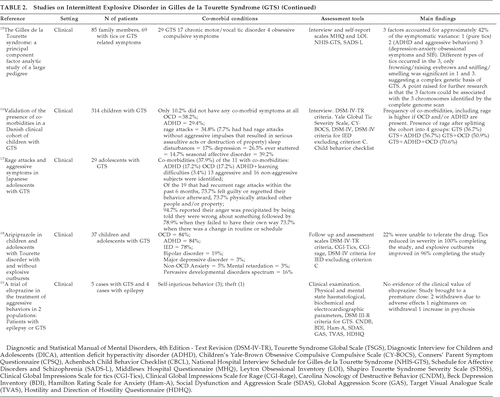
Of the nine patients who formed the basis of the original paper by Gilles de la Tourette in 1885, two were described as carrying out self-injurious behavior: a 24-year-old man had “characteristic” movements of his head and neck: “the teeth of both jaws gnashing violently. Quite often his tongue is caught between them and abruptly seized and lacerated;” and a 14-year-old boy “opened and shut his mouth with some force and abruptness, so that his lower lip was bitten (Gilles de la Tourette, 1885).”23 SIB has been observed in many GTS subjects subsequently: Table 4 summarizes the original studies from our literature review.
 |
A large study based on 6,805 GTS subjects from a worldwide clinical data-set estimated the prevalence of SIB in GTS to be 14.8%,29 although this study was severely limited by ascertainment bias. SIB was shown to be higher in those GTS individuals with comorbid ADHD; within this GTS+ADHD group, SIB prevalence was shown to increase with age. The age at onset of SIB was found to be 7.4 years in the GTS+ADHD group, as compared with 10 years in the GTS –ADHD group.29 When GTS patients were grouped for comparison into those with (“GTS-Plus”) and those without comorbidities (“pure GTS”), SIB was significantly higher in the GTS-Plus population (10% versus 40%).28 A study of 297 GTS subjects in both the clinical and community setting reported the prevalence of SIB to be 29%. This study also demonstrated that the rates of SIB differed in referral source and age, but not gender, with adults (≥18 years) referred by a healthcare provider having higher rates of self-harm than younger individuals (<18 years) recruited from other sources (school referral, media, family member referrals, and via the Tourette Syndrome Association).27 A study of 90 patients, 30 of whom self-harmed, listed the types of SIB in GTS patients. The five most common behaviors were 1) head-banging; 2) body punching/slapping; 3) head or face punching/slapping; 4) body-to-hard-object banging; 5) poking sharp objects into body.25
The searches carried out further identified 10 published case reports and a letter, summarized in Table 5. A large proportion of these described oral self-mutilation; it is interesting to note that this type of SIB is not reported as frequently in larger studies. A study of 75 patients did reveal lip-biting in 7; the same study reported onychophagia in 28, which has otherwise rarely been described in association with GTS.26 This could suggest a deficiency in the way that SIB data are collected; however, it may also be worth mentioning that oral self-mutilation is common in Lesch-Nyhan disorder,40 which could make one question the diagnosis of GTS.
 |
Compulsive touching and striking was described in 61% and 36% of patients, respectively, in a study of 53 GTS individuals in the U.K..24 This finding could suggest a link between SIB and sexually inappropriate behavior, since popular objects that were touched include hot things (fires, irons, hot plates, lighted cigarettes), fabrics with an erotic texture (fur, velvet, satin, silk), breasts, buttocks, and hair. This study also reported that striking was often directed to the patient's own body and was more common in female patients, supporting the previous observation by Hollander et al.9 that inwardly-directed ICDs are more common in women and girls.
GTS and Other ICDS
Very few original studies have been carried out comparing the remaining ICDs as listed by the DSM-IV-TR with GTS, although they were mentioned occasionally in the literature.
Trichotillomania (TTM)
In an analysis of 6,805 cases from a worldwide clinical data-set of GTS subjects, TTM was present in 2.6%.29 When clinical differences were compared between patients with and without comorbid ADHD, differences were insignificant in children and not appreciably different in adults. Another clinical study based on 126 cases of GTS28 compared several comorbidities between genders: TTM was present in 2% of male and 12% of female patients. When looking at TTM prevalence in general (i.e., not specific to GTS populations), it is interesting to note that both community sampling by Graber and Arndt41 and lifetime prevalence estimated by Christenson et al.42 revealed an equal gender distribution of TTM.
Impulsive-Compulsive Sexual Behavior
In a worldwide clinical data-set, sexually inappropriate behavior was present in 4.3%.29 When clinical differences were compared in GTS without ADHD (GTS–ADHD) versus GTS+ADHD, sexually inappropriate behavior was significantly higher in the GTS+ADHD group.29 In the GTS+ADHD group, sexually inappropriate behavior increased significantly with age, with a prevalence of 14% in the under-18 age-group, versus 27% in the 18+ age-group. Sexually inappropriate behavior is reported as a spectrum of commonly-observed behavioral problems, ranging from exposing genitals and inappropriate touching (of own parents, family members, or strangers) to frequent or open masturbation and excessive and unwanted sexual talk or joking.28 However, these behaviors do not fulfill the criteria for sexual dysfunctions, paraphilias, or gender-identity disorders, according to the DSM-IV classification.43 When a comparison was made between patients with GTS-Only and patients with GTS-Plus (i.e., GTS with comorbidities), sexually inappropriate behavior was found in 0% of the former and 11% of the latter group.28
Kleptomania, Pyromania, Pathological Gambling, Impulsive-Compulsive Internet Usage Behavior, Impulsive-Compulsive Buying Behavior
Our literature search yielded few original studies that directly looked at the prevalence, neurobiological mechanisms, and treatment implications of these other ICDs in GTS. It seems that this is an under-investigated area in need of further research.
A cluster analysis of obsessive-compulsive spectrum disorders in OCD found that TTM, pathological gambling, and hypersexual disorder clustered together with GTS in Cluster I, labeled “reward deficiency,” where individuals are looking for an increased sense of arousal.44 There is much evidence that tics in OCD are mediated at least partly by the dopaminergic system and some evidence that this system also plays a role in the other conditions belonging to this cluster.45–49 It has been argued that many obsessive-compulsive spectrum disorders are characterized by reward-deficiency, pleasure-seeking behavior, and dopaminergic deficits.50 Compulsive shopping, kleptomania, eating disorders, SIB, and IED were apparent as a second cluster, labeled “impulsivity.” An association has been established between impulsivity and OCD severity,51 and, in addition, Cluster II scores are associated with female gender and emotional abuse. Although Cluster II disorders are not being looked at in the context of patients with GTS, it could be significant to note the divide this cluster-analysis makes within the broad category of “impulse-control disorders,” which are increasingly being considered as a separate entity to obsessive-compulsive spectrum disorders in general. Therefore, it could be possible to further categorize the ICDs as defined by DSM-IV-TR into those associated with GTS and those less associated with GTS, as suggested by this cluster analysis. This hypothesis raises further questions: Will the GTS-associated ICDs share different features from those not associated with GTS? Is there a neurobiological basis to this clustering, and could this enhance our understanding of impulsive behavior in GTS?
Taking a different perspective, OCD and TTM were compared in terms of dissociative experiences (DE),52 defined as disruptions in the usually-integrated functions of consciousness, such as memory, identity, and perceptions of the environment.43 Demographic features of the “high dissociators” were lower age and a comorbidity profile like that of the ICDs. GTS was more common in the “high dissociators” of the OCD group, whereas kleptomania was more common in the “high dissociators” of the TTM group. A dissociative subtype of TTM was identified, where the individuals pull at their hair in an almost trance-like state.53 These findings suggest that subtypes of TTM exist and that DE may be a factor for consideration in the hypothesis made above, according to which different groups of ICDs may be more strongly associated with GTS.
DISCUSSION
This literature search shows that there is a lack of controlled data in the area of impulse-control in GTS. However, a range of impulse-control disorders appear to be common in Tourette syndrome. In general, impulsivity directed at the self appears to be more common in women, and impulsivity directed toward others appears to be more common in men. Moreover, impulsivity generally increases with the presence of other comorbidities in relation to GTS.
Cognitive Aspects of Impulsivity
There are three main cognitive components that play a role in modulating impulsivity:9 1) inability to delay gratification: decisions are made with the intention of immediate reward, irrespective of the size of the reward or consequences in the long-term; 2) distractibility: attention to a particular task cannot be maintained; 3) disinhibition: behavior is not restricted, as would normally occur, to comply with cultural norms.
From a dimensional standpoint, impulsivity can be considered at one end of a spectrum, with compulsivity at the other.54 On one end of the spectrum are the compulsive individuals who view the environment as risky and threatening and carry out behaviors or rituals to minimize threat and anxiety; on the opposite end of the spectrum are the impulsive individuals who tend to underestimate the degree of harm in the environment; hence, their repeated engagement in high-risk activities. This behavior is accompanied by a failure to learn from errors in their judgment. GTS can be considered as a mixed compulsive-impulsive disorder; patients characteristically display compulsively driven behaviors to reduce anxiety; however, careful history-taking can reveal a number of impulsive behaviors associated with arousal, pleasure, or gratification (Figure 1).9

A common factor in compulsive-impulsive disorders is the inability to refrain from carrying out repetitive behaviors; that is, a diminished capacity to control a motor response to an affective state. However, what distinguishes the two ends of the spectrum is the motive of the behavior; compulsions are driven by an attempt to reduce anxiety, whereas impulsions are driven by an attempt to obtain arousal and gratification.9,55 Consequently, impulsive disorders may be perceived to be ego-syntonic, whereas compulsive disorders tend to be more ego-dystonic. The spectrum is to be viewed as a dynamic continuum: there are often difficulties in deciding the motive of the behavior, so that ICDs may begin with an urge that has a pleasurable incentive, although, over time, the purpose of the behavior may be more centralized around avoiding anxiety and discomfort as a consequence of not carrying out the act.9
Impact of Gender on ICDS
Prevalence rates of all the ICDs have not been thoroughly researched; however, there do appear to be some differences in prevalence when men are compared with women: SIB, TTM, kleptomania, and compulsive buying are more associated with women, whereas IED, pathological gambling, pyromania, and sexual compulsions, with men. It seems to be the case that the ICDs more common in men are the outwardly-directed, aggressive behaviors, in contrast to the inwardly-directed nonaggressive disorders more common in women. Suggestions for causes underlying this observation include hormonal factors, genetic factors, different modulation of serotonin and vasopressin,56 and/or possibly sociocultural factors.9
Organicity of ICD and GTS
Several animal studies suggest that there is an organic origin to impulsive behavior. Studies of rats with lesions of the nucleus accumbens, an area of the brain associated with reward and reinforcement, showed a preference toward small, immediate rewards over larger, delayed rewards; that is, more impulsive, immediate action.57 The anterior cingulate cortex, medial, orbitofrontal, and ventromedial prefrontal cortices are all afferents to the nucleus accumbens; however, only lesions in the orbitofrontal and ventromedial prefrontal cortices have been shown to induce impulsivity, indicating that specific areas have an essential contribution to impulsivity. This is consistent with the observation that lesions in the ventromedial prefrontal cortices have also been associated with impaired decision-making and a lack of consideration of future consequences.58
Human studies with GTS, using positron emission tomography (PET) and single photon emission computed tomography (SPECT), measuring metabolism and blood-flow in patients, have displayed hypometabolism of the anterior cingulate, parahippocampal, and insular cortices, which are thought to be involved in executive functions, inhibition of unwanted behavior, and, therefore, impulse-control.59
A study looking into the neurobiological basis of GTS suggests that disturbances of the dopaminergic and serotonergic neurotransmitter systems play a key role in a defect in the circuitry that connects multiple areas of the cortex with the basal ganglia and thalamus, which are involved in motivation, inhibition of behavior, planning of motor acts, and detection of threats.60 A neuroimaging study using carbon-11 raclopride PET and amphetamine stimulation found increased dopamine release in the putamen of patients with GTS.61 Other functional-imaging studies have provided support for this finding, by demonstrating increased binding of monoamine transporter ligand [11C]dihydrotetrabenazine (DTBZ) in the ventral striatum in GTS subjects, as compared with age-matched controls.62 However, neither study has specifically examined impulsivity as a factor in the analysis.
Finally, over the last few decades, a series of parallel frontal-subcortical pathways that link specific regions of the frontal cortex to the basal ganglia have been identified.63 These cortico-striato-thalamo-cortical (CSTC) circuits comprise an integrative framework for understanding motor, cognitive, and emotional functions in a variety of neuropsychiatric disorders. With reference to the GTS spectrum, three different CSTC pathways have been proposed as the neuroanatomical bases for movement and behavior dysregulation: the sensorimotor circuit is thought to regulate tic urge and expression; the orbitofrontal circuit, which mediates impulse-control and emotional responses, has been linked to OCD and affective symptoms; and, finally, the dorsolateral circuit, conveying executive function, has been implicated in ADHD symptoms.64,65 One of the aims of future research is to identify the mechanisms of integration and transfer of information from one CSTC loop to another in subjects suffering from tics and tic-related behavioral problems.
Limitations
According to the results of our literature search, many of the relevant studies were carried out in a clinical setting; this has implications on our ability to apply the conclusions made to the general population. For example, comorbidities may appear higher in these cohorts than in GTS individuals in the community, as these individuals are more likely to present at clinics (referral bias). The age of the participants is also an influential variable on the results, especially in terms of rates of comorbid disorders; OCD typically begins in late childhood, and onset of TTM is usually before young adulthood;28 hence, some children diagnosed as “GTS-only” may acquire other disorders at a later stage. With particular reference to anxiety measurements, there was a lack of standardization in recording; subjective terms such as “often” and “sometimes” were used in some of the studies, with different definitions, making comparison difficult. Where interviews were conducted in the data collection, it is extremely likely that a degree of subjectivity was present. Recall bias may have contributed to limitations about the collected data, especially when parents and adult patients were questioned. Often, similar criteria and standardized rating scales were used between studies; however, some discrepancies were inevitably present, especially after the update of the DSM-III to the DSM-IV and DSM-IV-TR criteria. Overall, instability of some of the measurement factors, such as spontaneous fluctuations in tic severity, makes it difficult to estimate a definite level of severity for a single individual. Finally, the searches included in this article may have failed to identify all published literature relevant to the review. The papers missed may have been indexed in databases that were not accessed in the search, or the paper may have included terms other than the few search terms that were used here.
CONCLUSIONS
This review has attempted to give a comprehensive representation of what research has been carried out to-date on the subject of ICDs in Tourette syndrome. In the process of selecting this literature, many potential factors for further research have been identified; a paucity of information has become particularly apparent in the more recently defined ICDs, such as pathological gambling, impulsive-compulsive Internet usage, and impulsive-compulsive buying. It has been noted that certain externalizing ICDs, including temper dyscontrol and rage attacks associated with physical assault or destruction of property, are responsible for the potential impact of GTS on the legal system.66 In general, medical and legal professionals should be aware that, although GTS rarely leads to criminal behavior, patients with GTS who have comorbid ICDs can be at risk of potential mistreatment by the courts of justice. In conclusion, a more comprehensive understanding of the questions this article poses requires the systematic evaluation of ICDs in GTS, a topic that is becoming ever more important with changes in society and technological advancements.
1.
2. : Tourette Syndrome. N Engl J Med 2001; 345:16:1184–1192Crossref, Medline, Google Scholar
3. : Tourette syndrome: pieces of the puzzle. Adv Neurol 2001; 85:369–390Medline, Google Scholar
4. : Tourette syndrome, associated conditions, and the complexities of treatment. Brain 2000; 123:425–462Crossref, Medline, Google Scholar
5. : Current issues in Tourette syndrome. Mov Disord 2000; 15:1051–1063Crossref, Medline, Google Scholar
6. : The behavioral spectrum of Gilles de la Tourette syndrome. J Neuropsychiatry Clin Neurosci 2009; 21:13–23Link, Google Scholar
7. : Self-injurious behavior in Tourette's syndrome: correlates with impulsivity and impulse-control. J Neurol Neurosurg Psychiatry 2004; 75:1149–1155Crossref, Medline, Google Scholar
8. : Non-obscene, complex, socially-inappropriate behavior in Tourette's syndrome. J Neuropsychiatry Clin Neurosci 1996; 8:311–317Link, Google Scholar
9. : Clinical Manual of Impulse-Control Disorders. Arlington, VA, American Psychiatric Publishing, 2006Google Scholar
10. : Tourette syndrome and social functioning in a Canadian population. Neurosci Biobehav Rev 1988; 12:255–257Crossref, Medline, Google Scholar
11. : Tourette syndrome: associated symptoms and most disabling features. Neurosci Biobehav Rev 1993; 17:271–275Crossref, Medline, Google Scholar
12. : Rage attacks in children and adolescents with Tourette's disorder: a pilot study. J Clin Psychiatry 1998; 59:576–580Crossref, Medline, Google Scholar
13. : Explosive outbursts in children with Tourette's disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:1270–1276Crossref, Medline, Google Scholar
14. : Clinical phenomenology of episodic rage in children with Tourette syndrome. J Psychosom Res 2003; 55:59–65Crossref, Medline, Google Scholar
15. : The Gilles de la Tourette syndrome: a principal-component, factor-analytic study of a large pedigree. Psychiatr Genet 2007; 17:143–152Crossref, Medline, Google Scholar
16. : Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol 2008; 23:1017–1027Crossref, Medline, Google Scholar
17. : Rage attacks and aggressive symptoms in Japanese adolescents with Tourette syndrome. CNS Spect 2008; 13:325–332Crossref, Medline, Google Scholar
18. : Aripiprazole in children and adolescents with Tourette disorder with and without explosive outbursts. J Child Adolesc Psychopharmacol 2008; 18:509–515Crossref, Medline, Google Scholar
19. : A trial of eltoprazine in the treatment of aggressive behaviours in two populations: patients with epilepsy or Gilles de la Tourette's syndrome. Hum Psychopharmacol 1994; 9:253–258Crossref, Google Scholar
20. : Tourette's syndrome: what are the influences of gender and comorbid obsessive-compulsive disorder? J Am Acad Child Adolesc Psychiatry 1994; 33:795–804Crossref, Medline, Google Scholar
21. . Behavioral symptoms in Tourette syndrome, in Tourette's Syndrome and Tic Disorders: Clinical Understanding and Treatment. Edited by Cohen DBruun RLeckman J. New York, Wiley, 1988, pp 152–162Google Scholar
22. : Tourette's syndrome and attention deficit disorder, in Tourette's Syndrome and Tic Disorders: Clinical Understanding and Treatment. Edited by Cohen DBruun RLeckman J. New York, Wiley, 1988, pp 119–135Google Scholar
23. : Étude sur une affection nerveuse characterisée par de l'incoordination motrice accompagnée d′écholalie et de copralalie. Arch Neurol 1885; 9:19–42, 158–200Google Scholar
24. : A clinical study of Gilles de la Tourette syndrome in the United Kingdom. J Neurol Neurosurg Psychiatry 1984; 47:1–8Crossref, Medline, Google Scholar
25. : Self-injurious behavior and the Gilles de la Tourette syndrome: a clinical study and review of the literature. Psychol Med 1989; 19:611–625Crossref, Medline, Google Scholar
26. : Gilles de la Tourette syndrome: clinical features of 75 cases from Argentina. Behav Neurol 1995; 8:75–80Crossref, Medline, Google Scholar
27. : Self injurious behavior in Tourette syndrome: correlates with impulsivity and impulse-control. J Neurol Neurosurg Psychiatry 2004; 75:1149–1155Crossref, Medline, Google Scholar
28. : Clinical analysis of Gilles de la Tourette syndrome based on 126 cases. Neurol i Neurochir Polska 2007; 41:381–387Medline, Google Scholar
29. and
30. : A case of Tourette syndrome presenting with oral self-injurious behaviour. Int J Paediatr Dentistry 2005; 15:370–374Crossref, Medline, Google Scholar
31. : [Self-mutilation with crystalline lens dislocation in Gilles de la Tourette disease treated with retropupillary “iris claw” lens]. Klinische Monatsblatter fur Augenheilkunde 2004; 221:435–437Crossref, Medline, Google Scholar
32. : Case study: severe self-injurious behavior in comorbid Tourette's disorder and OCD. J Am Acad Child Adolesc Psychiatry 2004; 43:1298–1303Crossref, Medline, Google Scholar
33. : Tourette's syndrome with rapid deterioration by self-mutilation of the upper lip. J Clin Pediatr Dentistry 2003; 27:177–180Crossref, Medline, Google Scholar
34. : Oral self-mutilation associated with the Gilles de la Tourette syndrome. Irish J Psychol Med 1993; 10:105–111Crossref, Google Scholar
35. : Compulsive self-mutilation and hostility in Gilles de la Tourette's syndrome. Fortschritte der Neurologie-Psychiatrie 1992; 60:253–261Crossref, Medline, Google Scholar
36. : Tooth extraction as a form of self-mutilation in Tourette's disorder. Southern Med J 1986; 79:1466Crossref, Medline, Google Scholar
37. : Gilles de la Tourette syndrome with self-mutilation: a case report. Nervenarzt 1982; 53:670–673Medline, Google Scholar
38. : Self-mutilation and Tourette's disorder. J Child Neurol 1987; 2:265–267Crossref, Medline, Google Scholar
39. : Self-mutilation in Tourette's syndrome. J Child Neurol 1988; 3:147–148Crossref, Medline, Google Scholar
40. : Self-injurious behavior in young children with Lesch-Nyhan syndrome. Dev Med Child Neurol 2001; 43:745–749Crossref, Medline, Google Scholar
41. : Trichotillomania. Compr Psychiatry 1993; 34:340–346Crossref, Medline, Google Scholar
42. : Estimated lifetime prevalence of trichotillomania in college students. J Clin Psychiatry 1991; 52:415–417Medline, Google Scholar
43.
44. : Cluster analysis of obsessive-compulsive spectrum disorders in patients with obsessive-compulsive disorder: clinical and genetic correlates. Compr Psychiatry 2005; 46:14–19Crossref, Medline, Google Scholar
45. : Altered dopamine function in pathological gambling. Psychol Med 1997; 27:473–475Crossref, Medline, Google Scholar
46. : Pathological gambling. Psychiatr Clin North Am 2000; 23:629–642Crossref, Medline, Google Scholar
47. : Pathological gambling behavior: emergence secondary to treatment of Parkinson's disease with dopaminergic agents. Depr Anxiety 2000; 11:185–186Crossref, Medline, Google Scholar
48. : Abnormal dopamine uptake sites in post-mortem striatum from patients with Tourette's syndrome. Ann Neurol 1991; 30:558–562Crossref, Medline, Google Scholar
49. : Low-dose pimozide augmentation of serotonin reuptake blockers in the treatment of trichotillomania. J Clin Psychiatry 1992; 53:123–126Medline, Google Scholar
50. : Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs 2000; 32(suppl):1–112Crossref, Google Scholar
51. : Impulsivity scores in patients with obsessive-compulsive disorder. J Nerv Ment Dis 1994; 182:240–241Crossref, Medline, Google Scholar
52. : Dissociative experiences in obsessive-compulsive disorder and trichotillomania: clinical and genetic findings. Compr Psychiatry 2004; 45:384–391Crossref, Medline, Google Scholar
53. : Symptom subtypes of obsessive-compulsive disorder and their relation to dissociation. J Anxiety Disord 2004; 18:435–458Crossref, Medline, Google Scholar
54. : Impulsivity. J Psychopharmacol 2000; 14(suppl1):39–44Crossref, Google Scholar
55. : Neurobiology of impulsivity and the impulse-control disorders. J Neuropsychiatry Clin Neurosci 1993; 5:9–17Link, Google Scholar
56. : Cerebrospinal fluid vasopressin levels: correlates with aggression, serotonin function in personality-disordered subjects. Arch Gen Psychiatry 1998; 58:708–714Crossref, Google Scholar
57. : Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 2001; 292:2499–2501Crossref, Medline, Google Scholar
58. : Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci 1999; 19:5472–5481Crossref, Google Scholar
59. : A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry 1998; 55:326–333Crossref, Medline, Google Scholar
60. : Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog Brain Res 2008; 172:495–513Crossref, Medline, Google Scholar
61. : Elevated intrasynaptic dopamine release in Tourette's syndrome measured by PET. Am J Psychiatry 2002; 159:1329–1336Crossref, Medline, Google Scholar
62. : Increased ventral-striatal monoaminergic innervation in Tourette syndrome. Neurology 2003; 61:310–315Crossref, Medline, Google Scholar
63. : Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci 1994; 6:358–370Link, Google Scholar
64. : Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 2002; 53:647–654Crossref, Medline, Google Scholar
65. : Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev 2008; 32:408–422Crossref, Medline, Google Scholar
66. : Tourette's syndrome and the law. J Neuropsychiatry Clin Neurosci 2006; 18:86–95Link, Google Scholar



