Hippocampal and Subcortical Alterations of First-Episode, Medication-Naïve Major Depressive Disorder With Panic Disorder Patients
Abstract
The authors conducted a subcortical analysis of first-episode, medication-naïve patients with major depressive disorder comorbid with panic disorder. A sample of 15 patients were compared with 15 healthy controls to explore volumetric differences of subcortical regions and hippocampus, including the putamen and thalamus. The patient group had smaller volumes of left hippocampus, thalamus, and putamen. Subcortical and hippocampal volumes in the patient group showed significant negative correlations with symptom severity. Structural alterations of the putamen, hippocampus, and thalamus might represent the structural pathophysiology of major depressive disorder comorbid with panic disorder.
Subcortical structures, including hippocampus, putamen, and thalamus, are important for regulation of emotion and play critical roles in major depressive disorder (MDD). Hippocampal volumes decrease from the first episode and deteriorate as the disease progresses, to the greatest degree in first few years of illness.1,2 Deficits of hippocampal volumes are also observed in medication-free patients with MDD,3 and smaller hippocampal volume predicts poorer clinical responses and outcomes.4 Metaanalysis results also show moderate decreases of hippocampal volumes in patients with MDD.5,6 Moderate reductions of putaminal volumes are also observed in metaanalysis of volumetric magnetic resonance (MR) images of patients with MDD.5 The volumetric MR study of MDD also indicates that volumetric reductions of thalamus might be related to emotional, cognitive, and motivational deficits.7 According to the Sheline’s proposal, the limbic-cortico-striatal-pallidal-thalamic circuit is important for pathogenesis of MDD symptoms. Limbic and subcortical structures might interact with cortical regions to modulate activities within this circuit.8 These reports suggest that structures of hippocampus, putamen, and thalamus might be altered in MDD patients.
Panic disorder (PD) patients have no significant changes of hippocampal volumes and sizes, which are preserved after multiple episodes of panic attacks.9 Structural deficits of putamen are also observed in PD patients and gray matter volumes are negatively correlated with clinical rating scales of PD.10 Sexually dimorphic deficits in the thalamus of PD patients have also been mentioned in previous reports, which might provide a neurobiological background for PD.11 From these studies, structural deficits of thalamus and putamen might influence PD pathophysiology. Deficits of hippocampal volumes, however, seem to be a minor issue for the etiology of PD.
The studies of lifetime prevalences of MDD and PD show that these two disorders may often be comorbid.12 This comorbidity has been reported to be associated with more severe symptoms, longer illness duration, treatment-resistance, worse outcomes, and poorer functioning.13 Also, MDD comorbid with anxiety disorders may predict a higher possibility of treatment-resistant MDD.14 Comorbidity between MDD and anxiety disorders is associated with increased suicide risk.15 From these reports, MDD with PD may represent a distinct disorder, composed of complex symptoms and significant genetic-loading phenomena.16,17 In this point of view, this comorbidity should be an important issue for clinical research and treatment.
Automated segmentation and estimation of subcortical and hippocampal structures is a critical issue in neuroimaging analysis, partly because of deep locations and technical difficulty in delineating these structures. FSL FIRST toolbox (FMRIB Software Library, FMRIB’s Integrated Registration and Segmentation Tool), a new function of FSL, was developed to evaluate the volumes of subcortical structures. It was used to find that volumes of thalamus and putamen are progressively reduced in patients with Alzheimer’s disease, and the abnormalities are related to cognitive declines.18 FIRST toolbox has also been used in the study of Creutzfeldt-Jakob disease, to assist in the analysis of subcortical structures, such as the thalamus and putamen.19 These articles show that FIRST Toolbox can help researchers determine volumes and shapes of subcortical and hippocampal structures, including our regions of interest: hippocampus, putamen, and thalamus.
From the above literature review, we hypothesized that MDD-with-PD patients might have structural deficits of hippocampus, thalamus, and putamen, as compared with healthy-controls. However, from the literature review of PD, hippocampal deficits may not be as significant as those in pure MDD. Structural deficits will be detected with assistance of FIRST analysis, under a shape/appearance model. The patient group might have lower volumes in these regions, and volumes of these regions might be correlated with symptom severity of MDD and PD.
Methods
Participants
This study was approved by Institutional of Review Board, Buddhist Tzu-Chi Hospital Taipei Branch. A group of 15 first-episode, drug-naïve MDD-with-PD patients (6 subjects with agoraphobia) and 15 healthy-controls were recruited from the Department of Psychiatry, Buddhist Tzu-Chi General Hospital Taipei Branch, Taiwan. Each participant already signed the informed consent and agreed to join this project. The criteria of selection for patients were as follows: 1) DSM-IV and the Structured Clinical Interview for DSM-IV-diagnosed first-episode, drug-naïve concurrent MDD-with-PD diagnosis, which emerged together; 2) no other psychiatric illnesses or medical illnesses suggested; 3) MDD and PD with moderate severity (Clinician Global Impression of Severity >4, Quick Inventory for Depressive Symptoms-Self Rating, 16-item version (QIDS-SR16) >19, Hamilton Rating Scale for Depression, 21-item version (Ham-D−21) score >24; Hamilton Rating Scales for Anxiety (Ham-A) score >22; Panic Disorder Symptom Severity Scale (PDSS) >15; full-blown symptom panic attacks >4 times within the previous 4 weeks before the baseline visit; 4) no previous cognitive-behavioral therapy or other psychotherapies; 5) no alcohol or substance abuse or dependence; 6) no past history of claustrophobia or discomforts while receiving MR scanning. The healthy-controls had no psychiatric illnesses or medical illnesses according to the Mini-International Neuropsychiatric Interview, medical record, and self-report. Handedness was determined by using the Edinburgh Inventory of Handedness.20 These patients and controls were the same participants in our previous published works about neuroimaging findings in MDD comorbid with PD, which show structural deficits of fronto-limbic regions.21,22
MR Imaging Procedure
Data Acquisition
The structural brain MR images were obtained with a 3T GE version scanner housed at Buddhist Tzu-Chi Hospital Taipei Branch. Scans with three-dimensional, fast-spoiled, gradient-echo recovery (3D-FSPGR) T1W1 (TR: 11.2 msec; TE: 5.2 msec; matrix: 256 × 256; field of view: 260 mm; number of excitations: 1; slice thickness: 1 mm; 180 slices; no gap) were performed at the first visit.
FIRST Analysis of Subcortical Structures
We used FIRST function of FSL to analyze the subcortical structures of these participating subjects. The theory of FIRST Toolbox is based on the shape/appearance models, which are constructed from manually-segmented images from the Center for Morphometric Analysis (Massachusetts General Hospital; Boston, MA). The shape-and-appearance model is based on multivariate Gaussian assumptions where deformable surfaces will be transformed to surface meshes containing volumetric labels. Principal components of variations and highly probable shape in the model will be used to construct the shape. The processing steps were the following: First, the subcortical structures of each participating subject were registered and segmented to produce mesh and volumetric outputs with boundary correction. Second, the summary images of the segmentation outputs were checked for quality and registration. Third, the segmented images (.bvars file) of subcortical structures in each group were concatenated to a single file. The two concatenated files were analyzed by vertex-wise statistics to investigate localized shape differences between groups. The total brain volumes of all participants were included as covariates for the comparisons between groups. The output files consisted of mean mesh and displacement of vectors. The surface coloring of output mesh vtk files is the multivariate F statistic (based on Pillai’s Trace), which indicated the group differences of shapes and volumes.
Volumetric Analysis by SPSS
The mesh files produced by FIRST Toolbox were converted to volumetric files of each subcortical structure and hippocampal region. Volumes of these regions were measured in cubic millimeters (mm3) and analyzed by SPSS (Version 16; Chicago, IL). Three parts of analysis were performed. First, subcortical volumes of different groups were compared, using nonparametric independent t-test (Mann-Whitney U test) because of the relatively small sample size (with Bonferroni correction). The significant threshold is p <0.05. Second, we also divided subcortical volumes by total brain volume (TBV), which is also called “scaling by TBV.” This method is frequently used in structural imaging to estimate the relative volume differences. The ratios (subcortical structures/TBV) of patients and controls were also compared, using nonparametric, independent t-tests (with Mann-Whitney U correction) to reconfirm the results of previous steps. The results were corrected for differences in head size, gender, body mass index, age, etc. (Bonferroni correction) as covariates. Third, the subcortical volumes were correlated with clinical rating scales of MDD and PD (threshold p <0.05) to estimate the relationship between structures and severity of symptoms. The correlations were also corrected for age, years of education, total brain volume, and gender. The TBV, gray-matter volumes, and white-matter volumes of patients were also compared with controls by nonparametric, independent t-test (Mann-Whitney U test) to determine whether there is any sign of brain atrophy in the patient group, which probably influenced our interpretations about relationship between subcortical volumes and brain volumes.
Results
Demographic Data
There were no significant differences in age, gender, height, or weight between Patients and Controls. All the subjects were right-handed. Scores on the QIDS-SR16, Ham-D, Ham-A, and PDSS confirm that patients had moderately severe MDD-with-PD. Patients’ scores were significantly different from those of Controls (Table 1). The education level ranged from high school graduate (1 Patient and 1 Control) to college graduate (14 patients and 14 controls). The durations of the current episode in the Patient group were 1–6 months (mean: 3.12; standard deviation [SD]: 1.05 months).
| Patients (N=15) | Controls (N=15) | p | |
|---|---|---|---|
| Age, years | 36.82 (7.87) | 34.30 (9.87) | NS |
| Height, cm | 160.69 (9.05) | 160.80 (5.98) | NS |
| Body weight, kg | 64.84 (16.04) | 64.80 (15.11) | NS |
| Gender (N) | F (11), M (4) | F (11), M (4) | |
| Handedness | R (15) | R (15) | |
| QIDS-SR16 | 26.12 (6.57) | 1.7 (1.34) | <0.0001* |
| Ham-D | 36.43 (8.43) | 2 (1.41) | <0.0001* |
| Ham-A | 33.21 (6.78) | 2.7 (1.34) | <0.0001* |
| PDSS | 19.62 (2.84) | 0.5 (0.71) | <0.0001* |
FIRST Vertex Analysis of Hippocampus, Putamen, and Thalamus Between Patients and Controls
Colors and numbers of the results from vertex analysis represented the multivariate F statistic (based on Pillai’s Trace) results (see Figure 1). Shape/appearance model analysis of putamen and thalamus revealed larger areas of blue colors, which indicated different areas of shape with highest significance between the two groups. These areas were mostly located in the medial surface of putamen and thalamus. Green regions represented moderate deficits, and red areas showed mild deficits of patients as compared with controls. The differences of left hippocampus seemed to be more significant than those of right hippocampus between two groups. The results of shape model analysis of right hippocampus suggested that the degrees of differences were just mild-to-moderate. No significant differences of amygdalar volumes were observed between Patients and Controls.
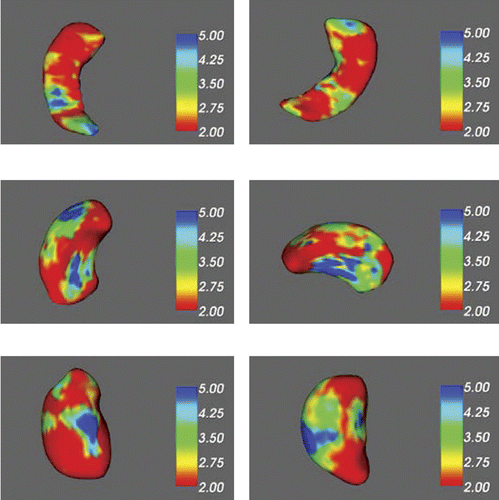
The subcortical structures include left hippocampus (upper left), right hippocampus (upper right), left putamen (middle left), right putamen (middle right), left thalamus (lower left), and right thalamus (lower right). The color and number (right color scale) represented the F test for group differences.
Differences Between Patients and Controls by SPSS Nonparametric t-tests
The volumes of putamen and thalamus in Patients were significantly different from those of Controls (Table 2)—left thalamus: two-tailed p=0.029; right thalamus: two-tailed p=0.033; left putamen: two-tailed p=0.016; right putamen: two-tailed p=0.041 [df: 28]). Left hippocampal volumes of the Patient group also showed significant differences from those of the Control group (Table 2)—left hippocampus: two-tailed p=0.010 [df: 28]). Mean differences and SDs of these regions showed that Patients had smaller volumes of putamen and thalamus than those of Controls; the 95% confidence interval (CI) also suggested that there was a trend for lower volumes of left hippocampus, putamen, and thalamus of patients. However, the differences of right hippocampal volume did not reach a significant level (two-tailed p=0.056 [df: 28]) with a borderline 95% CI upper limit (Table 2). The differences of total brain volume (TBV) and white-matter volumes between groups was not significant (two-tailed p value: 0.061 [df: 28]; effect size [η]: 0.024; two-tailed p value: 0.935 [df: 28], η: 0.0102, respectively). Patients had lower gray-matter volumes than those of Controls (two-tailed p value: 0.029 [df: 28]; η: 0.427). No significant differences in amygdalar volumes were observed between Patients and Controls on SPSS nonparametric t-test (Table 2).
| Volumes, Patient Group | Volumes, Control Group | Group Differences | 95% CI | Effect Size (η) | p | |
|---|---|---|---|---|---|---|
| Left hippocampus | 4,264.46 (484.55) | 4,861.27 (618.20) | –596.81 (202.81) | –1,012.24 to –181.37 | 0.486 | 0.010* |
| Right hippocampus | 4,360.32 (670.38) | 4,838.41 (602.79) | –478.09 (232.78) | –954.91 to –1.27 | 0.362 | 0.056 |
| Left thalamus | 7,246.23 (870.13) | 8,111.80 (766.56) | –684.38 (299.42) | –1,297.71 to –71.05 | 0.396 | 0.029* |
| Right thalamus | 7,246.23 (806.20) | 7,855.88 (704.12) | –609.65 (276.38) | –1,175.78 to –43.52 | 0.384 | 0.033* |
| Left putamen | 5,013.77 (555.02) | 5,468.57 (538.20) | –454.79 (199.62) | –863.70 to –45.89 | 0.395 | 0.016* |
| Right putamen | 4,705.23 (506.73) | 5,162.99 (606.13) | –457.75 (203.99) | –875.61 to –39.90 | 0.390 | 0.041* |
| Left amygdala | 1,791.22 (116.96) | 1,812.71 (129.41) | –32.49 (23.17) | –70.76 to 113.76 | 0.089 | NS |
| Right amygdala | 1,778.51 (177.88) | 1,825.99 (167.07) | –47.48 (27.91) | –66.55 to 191.60 | 0.111 | NS |
Differences Between Patients and Controls by SPSS Nonparametric t-tests (after scaling by total brain volume [TBV])
The ratio of hippocampus, putamen, and thalamus, divided by TBV all showed significant differences between the two groups (Table 3—left hippocampus: two-tailed p=0.008; right hippocampus: two-tailed p=0.023; left thalamus: two-tailed p=0.023, right thalamus: two-tailed p=0.019; left putamen: two-tailed p=0.016; right putamen: two-tailed p=0.006 [df: 28]). The 95% confidence interval (CI) also suggested that there was a trend for lower volumes of bilateral hippocampus, putamen, and thalamus of Patients.
| Ratio of Patient Group, Mean (SD) | Ratio of Control Group, Mean (SD) | Mean Differences (SD) | 95% CI | p | |
|---|---|---|---|---|---|
| Left hippocampus/TBV | 0.00359 (0.00071) | 0.00422 (0.00052) | –0.00063 (0.00023) | –0.00110 to –0.00016 | 0.008* |
| Right hippocampus/TBV | 0.00365 (0.00072) | 0.00421 (0.00056) | –0.00056 (0.00023) | –0.00105 to –0.00007 | 0.023* |
| Left thalamus/TBV | 0.00623 (0.00011) | 0.00706 (0.00078) | –0.00083 (0.00035) | –0.00156 to –0.00110 | 0.023* |
| Right thalamus/TBV | 0.00606 (0.00010) | 0.00683 (0.00073) | –0.00077 (0.00032) | –0.00144 to –0.00099 | 0.019* |
| Left putamen/TBV | 0.00419 (0.00071) | 0.00475 (0.00051) | –0.00056 (0.00022) | –0.00102 to –0.00009 | 0.016* |
| Right putamen/TBV | 0.00394 (0.00063) | 0.00450 (0.00063) | –0.00056 (0.00023) | –0.00104 to –0.00009 | 0.006* |
Correlations Between Volumes of Subcortical Structures and Clinical Rating Scales
The volumes of putamen, thalamus, and hippocampus (except right hippocampus) of Patients were well correlated with the scores of clinical rating scales for MDD or PD. The volumes of these areas of patients seemed to be negatively correlated with Ham-D scores. The correlated areas included left thalamus (Kendall’s tau (τ), r: −0.811; p: 0.000), right thalamus (Kendall’s τ, r: −0.831; p: 0.000), left putamen (Kendall’s τ, r: −0.671; p: 0.000), right putamen (Kendall’s τ, r: −0.651; p: 0.001), and left hippocampus (Kendall’s τ, r: −0.431; p: 0.031). Right hippocampal volumes of the Patient group were not correlated with Ham-D scores (Kendall’s τ, r: −0.270; NS). Also, the volumes of bilateral putamen, thalamus, and left hippocampus of Patients also showed negative correlation with PDSS scores. For left thalamus (Kendall’s τ, r: −0.875; p: 0.000), right thalamus (Kendall’s τ, r: −0.834; p: 0.000), left putamen (Kendall’s τ, r: −0.590; p: 0.003), right putamen (Kendall’s τ, r: −0.692; p: 0.001), left hippocampus (Kendall’s τ, r: −0.407; p: 0.043). The volumes of right hippocampus of the Patient group were not correlated with PDSS scores (Kendall’s τ, r: −0.224; NS). All the above statistical values were derived after Bonferroni correction. Scatterplots also showed a similar trend of negative correlation with symptom severity. (See Data Supplement materials).
Discussion
The results of this study replicate our previous findings of gray-matter deficits in fronto-limbic regions, which included right parahippocampal gyrus, thalamus, and striatum.21,22 The current subcortical results enhanced our confidence about deep structural deficits in patients with MDD-and-PD. Volumetric reductions of subcortical and hippocampal structures, such as putamen, thalamus, and hippocampus, have been reported in MDD patients. Also, chronological ages of these patients are correlated with volumes of thalamus and putamen.23 In our study, structural alterations of thalamus and putamen were found, which suggests shared pathogenesis in these comorbid patients with MDD. The thalamus is important for visual and acoustic attention of MDD patients, and attentive ability is correlated with thalamic gray-matter volumes.24 Structural deficits of thalamus have been reported in many articles on MDD patients, which cite reduced gray-matter volumes.25 Bielau et al. reported structural differences of thalamus and putamen in MDD patients.26 A similar postmortem study found abnormal neuronal numbers in the pulvinar nucleus of thalamus of MDD patients, which might enhance subcortical inputs of emotionally relevant stimuli to the limbic system, to produce MDD symptoms.27 Decreases of thalamic volumes might also be responsible for MDD pathogenesis of the cortico-striatal-pallido-thalamic circuit.28
Reductions of putaminal volumes of our patients also replicate previous studies on MDD or PD. Lacerda et al. found that female MDD patients have neurodegenerative changes of basal ganglion.29 Recent metaanalysis of MR images of MDD patients shows these moderate reductions of volumes of putamen and thalamus.5 A study of medication-free pediatric MDD patients indicates that volumes of putamen might be inversely correlated with age. Striatal alterations of these early-onset patients might suggest that deficits of putaminal volumes might start from the onset of MDD.30 Reduced brain activities of putamen toward reward stimuli are found in MDD patients, and reduced volumes of caudate were associated with severity of symptoms, which suggested that the basal ganglion might be responsible for hedonic motivation.31 Our previous article also shows volumetric alterations of thalamus in the patients with MDD-and-PD, which also supports the findings in this study.21 These studies suggested that MDD patients might have volumetric deficits of putamen and thalamus, which might be associated with clinical symptoms and pathophysiology of MDD.
Volumetric deficits of thalamus of PD patients are only mentioned in Asami’s study specific for female gender.11 Structural deficits of putamen are also negatively correlated with symptomatic severity of PD patients.10 Thalamic alterations in this study also corresponded to our previous published work on the same subjects, which also showed deficits of gray-matter volumes of thalamus.22 From these literature reviews, the structural deficits of putamen and thalamus might also appear in PD patients. Also, negative correlations between volume and symptom severity can support the idea that structural anomalies of thalamus and putamen might represent underlying pathogenesis for MDD-with-PD.
The differences in hippocampal volumes in our study were not as significant between patients and controls, either in t-test or FIRST results (but remained significant after scaling for TBV). Structural deficits of hippocampi are related to MDD. Kronmuller et al. found a negative correlation between life events and left hippocampal volumes, which might suggest that stress could influence the hippocampus of patients with MDD.32 The reductions of hippocampal volumes will be related to the course of MDD, and the decline reaches maximum during the early phase.1 Reductions in hippocampal volumes are also correlated with symptom severity, and left-sided abnormalities are more obvious,3 which supports our study results of volumetric anomalies in left hippocampus. A study of first-onset and drug-naïve MDD patients showed that patients had volumetric reductions of hippocampus, which might shrink with the onset of MDD.33 However, the role of hippocampus in PD pathophysiology is not very obvious. Vythilingam et al. reported that PD patients had no significant differences in hippocampal volumes when they were compared with healthy controls.34 Protopoescu et al found that hippocampus was enlarged in PD patients.35 In a quantitative MR imaging study, amygdala–hippocampus complex areas of patients with PD were not different from those of healthy controls.36 A review of MR-based in-vivo studies of hippocampal volumes concludes that hippocampal volumes are preserved in patients with PD.9 These studies suggested that the hippocampus alterations may not be so obvious for patients with pure PD. In this study, volumetric deficits of left hippocampus in MDD-with-PD patients still remained significant in all the statistical tests. However, these patients had similar volumes of right hippocampus to those of healthy controls. From these results, we suggested that the hippocampus (especially right hippocampus) of patients with MDD-and-PD might maintain original volumes and not shrink with the onset of illness. The volume loss might be limited to left hippocampus and not spread to right hippocampus in these patients, which may correspond to the findings of several published works.37,38 However, the findings in the right hippocampus may generate from limited statistical power because of the small sample size. The group difference in the right hippocampus is trend-level, and the results should be interpreted with caution.
Limitations
The major limitations of this study were the following: 1) a relatively small sample size of participating subjects, which might limit interpretations of our results; 2) a relatively liberal statistical threshold, which might decrease the reliability of our findings; 3) the age at illness onset of our patients was older than typical age at onset of MDD (20–30 years old) and PD (20–25 years old; it is possible that this age at onset might be more typical of those patients with MDD-and-PD); 4) we did not correct for number of comparisons we made (six regions: bilateral hippocampus, bilateral putamen, and bilateral thalamus). Therefore, a p value for one region of p=0.045 would be needed to reach significance when all six regions were tested; 5) the lack of a pure MDD or PD comparison group limited us to confirm the specificity of brain pathology of MDD-with-PD comorbidity; and 6) we did not perform a manual region-of-interest analysis to validate our results of the subcortical structures, which might bias our findings. These concerns might be relieved by the reports of the validating FIRST results, 39–40 which showed that FIRST is comparable to autopsy results or better than other automated methods because there is less bias from tissue classification or arbitrary smoothing. However, our study had several strengths: 1) Our patients were first-episode and drug-naïve, which could exclude the confounding effects of past medications on brain structures. 2) The comorbid MDD and PD could provide a specific model of subcortical and hippocampal deficits for this group of patients. 3) Volumes of putamen, thalamus, and hippocampus were also analyzed to support the results of FIRST vertex analysis. 4) Putaminal and thalamic volumes were negatively correlated with the scores of clinical rating scales for MDD or PD, which suggests that there is a relationship between subcortical volumes and symptom severity.
Conclusions
According to our study results, patients with MDD-and-PD may have alterations in subcortical structures such as putamen and thalamus. Also, limited deficits of hippocampal volumes were observed. The volumetric deficits of these regions may be related to severity of symptoms.
1 : Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A 2003; 100:1387–1392Crossref, Medline, Google Scholar
2 : Depression-related variation in brain morphology over 3 years: effects of stress? Arch Gen Psychiatry 2008; 65:1156–1165Crossref, Medline, Google Scholar
3 : Reduced hippocampal volume in drug-free depressed patients. Surg Radiol Anat 2006; 28:82–87Crossref, Medline, Google Scholar
4 : Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci 2008; 33:423–430Medline, Google Scholar
5 : Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 2009; 30:3719–3735Crossref, Medline, Google Scholar
6 : Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004; 161:1957–1966Crossref, Medline, Google Scholar
7 : Neuroimaging studies of mood disorders. Biol Psychiatry 2000; 48:813–829Crossref, Medline, Google Scholar
8 : 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry 2000; 48:791–800Crossref, Medline, Google Scholar
9 : MR-based in-vivo hippocampal volumetrics, 2: findings in neuropsychiatric disorders. Mol Psychiatry 2005; 10:160–184Crossref, Medline, Google Scholar
10 : Putaminal gray-matter volume decrease in panic disorder: an optimized voxel-based morphometry study. Eur J Neurosci 2005; 22:2089–2094Crossref, Medline, Google Scholar
11 : Sexually dimorphic gray-matter volume reduction in patients with panic disorder. Psychiatry Res 2009; 173:128–134Crossref, Medline, Google Scholar
12 : Lifetime panic-depression comorbidity in the National Comorbidity Survey. Arch Gen Psychiatry 1998; 55:801–808Crossref, Medline, Google Scholar
13 : Lifetime panic-depression comorbidity in the National Comorbidity Survey: association with symptoms, impairment, course, and help-seeking. Br J Psychiatry 2000; 176:229–235Crossref, Medline, Google Scholar
14 ;
15 : Suicide attempts in the Hungarian adult population: their relation with DIS/DSM-III-R affective and anxiety disorders. Eur Psychiatry 2000; 15:343–347Crossref, Medline, Google Scholar
16 : The familial relationship between panic disorder and unipolar depression. J Psychiatr Res 1995; 29:375–388Crossref, Medline, Google Scholar
17 : Familial transmission of panic disorder: effect of major depression comorbidity. Anxiety 1994-1995; 1:180–185Crossref, Medline, Google Scholar
18 : Strongly reduced volumes of putamen and thalamus in Alzheimer’s disease: an MRI study. Brain 2008; 131:3277–3285Crossref, Medline, Google Scholar
19 : Enhanced detection of diffusion reductions in Creutzfeldt-Jakob disease at a higher B factor. AJNR Am J Neuroradiol 2010; 31:49–54Crossref, Medline, Google Scholar
20 : The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
21 : A subtle grey-matter increase in first-episode, drug-naive major depressive disorder with panic disorder after 6 weeks’ duloxetine therapy. Int J Neuropsychopharmacol 2011; 14:225–235Crossref, Medline, Google Scholar
22 : First episode, drug-naïve major depressive disorder with panic disorder: gray-matter deficits in limbic and default network structures. Eur Neuropsychopharmacol 2010; 20:676–682Crossref, Medline, Google Scholar
23 : Magnetic-resonance morphometry in patients with major depression. Psychiatry Res 1998; 84:7–15Crossref, Medline, Google Scholar
24 : Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage 2010;50:347–356Crossref, Medline, Google Scholar
25 : Gray-matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord 2008; 109:107–116Crossref, Medline, Google Scholar
26 : Volume deficits of subcortical nuclei in mood disorders: a postmortem study. Eur Arch Psychiatry Clin Neurosci 2005; 255:401–412Crossref, Medline, Google Scholar
27 : 5HTTLPR polymorphism and enlargement of the pulvinar: unlocking the backdoor to the limbic system. Biol Psychiatry 2007; 61:813–818Crossref, Medline, Google Scholar
28 : Reduced caudate gray-matter volume in women with major depressive disorder. Psychiatry Res 2008; 164:114–122Crossref, Medline, Google Scholar
29 : Anatomical MRI study of basal ganglia in major depressive disorder. Psychiatry Res 2003; 124:129–140Crossref, Medline, Google Scholar
30 : Striatal volume abnormalities in treatment-naïve patients diagnosed with pediatric major depressive disorder. J Child Adolesc Psychopharmacol 2008; 18:121–131Crossref, Medline, Google Scholar
31 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Crossref, Medline, Google Scholar
32 : Life events and hippocampal volume in first-episode major depression. J Affect Disord 2008; 110:241–247Crossref, Medline, Google Scholar
33 : Changes of brain morphometry in first-episode, drug-naïve, non-late-life adult patients with major depression: an optimized voxel-based morphometry study. Biol Psychiatry 2010; 67:186–188Crossref, Medline, Google Scholar
34 : Temporal lobe volume in panic disorder: a quantitative magnetic resonance imaging study. Psychiatry Res 2000; 99:75–82Crossref, Medline, Google Scholar
35 : Increased brainstem volume in panic disorder: a voxel-based morphometric study. Neuroreport 2006; 17:361–363Crossref, Medline, Google Scholar
36 Temporal and right frontal lobe alterations in panic disorder: a quantitative volumetric and voxel-based morphometric MRI study. Psychol Med 2010; 40:1879–1886Google Scholar
37 : Hippocampal volume in first-episode and recurrent depression. Psychiatry Res 2009; 174:62–66Crossref, Medline, Google Scholar
38 : Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 2002; 159:1112–1118Crossref, Medline, Google Scholar
39 : A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 2011; 56:907–922Crossref, Medline, Google Scholar
40 MRI volumetric analysis techniques, including hippocampus extraction, based on data from the Honolulu Asia Aging Study (HAAS). Ethn Dis 2010; 20(S1):104–106Google Scholar



