Hippocampal Volume Reduction Correlates With Apathy in Traumatic Brain Injury, But Not Schizophrenia
Abstract
Apathy commonly accompanies both traumatic brain injury (TBI) and deficit syndrome schizophrenia (DSZ), despite unclear neurological bases. The authors examined differences in cortical thickness and subcortical/cerebellar regional volumes between adult TBI survivors, patients with DSZ, and healthy-control subjects by use of 3-D magnetic resonance imaging (MRI), and correlated imaging findings with clinical ratings of apathy and selected cognitive test scores. Imaging findings revealed specific areas of volume reduction in TBI survivors and areas of cortical thinning among patients with DSZ. The severity of apathy symptoms was similar across patient groups; however, severity of apathy was only correlated with imaging findings in TBI survivors.
Apathy refers to a loss of motivation, in combination with impairments of behavioral, cognitive, and emotional aspects of goal-directed behavior.1 Individuals with apathy can be misperceived as “lazy” because of difficulty marshaling the effort required to initiate and complete activities. Apathy is seen under various medical or psychiatric conditions, such as traumatic brain injury (TBI),2 schizophrenia (SZ),3 Alzheimer’s disease (AD),4 and Parkinson’s disease (PD).5 TBI apathy among TBI survivors may be as high as 71%,2 and can emerge immediately after a TBI or up to several years later.6–8 The negative symptoms of schizophrenia include apathy. Those with severe and enduring negative symptoms might represent a homogeneous subgroup, termed the “deficit syndrome” of schizophrenia (DSZ), characterized by restricted affect, poverty of speech, anhedonia, diminished sense of purpose, and social withdrawal.3,9 Previously, we found similar levels of apathy among patients with DSZ and TBI survivors selected on the basis of prominent apathy symptoms. However, compared with those with TBI, the DSZ group showed higher rates of other negative symptoms, including anhedonia, blunted affect, and alogia.10 Those findings suggest that individuals with DSZ have a constellation of apathy symptoms that differs from that of TBI patients, and possibly associated with different neuroanatomic correlates.
Numerous MRI studies have demonstrated broad regional brain volume reductions after TBI.11–21 These changes are thought to result from the acute injury, as well as delayed neuronal death attributable to more widespread processes that occur after TBI.18,21 Likewise, structural MRI studies have shown volumetric differences in the prefrontal and temporal regions among DSZ patients relative to healthy controls (HCs),22–24 and we previously found gray matter (GM) volume reductions among adults with DSZ in the insula and superior temporal, superior frontal, and fusiform gyri.25
Cognitive performance is also substantially affected in both TBI20 and DSZ.26 Specifically, DSZ patients show poorer performance on a variety of cognitive tests, including memory, as compared with HCs, and the severity of negative symptoms (psychomotor poverty, alogia) significantly relates to the amount of decrement in performance on cognitive tests.27,28 A relationship between cognitive deficits, including verbal memory, and apathy symptoms has also been shown among TBI survivors.29 We have been interested in delineating the clinical and biological correlates of apathy across specific neuropsychiatric diseases in order to assess the validity of its syndromal construct.10 This interest has been initially driven by our study of DSZ and the need to better define the biological characteristic of this syndrome, including brain structure abnormality. We thought that TBI apathy would constitute a “model” for DSZ because of the high prevalence of apathy among TBI survivors and because TBI apathy could be more easily related to localized brain abnormality with potential pathophysiological relevance to DSZ.
Therefore, the goals of the present exploratory study were to 1) compare brain morphology among DSZ, TBI, and HC participants; and 2) assess whether specific differences in cortical thickness or regional brain volume correlate with the severity of apathy and performance on tests of cognitive functioning in TBI and DSZ.
Methods
Study Participants
A group of 10 TBI patients were recruited from the Brain Injury Clinic at Johns Hopkins Hospital and from local rehabilitation centers. Injury details were obtained from the patient, a collateral informant when available, and medical records. All TBI patients survived a closed head injury secondary to either a motor vehicle accident (N=8) or a fall (N=2) sustained at least 18 months (mean: 72 [standard deviation {SD}: 47] months; range: 18–157 months) before study participation. Based on available data, including Glasgow Coma Scale scores and the duration of loss of consciousness, 6 individuals were rated as having sustained a severe TBI; 2 had a moderate brain injury; and 2 had a mild TBI. These participants all met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for a “personality change secondary to TBI, apathetic subtype.”
A group of 15 adults with schizophrenia, diagnosed according to DSM-IV criteria, were recruited from outpatient clinics, inpatient services, and psychiatric day hospitals affiliated with the Johns Hopkins University and Hospital. All participants in the TBI and DSZ groups met criteria for the “deficit syndrome” on the basis of the Schedule for Deficit Syndrome (SDS).30 None of the DSZ or TBI patients met criteria for either a mood disorder or substance abuse/dependence during the preceding 6 months.
The HC group comprised 47 healthy adults recruited for a study of normal aging from the Baltimore metropolitan region via random-digit dialing or telephone calls to randomly selected telephone numbers in the residential telephone directory. Exclusion criteria included any reported history of neurological disorders, neurodegenerative disorders, psychiatric illness, and current alcohol/drug abuse or dependence. These studies were reviewed and approved by the Johns Hopkins Hospital Institutional Review Board, and all participants provided written informed consent before participation.
Procedure
The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) was used to diagnose both schizophrenia and personality change secondary to TBI. The presence and severity of apathy in TBI and DSZ participants were assessed based upon the Apathy Evaluation Scale, Clinician Version (AES–C).31 The SDS and the AES–C were administered by one of two board-certified psychiatrists (NC and VR). Acceptable interrater reliability for the SDS (κ=0.73) was established between raters. The AES–C has been shown to have good internal reliability, test–retest reliability, and interrater reliability (reviewed by Clarke et al.32). We used the Scale for the Assessment of Positive and Negative Symptoms (SAPS and SANS) and the Hamilton Rating Scale for Depression (Ham–D) to assess severity of psychopathology and depressive symptoms. The National Adult Reading Test–Revised Version (NART–R) was used to estimate premorbid IQ.33
The HC group was administered diagnostic and clinical assessments, including the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) interview,34 review of medical history, and physical and neurological examinations. Whereas the majority of TBI survivors and HCs underwent physical and neurological examination, psychiatric interview, a brain MRI scan, and cognitive assessment on the same day, most of the DSZ patients needed several days (mean: 4.1 [7.5] days) to complete these procedures.
Cognitive Measures
Each participant was administered a brief neuropsychological test battery detailed in our previous study.20 This battery assessed functions including psychomotor speed and dexterity, psychomotor processing speed, auditory divided attention, letter–word fluency, visual construction, verbal learning and memory, visual learning and memory, and concept-formation.
MRI Acquisition and Pre-Processing
All participants underwent brain MRI on the same 1.5-tesla GE Signa scanner (Milwaukee, WI). We acquired 124 contiguous 1.5-mm, 3-D SPGR slices in the coronal plane. The parameters were the following: repetition time: 35 seconds, echo time: 5 seconds, flip angle: 45°, and image matrix: 256×256. Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer Image Analysis Suite, Version 5.0 (http://surfer.nmr.mgh.harvard.edu/fswiki). Detailed methodology of the Freesurfer program is available elsewhere.35
Statistical Analysis
One-way analysis of variance (ANOVA) was used to compare all three groups with regard to age, years of education, estimated full-scale IQ, and cognitive test results. The Scheffé test was used for post-hoc pairwise comparisons. Chi-square tested for differences in sex and handedness between the TBI and DSZ groups. Finally, independent-sample t-tests assessed for differences between the TBI and DSZ groups with respect to AES scores, SANS/SAPS total scores, SANS subscale scores, and Ham–D scores.
Subcortical and cerebellar volumes were compared across the three diagnostic groups via analysis of covariance (ANCOVA), with diagnosis as a between-subject factor, and age, gender, and intracranial volume (ICV) as covariates. Scheffé tests were used for post-hoc group comparisons. When significant volumetric changes were found between either patient group and the HC group, we calculated partial correlation coefficients between the region-of-interest (ROI) volumes of that patient group and AES scores and neurocognitive measures while controlling for age, sex, and ICV.
We used FreeSurfer’s general linear model with a 10-mm, full-width, half-maximum Gaussian kernel to compare cortical thickness across groups (DSZ versus HC, TBI versus HC, and DSZ versus TBI) and to correlate AES ratings and neurocognitive scores, while treating age and sex as nuisance variables. When significant cortical thickness changes were observed between either (or both) clinical groups (i.e., DSZ or TBI) and the HC group, additional correlation analyses were conducted independently for each patient group. Monte Carlo simulation was used to correct multiple comparisons.
Separate associations were explored between apathy symptoms and cognitive performance for each patient group by calculating partial correlation coefficients between AES scores and cognitive measures while covarying for age and sex.
The statistical significance level was set at p <0.01 for most statistical analyses, to control for type I error. Based on the anticipated loss of statistical power secondary to modest sample sizes of the patient groups, the statistical significance level was set at p <0.05 for t-tests and post-hoc comparisons (i.e., Scheffé’s test and Monte Carlo simulation). All statistical analyses were performed with SPSS Version 19, with the exception of the cortical thickness analyses, which were conducted in FreeSurfer.
Results
Neurocognitive Performance
DSZ patients, as compared with HCs, demonstrated worse performance on all 12 cognitive measures. The TBI group performed worse than HCs on tests of motor speed and dexterity (Grooved Pegboard Test; GPT), divided attention (Brief Test of Attention; BTA), verbal memory (Hopkins Verbal Learning Test [HVLT–R] recall), and visual memory (Brief Visuospatial Memory Test–Revised [BVMT–R] learning and recall). Compared with patients with DSZ, the TBI group showed better concept-formation (Modified Wisconsin Card-Sorting Test [M–WCST]). There were no cognitive tests on which patients with DSZ outperformed those in the TBI or HC groups (Table 1).
| Control Subjects (N=47) | DSZ Patients (N=15) | TBI Patients (N=10) | Statistical Findings | Post-hoc Pairwise Comparison | |||||
|---|---|---|---|---|---|---|---|---|---|
| Demographic variables | N | % | N | % | N | % | p | ||
| Men | 38 | 81 | 12 | 80 | 8 | 80 | χ2=0.08 | NS | |
| Handedness, right | 37 | 78 | 14 | 93 | 9 | 90 | χ2=2.26 | NS | |
| On typical antipsychotics | 4 | 27 | 0 | 0 | |||||
| On atypical antipsychotics | 13 | 87 | 1 | 10 | |||||
| On antidepressants | 1 | 7 | 0 | 0 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Age, years | 41.4 | 12.2 | 33.5 | 10.6 | 43.7 | 11.1 | F=3.079 | 0.052 | |
| Education, years | 14.7 | 2.3 | 11.7 | 1.8 | 14.8 | 2.5 | F=10.67 | <0.001 | HC > DSZ; TBI > DSZ |
| NART IQ | 105.3 | 8.7 | 92.5 | 7.5 | 104.1 | 12.3 | F=11.17 | <0.001 | HC > DSZ; TBI > DSZ |
| Duration of illness, years | 12.1 | 8.4 | 6.0 | 3.9 | |||||
| Clinical and neurocognitive variables | Mean | SD | Mean | SD | Mean | SD | |||
| AES score | 49.7 | 8.5 | 55.7 | 8.6 | t = –1.736 | 0.096 | |||
| SAPS sum | 2.9 | 2.8 | 1.4 | 2.5 | t=1.409 | NS | |||
| SANS sum | 17.1 | 2.3 | 12.7 | 5.3 | t=2.845 | 0.009 | |||
| SANS Affect | 3.8 | 0.6 | 2.3 | 1.6 | t=3.423 | 0.002 | |||
| SANS Alogia | 3.5 | 0.8 | 1.7 | 1.8 | t=3.501 | 0.002 | |||
| SANS Anhedonia | 4.0 | 0.4 | 3.6 | 0.7 | t=3.033 | 0.006 | |||
| SANS Apathy | 4.0 | 0.0 | 3.6 | 0.5 | t=1.857 | 0.076 | |||
| SANS Attention | 1.7 | 1.3 | 1.5 | 1.4 | t=0.426 | NS | |||
| Hamilton Depression score | 6.0 | 2.3 | 5.1 | 2.1 | t=0.978 | NS | |||
| Grooved Pegboard (DH) | 68.3 | 11.9 | 88.2 | 24.0 | 87.8 | 24.2 | F=10.44 | <0.001 | HC < DSZ, TBI |
| Grooved Pegboard (NDH) | 74.5 | 14.5 | 103.1 | 29.1 | 98.2 | 31.4 | F=12.65 | <0.001 | HC < DSZ, TBI |
| Trail-Making, Part A, sec. | 29.1 | 14.6 | 49.7 | 25.3 | 45.3 | 16.8 | F=9.51 | <0.001 | HC < DSZ |
| Trail-Making, Part B, sec. | 75.1 | 36.0 | 162.0 | 115.1 | 108.5 | 58.9 | F=10.2 | <0.001 | HC < DSZ |
| Brief Test of Attention | 16.4 | 2.6 | 10.3 | 3.8 | 10.4 | 5.9 | F=25.33 | <0.001 | HC > DSZ, TBI |
| Letter Fluency | 28.6 | 8.7 | 17.6 | 7.5 | 20.9 | 11.0 | F=10.3 | <0.001 | HC > DSZ |
| RCFT Copy | 32.4 | 3.4 | 26.4 | 7.7 | 30.6 | 5.5 | F=8.6 | <0.001 | HC > DSZ |
| HVLT–R Learning | 25.6 | 3.6 | 18.2 | 4.5 | 22.0 | 8.2 | F=15.44 | <0.001 | HC > DSZ |
| HVLT–R Recall | 9.3 | 2.1 | 4.6 | 2.7 | 6.6 | 4.1 | F=20.4 | <0.001 | HC > DSZ, TBI |
| BVMT–R Learning | 25.4 | 6.1 | 13.2 | 9.1 | 18.2 | 8.5 | F=18.23 | <0.001 | HC > DSZ, TBI |
| BVMT–R Recall | 9.6 | 2.1 | 5.5 | 3.7 | 6.7 | 4.2 | F=14.4 | <0.001 | HC > DSZ, TBI |
| m-WCST Categories | 5.4 | 1.3 | 3.2 | 2.2 | 5.1 | 1.7 | F=9.29 | <0.001 | HC > DSZ; TBI > DSZ |
Subcortical and Cerebellar Analyses
Results of the ANCOVAs revealed significant main effects of diagnosis for volumes in the bilateral hippocampi, the right thalamus, and the brainstem. Post-hoc analyses demonstrated that, compared with HCs, TBI survivors showed reduced volumes (p <0.001) of the hippocampi bilaterally, right thalamus, and brainstem. Compared with the DSZ group, TBI survivors also had smaller volumes of the left hippocampus (p=0.016), right thalamus (p=0.025), and brainstem (p=0.018). There were no significant volume differences between HCs and DSZ in subcortical and cerebellar ROIs (Table 2).
| Healthy Controls (N=47) | DSZ Patients (N=15) | TBI Survivors (N=10) | ANCOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROIs | Hemisphere | Volume | SD | Volume | SD | Volume | SD | F | p | Post-hoc Pairwise Comparison |
| Intracranial volume (ICV)a | 1,537.3 | 134.4 | 1,488.7 | 186.9 | 1,480.4 | 137.8 | 1.056 | NS | ||
| Hippocampus | Left | 4.6 | 0.4 | 4.3 | 0.4 | 3.8 | 0.8 | 10.378 | <0.001 | HC > TBI (p <0.001), DSZ > TBI (p=0.016) |
| Right | 4.3 | 0.4 | 4.1 | 0.4 | 3.7 | 0.7 | 7.122 | 0.0016 | HC > TBI (p <0.001) | |
| Amygdala | Left | 1.6 | 0.2 | 1.6 | 0.2 | 1.4 | 0.2 | 3.189 | 0.048 | |
| Right | 1.6 | 0.2 | 1.6 | 0.3 | 1.5 | 0.3 | 0.510 | NS | ||
| Thalamus | Left | 7.3 | 0.8 | 7.2 | 0.9 | 6.6 | 0.8 | 3.189 | 0.048 | |
| Right | 7.1 | 0.8 | 6.8 | 0.8 | 6.1 | 0.9 | 7.645 | 0.001 | HC > TBI (p <0.001); DSZ > TBI (p=0.025) | |
| Caudate | Left | 3.3 | 0.5 | 3.6 | 0.7 | 3.2 | 0.4 | 2.065 | NS | |
| Right | 3.4 | 0.5 | 3.7 | 0.6 | 3.3 | 0.4 | 2.274 | NS | ||
| Putamen | Left | 5.3 | 0.6 | 5.5 | 0.5 | 4.9 | 0.6 | 1.257 | NS | |
| Right | 5.1 | 0.6 | 5.2 | 0.9 | 4.6 | 0.5 | 2.252 | NS | ||
| Pallidum | Left | 1.8 | 0.2 | 1.8 | 0.3 | 1.6 | 0.3 | 3.245 | 0.045 | |
| Right | 1.6 | 0.2 | 1.7 | 0.3 | 1.4 | 0.2 | 3.648 | 0.031 | ||
| Cerebral white matter | Left | 264.3 | 30.5 | 251.8 | 38.3 | 239.4 | 33.0 | 2.364 | NS | |
| Right | 266.2 | 30.9 | 253.6 | 37.1 | 241.3 | 31.8 | 2.233 | NS | ||
| Corpus callosum | 3.2 | 0.5 | 3.1 | 0.7 | 2.6 | 0.5 | 3.826 | 0.027 | ||
| Ventral diencephalon | 8.1 | 0.9 | 8.0 | 0.8 | 7.1 | 0.9 | 4.294 | 0.018 | ||
| Brainstem | 22.6 | 2.5 | 21.3 | 2.8 | 19.2 | 2.1 | 9.000 | <0.001 | HC > TBI (p <0.001); DSZ > TBI (p=0.018) | |
| Cerebellar gray matter | Left | 56.8 | 5.5 | 54.1 | 5.7 | 52.9 | 7.5 | 1.959 | NS | |
| Right | 57.9 | 5.3 | 55.2 | 6.4 | 53.6 | 7.0 | 2.349 | NS | ||
| Cerebellar white matter | Left | 14.6 | 2.1 | 14.1 | 2.9 | 13.9 | 2.3 | 0.161 | NS | |
| Right | 14.2 | 1.8 | 13.8 | 2.2 | 12.9 | 1.7 | 1.215 | NS | ||
Correlational analyses controlling for age, sex, and ICV revealed a significant inverse correlation between left hippocampal volume and Apathy Evaluation Scale (AES) scores among TBI survivors (r = −0.93, p=0.002; Figure 1).
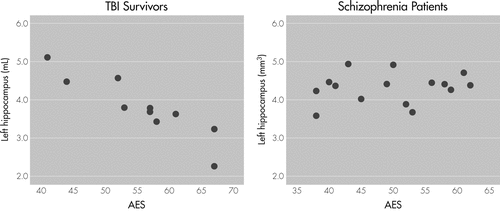
There was an inverse correlation of AES ratings and volume of the left hippocampus in TBI patients (partial correlation coefficient = −0.93; p=0.002, adjusted for age, sex, and intracranial volume).
AES: Apathy Evaluation Scale; DSZ: deficit schizophrenia; TBI: traumatic brain injury.
Cortical Analysis
Compared with the HC group, the DSZ group demonstrated widespread cortical thinning, predominantly in prefrontal and temporo-paralimbic regions, including the bilateral superior, middle, and inferior frontal gyri, as well as the left superior temporal gyrus, right fusiform gyrus, right anterior and posterior cingulate gyrus, bilateral insular cortices, and right supramarginal gyrus (Figure 2). There was no significant difference in cortical thickness between TBI survivors and HCs. Comparisons of cortical thickness between the DSZ and TBI groups demonstrated that TBI survivors had thicker cortices in the pars orbitalis and in the lateral orbitofrontal regions bilaterally (Figure 3). In the DSZ group, additional correlation analyses did not reveal any significant associations between cortical thickness and either AES ratings or cognitive test performance.

DSZ: deficit schizophrenia; HC: healthy controls; RH: right hemisphere; LH: left hemisphere.
ap <0.01, corrected.
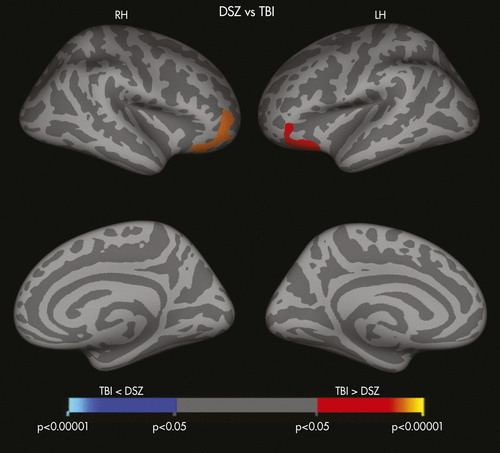
DSZ: deficit schizophrenia; TBI: traumatic brain injury; RH: right hemisphere; LH: left hemisphere.
ap <0.05, corrected.
Correlation of Apathy Evaluation Scale (AES) Ratings With Cognitive Measures
Patients with DSZ did not demonstrate any significant correlation between cognitive test performance and apathy severity. In contrast, in the TBI group, higher AES ratings were associated with worse performance on measures of verbal learning (HVLT−R learning r = −0.88; p=0.004), verbal memory (HVLT−R recall r = −0.84; p=0.0094), and phonemic fluency (Letter Fluency r = −0.85; p=0.0076) even after controlling for age and sex.
Discussion
To our knowledge, this is the first study to demonstrate an association between brain structure and post-TBI apathy. We observed volume reductions of the hippocampus, the thalamus, and the brainstem among TBI survivors. Among these patients, greater reductions of left hippocampal volume correlated strongly with more severe apathy, and the severity of apathy was negatively associated with performance on measures of verbal learning and memory in TBI patients. In contrast, subcortical volume change was not seen in DSZ patients as compared with HCs. Taken together, these findings suggest that apathy resulting from TBI might have a different neural basis from apathy resulting from schizophrenia. The hippocampus plays a critical role in memory function, including verbal and narrative memory.7,36,37 Ariza and colleagues17 reported an association between verbal memory deficits and left hippocampal atrophy in TBI. Andersson and Bergedalen previously reported a relationship between cognitive deficits, including verbal memory, and apathy symptoms among TBI survivors.29 Although we acknowledge that apathy may affect patients’ cognitive performance, it could be possible that, taken together with our data, the lesions to the left hippocampus might be causally related to the development of apathy and verbal memory impairment in TBI. However, given that subcortical and cortical areas of the brain are interconnected both structurally and functionally, it is possible that, in TBI, the cortical areas associated with the development of apathy could be functionally impaired as a result of structural lesions of subcortical regions such as the thalamus, the brainstem, or the hippocampus. An apathy syndrome has been previously described both after thalamic stroke38 and brainstem lesions,39 indicating that such a mechanism is possible. In addition, or as an alternative to, the above explanations, our data showing a correlation of left amygdala volume with AES score (r = −0.81 [p=0.03], controlling for age, gender, and ICV) suggest, if confirmed in a larger sample, a role of the left amygdala−hippocampus complex in mediating apathy symptoms in TBI.
Findings in this study also highlight differences in structural and functional brain changes between TBI survivors and patients with DSZ. Whereas TBI survivors exhibited several regions of subcortical volume loss as compared with HCs, patients with DSZ demonstrated thinner cortices, particularly in fronto-temporo-paralimbic regions, as compared with HCs. Patients with DSZ also showed significantly reduced cortical thickness of the pars orbitalis bilaterally and lateral orbitofrontal cortices (LOFC) relative to the TBI group. Lesions to the LOFC, one of the frontal lobe areas that is connected with subcortical limbic regions, results in personality blunting and change, possibly due to the absence or impairment of limbic affective input.40 Furthermore, LOFC is particularly sensitive to the implementation of reversal learning,42 and schizophrenia patients with “residual” negative symptoms are more likely to fail the set-shifting stage of the reversal learning task.43 Our results revealed that, DSZ patients completed significantly fewer categories on the M-WCST, compared with TBI survivors with equally severe apathy.
Although Roth and colleagues44 reported smaller bilateral frontal lobe volumes among SZ patients with severe apathy, based on the SANS Apathy subscale, as compared with HCs, we failed to find any significant correlations between structural brain changes and apathy in DSZ patients. A larger sample size may be required in order to replicate the findings of Roth and colleagues with respect to the relationship between apathy and frontal cortex integrity.
It has been previously proposed that apathy may not be a single syndrome, but one that could be divided into subtypes on the basis of the underlying neural mechanism affected by the pathologic processes.40 An example of such an approach, and relevant to our findings involving lateral orbitofrontal cortices in DSZ, can be seen in the apathy associated with frontal system dysfunction. Damage to each of the motor and behavioral cortical–subcortical circuits41 that involve frontal lobe, striatum, globus pallidus, substantia nigra, and thalamus, possibly give rise to different forms of apathy. Given that we thought that TBI apathy would constitute a “model” for DSZ because TBI apathy could be more easily related to localized brain abnormality with potential pathophysiological relevance to DSZ, our data suggest that the pathophysiology associated with TBI is different from that involved with DSZ.
Study Limitations
First, this study is limited by the small sample size, as described above, and our findings need to be validated with a larger number of participants. Second, since the TBI patients of this study were heterogeneous in terms of the severity of TBI and the time since head injury, such heterogeneity may have affected their neurocognitive performance or the severity of apathy. Because of such heterogeneity, we were unable to detect differences between patient groups on some cognitive domains. Third, we were unable to address the question as to whether patients in either group showed signs of apathy before they were injured or developed SZ. Fourth, although we excluded patients who had alcohol/drug abuse or dependence within the past 6 month before the study, we were not able to evaluate the lifetime history of alcohol/drug problems, which may have confounded our results. Finally, we did not have reference patient-groups, such as TBI patients without apathy or SZ patients without apathy.
In conclusion, unique changes in brain morphology differentiated our TBI survivors from patients with DSZ, despite similar degrees of apathy across groups. In TBI survivors, greater apathy was associated with reductions in left hippocampal volumes and more severe verbal memory deficits, suggesting that in TBI, as opposed to DSZ, the development of an apathy syndrome may be precipitated by damage to particular subcortical brain regions and may accompany specific cognitive deficits.
1 : Disorders of diminished motivation. J Head Trauma Rehabil 2005; 20:377–388Crossref, Medline, Google Scholar
2 : Prevalence of apathy following head injury. Brain Inj 1998; 12:87–92Crossref, Medline, Google Scholar
3 : Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Crossref, Medline, Google Scholar
4 : Apathy in Alzheimer’s disease. J Am Geriatr Soc 2001; 49:1700–1707Crossref, Medline, Google Scholar
5 : Psychiatric aspects of Parkinson’s disease: an update. J Neurol 2004; 251:795–804Crossref, Medline, Google Scholar
6 : Residual complaints of patients two years after severe head injury. J Neurol Neurosurg Psychiatry 1985; 48:21–28Crossref, Medline, Google Scholar
7 : The human hippocampus and spatial and episodic memory. Neuron 2002; 35:625–641Crossref, Medline, Google Scholar
8 : Social adjustment after closed head injury: a further follow-up seven years after injury. J Neurol Neurosurg Psychiatry 1985; 48:564–568Crossref, Medline, Google Scholar
9 : The deficit syndrome in schizophrenia: implications for the treatment of negative symptoms. Eur Psychiatry 2004; 19:21–26Crossref, Medline, Google Scholar
10 : Apathy syndrome after traumatic brain injury compared with deficits in schizophrenia. Psychosomatics 2007; 48:217–222Crossref, Medline, Google Scholar
11 : Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 1997; 18:11–23Medline, Google Scholar
12 : Quantitative magnetic resonance imaging in traumatic brain injury. J Head Trauma Rehabil 2001; 16:117–134Crossref, Medline, Google Scholar
13 : Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. AJNR Am J Neuroradiol 2002; 23:1509–1515Medline, Google Scholar
14 : Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1-weighted MRI study. J Neurol Neurosurg Psychiatry 2004; 75:1314–1322Crossref, Medline, Google Scholar
15 : Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. J Neurotrauma 2005; 22:76–82Crossref, Medline, Google Scholar
16 : Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J Neurol Neurosurg Psychiatry 2005; 76:984–988Crossref, Medline, Google Scholar
17 : Hippocampal head atrophy after traumatic brain injury. Neuropsychologia 2006; 44:1956–1961Crossref, Medline, Google Scholar
18 : Cerebral atrophy after traumatic white matter injury: correlation with acute neuroimaging and outcome. J Neurotrauma 2008; 25:1433–1440Crossref, Medline, Google Scholar
19 : Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage 2009; 44:1–8Crossref, Medline, Google Scholar
20 : A morphometric analysis of neuroanatomic abnormalities in traumatic brain injury. J Neuropsychiatry Clin Neurosci 2010; 22:173–181Link, Google Scholar
21 : Regionally selective atrophy after traumatic axonal injury. Arch Neurol 2010; 67:1336–1344Crossref, Medline, Google Scholar
22 : Frontal and temporal lobe brain volumes in schizophrenia: relationship to symptoms and clinical subtype. Arch Gen Psychiatry 1995; 52:1061–1070Crossref, Medline, Google Scholar
23 : Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry 2000; 57:471–480Crossref, Medline, Google Scholar
24 : Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white-matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 2001; 158:234–243Crossref, Medline, Google Scholar
25 : Gray-matter abnormalities in deficit schizophrenia. Schizophr Res 2010; 120:63–70Crossref, Medline, Google Scholar
26 : Neuropsychological impairment in deficit vs. non-deficit schizophrenia. J Psychiatr Res 2008; 42:930–937; available at doi: 10.1016/j.jpsychires.2007.10.002Crossref, Medline, Google Scholar
27 : Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry 1991; 158:340–345Crossref, Medline, Google Scholar
28 : Neuropsychological correlates of alogia and affective flattening in schizophrenia. Biol Psychiatry 1994; 35:164–172Crossref, Medline, Google Scholar
29 : Cognitive correlates of apathy in traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 2002; 15:184–191Medline, Google Scholar
30 : The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
31 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
32 : Are the available apathy measures reliable and valid? a review of the psychometric evidence. J Psychosom Res 2011; 70:73–97Crossref, Medline, Google Scholar
33 : Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol 1989; 3:129–136Crossref, Google Scholar
34 : Schedules for Clinical Assessment in Neuropsychiatry (SCAN), Version 2.1. Geneva, Switzerland, World Health Organization, 1996Google Scholar
35 : Classification of first-episode schizophrenia patients and healthy subjects by automated MRI measures of regional brain volume and cortical thickness. PLoS ONE 2011; 6:e21047Crossref, Medline, Google Scholar
36 : The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007; 30:123–152Crossref, Medline, Google Scholar
37 : Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke 2009; 40:2042–2045Crossref, Medline, Google Scholar
38 : Apathy following stroke. Can J Psychiatry 2010; 55:350–354Crossref, Medline, Google Scholar
39 : Nasu-Hakola’s disease (membranous lipodystrophy): a case report. Acta Neuropathol 1981; 54:89–93Crossref, Medline, Google Scholar
40 : Differentiation of states and causes of apathy, in The Neuropsychology of Emotion. Edited by Borod J. New York, Oxford University Press, 2000Google Scholar
41 : Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
42 : Dissociable roles for lateral orbitofrontal cortex and lateral prefrontal cortex during preference driven reversal learning. Neuroimage 2012; 59:4102–4112Crossref, Medline, Google Scholar
43 : Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry 2009; 66:586–593Crossref, Medline, Google Scholar
44 : Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry 2004; 161:157–159Crossref, Medline, Google Scholar



