Update on Mild Traumatic Brain Injury: Neuropathology and Structural Imaging
Abstract
Traumatic brain injury (TBI) is a common occurrence in the United States in both the civilian and military populations. The Centers for Disease Control and Prevention (CDC) estimates that at least 1.7 million people receive urgent medical care for TBI each year.6 Of these, 80% likely experienced a mild TBI, or concussion, based on symptoms at the time of injury (e.g., dazed/confused/“saw stars;” at most, a short loss of consciousness or brief period of amnesia).7 It is known that many individuals do not seek medical care after a mild TBI. The CDC estimates that the true incidence may be as high as 3.8 million annually.6 Also, it is estimated that 12%–35% of the military personnel who have deployed to recent combat operations (2.4 million as of June 30, 2012) have sustained at least one mild TBI.8,9 Although most people recover fully, needing only appropriate education and rest, studies in civilian populations suggest that 10%–30% of individuals experiencing a single mild TBI may develop some long-term sequelae,10–12 and recent studies are now proposing possible long-term adverse health effects emerging years after mild TBI, including the development of chronic traumatic encephalopathy (CTE), thus increasing the importance of prompt and accurate identification of injuries.13–17 This review will focus on neuropathology and imaging of mild TBI, with particular emphasis on neuroimaging methods that are noninvasive and thus have potential for future clinical use in tracking neuronal changes over time.
NEUROPATHOLOGY
The challenges to studying mild TBI are considerable, as this is a highly heterogeneous condition.2,12,18 Each TBI involves a unique combination of forces interacting with the individual’s unique anatomy. Multiple factors, such as presence of protective equipment, head position, head movement relative to direction of force(s), and magnitude and/or duration of forces (e.g., proximity to explosion, distance of fall) can all influence the outcome of an event. Mild TBI is also an evolving, dynamic injury.1–3,19–21 In brief, traumatic forces (e.g., linear, angular, pressure) induce localized physical stress in the brain that instigates a complex series of neurochemical and metabolic changes that may include substantial ionic fluxes, release of excitotoxic levels of neurotransmitters, hypermetabolism leading to energy depletion and hypometabolism, altered cerebral bloodflow and vascular reactivity, edema, and neuroinflammation. Multiple lines of evidence indicate that full return to baseline physiological state can be a prolonged process, requiring weeks or even months, rather than the few days once thought sufficient.2 Diffuse axonal injury (DAI) can be the only pathological injury present in mild TBI. Foci of DAI are generally very small relative to current imaging resolution and may be present in multiple areas. Although some areas of the brain are more vulnerable to injury than others, there is no single pattern of injury characteristic of mild TBI.
Both direct measurements and improved modeling have contributed to a better understanding of the biomechanics of mild TBI.1–3,19–23 The direction, speed, and extent of the induced local deformation(s) are critical factors. Primary (immediate) axon transaction (axotomy) is unlikely to occur. An axon may be unaffected, may undergo a transient disruption, or an injury cascade may be triggered that can eventually lead to secondary axotomy. Focal damage to the axon membrane (axolemma) allows influx of sodium and calcium ions (and hyperpolarization) and triggers localized disruption of fast axonal transport. (This may be due to direct mechanical injury of the intra-axonal cytoskeletal components or may be secondary to entry of ions causing activation of proteases.) The beaded appearance of injured nerve fibers results from focal compaction of the cytoskeleton and axonal swelling (Figure 1). A recent study utilizing an in-vitro model for stretch injury reported that stretch resulted in periodic breaks affecting only a portion of microtubules at that location.24 These breaks were associated with partial interruption of axonal transport, indicating that the beaded appearance of injured nerve fibers may result from development of swelling in areas in which axonal transport is impaired but not halted. Post-injury changes may be complex and individual, as there is evidence that some neurons may re-seal and recover, whereas others may exhibit delayed membrane disruption. If local swelling is sufficient, the axon separates (secondary axotomy); an axon bulb forms; and the distal fiber dies back (Figure 1). Although it has been thought that axotomy was always followed by cell death, studies from one group have recently brought this into question.25,26 Utilizing a moderate midline fluid percussion injury in mice that induces mild TBI without contusion, they found that whereas reactive changes (e.g., truncated axons, decreased cortical neuron soma size, intracellular accumulation of lysosomal debris) were clearly present, axotomized cortical neurons did not show evidence of apoptosis or necrosis by 28 days post-injury.

A key aspect of mild TBI is that studies in both animal models and humans have consistently found that only a small percentage of axons in any area will be affected. Multiple factors associated with increased vulnerability of individual axons to injury have been identified.1–3,22,23 Larger caliber and a high degree of alignment with surrounding axons may be protective. Local change in trajectory increases the risk of injury, particularly if the axon is bending around another structure (e.g., blood vessel). Although most studies have focused on larger-caliber, myelinated axons, there is in-vitro evidence indicating greater vulnerability of unmyelinated axons, even when matched for caliber.27 Of particular relevance to mild TBI, a recent study in rats, utilizing the moderate midline fluid percussion injury model, reported that unmyelinated axons in the corpus callosum were much more profoundly affected than myelinated axons.28 The largest unmyelinated axons were particularly affected. The authors proposed several factors that might underlie this enhanced vulnerability. Myelin itself is protective, providing both physical support and insulation from the extracellular environment. Thus, axons lacking myelin may be at higher risk of injury from both force trauma and biochemical trauma. Unmyelinated axons are also thinner, with a much higher surface area-to-volume ratio, and so may be more likely to experience injurious increases in intracellular calcium. Differential vulnerability could have profound functional implications, as smaller-diameter axons in the corpus callosum are known to preferentially connect higher-order processing areas.29 Most importantly, the region of the corpus callosum with the highest density of small fibers and the lowest density of large fibers is the genu, where fibers interconnecting the prefrontal areas cross.4
STRUCTURAL IMAGING
Studies of mild TBI in both animal models and humans indicate that only a small percentage of axons in any area will undergo secondary axotomy. Thus, most areas of DAI are very small compared with the size of an imaging voxel (Figure 2), making them difficult to visualize, even by clinical magnetic resonance imaging (MRI). If there was sufficient focal shear/strain to cause a breach in the microvasculature, the resultant small area of hemorrhage (microbleed, petechial hemorrhage) may be visible with the use of gradient echo or susceptibility-weighted imaging.2,12,30,31 In addition to identifying areas in which DAI has likely occurred, microbleeds also serve as a reminder that neurons are not the only brain constituent that can be injured in mild TBI. Other neuroimaging findings have also been associated with mild TBI, particularly when repetitive injuries have occurred.32,33 A recent retrospective study assessed presence of neuroimaging findings commonly reported in mild TBI in a group of combat sports (e.g., boxing, mixed martial arts) participants.34 The majority (76/100) had at least one finding (59% hippocampal atrophy, 43% cavum septum pellucidum, 32% dilated perivascular [Virchow-Robbins] spaces, 24% cerebral atrophy). Although DAI was found in 29%, none had evidence of hemosiderin, indicating that all were nonhemorrhagic lesions. Of potential importance, whereas most DAIs were located in the frontal subcortical white matter, the major location for dilated perivascular spaces was the parietal subcortical white matter. Thus, these may represent different mechanisms or types of diffuse injury. As noted by the authors, the checklist approach developed for this study may help systematize capture of neuroimaging findings.34

Diffusion tensor imaging (DTI), an MRI method that provides metrics of the speed and direction of water diffusion within the voxel, is presently the most promising method for identifying areas of injury in mild TBI.2,12,29–31,35 Multiple DTI studies have reported abnormalities in specific metrics at various times after injury, as recently reviewed in detail.12 The most commonly reported metrics are fractional anisotropy and mean diffusivity. Fractional anisotropy (FA) is a measure (scale of 0 to 1) of the average directionality of water diffusion. Areas in which diffusion is similar in all directions (e.g., gray matter) are termed isotropic, and have a low FA. Areas in which diffusion is faster in one direction than another (e.g., white matter) are termed anisotropic, and have a higher FA. FA is highest when the voxel contains only axons, all axons are parallel (coherent), densely packed, and myelinated. Mean diffusivity (MD) is a measure of the averaged speed of diffusion along the three main directions. In an area in which diffusion is anisotropic, the speed of diffusion in the fastest direction is termed the longitudinal or axial diffusivity, and the speed of diffusion in the two directions perpendicular to the fastest direction is termed the radial or tangent diffusivity.
The most common pattern of findings in mild TBI is multiple small areas of reduced FA and increased MD, but both the opposite pattern and changes in one metric only have also been reported.2,12,29–31,35 Reductions in white-matter FA have been attributed to loss of structural integrity of some/all axons, reduced axon packing density, and/or reduced axonal coherence within a voxel. Elevations in white-matter FA have been attributed to axonal swelling, inflammation, presence of reactive astrocytes (gliosis), and/or selective loss of fibers oriented differently from the majority in the voxel. It has been suggested that decreased FA coupled with increased MD indicates vasogenic (extracellular) edema, whereas the opposite pattern indicates cytotoxic (intracellular) edema. An alternative suggestion is that increased FA may be due to compensatory or neuroplastic changes. In some studies, increased axial diffusivity has been correlated with pathology in axons and increased radial diffusivity with pathology in myelin. Although there are considerable differences across these studies in both the changes reported and the anatomic locations of abnormalities, this is not surprising, given that most studies captured data at a single time-point and utilized primarily group-wise comparisons, both of which are problematic with a heterogeneous condition such as mild TBI.12
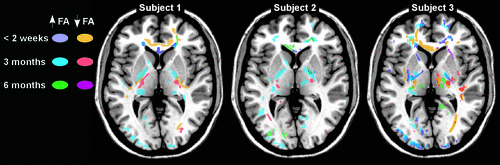
Recent studies of mild TBI in both civilian and military populations have demonstrated the value of utilizing analytic approaches that allow comparisons to be based on individual data and the value of obtaining longitudinal measures. The importance of both were clearly illustrated by a recent study comparing DTI metrics of the cingulum bundle (tractography-based region of interest [ROI]) at four times in the first 8 days after mild TBI; this study reported considerable individual differences in both metric values and the temporal pattern of changes.36 In another study, active-duty military personnel evacuated to Landstuhl Regional Medical Center with and without mild TBI (median time post-injury: 14 days; range: 1–90), with exposure to blast, were compared using 12 manually-defined ROIs.37 Group-wise comparisons indicated that mild TBI was associated with reduced average FA in several ROIs. Individual comparisons indicated that average FA was significantly reduced (z score threshold: −2.0) in at least two ROIs in 29% and in one ROI in 32% of the mild TBI group. Repeat neuroimaging 6–12 months later indicated some normalization. A study comparing civilians with mild TBI in the subacute stage (15.6 [SD: 4.3] days after injury) with matched controls (gender, age, education) reported a general pattern of increased FA and reduced MD, but found no significant differences based on most group-wise comparisons (voxel-wise white matter, tract-based spatial statistics), with FA significantly increased in a single ROI (genu of the corpus callosum).38 There was a trend toward increased number and total volume of voxels with significantly altered FA (z score threshold: ±2.0) in the mild TBI group, but clusters were present in both groups. However, these metrics were unchanged at the follow-up neuroimaging for the controls and significantly improved in the mild TBI group. Another recent longitudinal study in civilians assessing changes in FA after mild TBI reported areas of abnormally high and low FA (z score threshold: >1.96) that were unique to each individual and changed considerably across time-points (Figure 3).5 The most common pattern was fewer voxels with reduced FA and more with elevated FA when neuroimaging at 2 weeks was compared with 3 months. Decreases in both were seen by 6 months. The authors suggested that elevated FA may represent neuroplastic responses to injury, rather than pathology. A case report of a Marine with multiple exposures to primary blast forces also supports the value of DTI on an individual level.39 Group and individual analytic approaches were compared in two recent DTI studies of Iraq and Afghanistan veterans with and without a history of mild TBI (chronic stage; all more than 2 years post-injury) that included exposure to blast as an injury mechanism.40,41 In one study, no differences were found in group-wise comparisons (tractography-based ROI averages), but mild TBI was associated with presence of more voxels with significantly reduced FA (z score threshold: −2.0) in 10 of the 20 ROIs. Only a few voxels were common across the mild TBI group. The second study also found no differences based on group-wise comparisons (tract-based spatial statistics), but more voxels with significantly reduced FA (z score threshold: −3.0) were present in the mild TBI group, based on individual analyses.41 Importantly, the differences were not related to variables such as coexisting psychiatric conditions. As noted in an editorial accompanying the second study, further research is required to determine the pathophysiological and prognostic significance of such findings.42
Conclusions
DTI use in mild TBI is still at an early stage of development, as evidenced by the diversity of findings. The majority of studies have been cross-sectional, rather than longitudinal, and utilized group-wise comparisons and ROIs, rather than investigating the whole brain on an individual basis. Also, there are no commonly agreed-upon methods for acquisition, processing, or analysis of DTI data, which may be a source of considerable variability across studies.12,43 Recommendations have been developed that may improve standardization of neuroimaging methods and data collection in TBI research.44–46 The more subtle injuries characteristic of mild TBI require sensitive analytic approaches, which all have strengths and weaknesses that must be considered.12,35 To have clinical utility, it is essential that methods be applicable on an individual basis. For DTI to become a clinically meaningful technique, it must provide a quantitative, individualized, reliable assessment. There are potentially multiple changes in the brain that, if reliably quantified, might be of value in diagnosis and clinical management of mild TBI. These include the actual areas of injury, as well as areas undergoing changes as a result of metabolic, degenerative, adaptive, and/or compensatory processes.
1 : Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res 2007; 161:43–59Crossref, Medline, Google Scholar
2 : Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav 2012; 6:108–136Crossref, Medline, Google Scholar
3 : Axonal pathology in traumatic brain injury. Exp Neurol 2012; (e-pub ahead of print).Google Scholar
4 : Fiber composition of the human corpus callosum. Brain Res 1992; 598:143–153Crossref, Medline, Google Scholar
5 : Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time, and bidirectional changes in anisotropy. Brain Imaging Behav 2012; 6:329–342Crossref, Medline, Google Scholar
6 : Epidemiology of concussion and mild traumatic brain injury. PM R 2011; 3(Suppl 2):S354–S358; http://www.ncbi.nlm.nih.gov/pubmed/22035677Crossref, Medline, Google Scholar
7
8 : Concussions and the military: issues specific to service members. PM R 2011; 3(Suppl 2):S380–S386; http://www.ncbi.nlm.nih.gov/pubmed?term=rigg%20concussionCrossref, Medline, Google Scholar
9
10 : Neuropsychology and clinical neuroscience of persistent post-concussive syndrome. J Int Neuropsychol Soc 2008; 14:1–22Crossref, Medline, Google Scholar
11 : Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010; 25:241–255Crossref, Medline, Google Scholar
12 : A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 2012; 6:137–192Crossref, Medline, Google Scholar
13 : Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke 2011; 42:2733–2739Crossref, Medline, Google Scholar
14 : Death after head injury: the 13-year outcome of a case–control study. J Neurol Neurosurg Psychiatry 2011; 82:931–935Crossref, Medline, Google Scholar
15 : Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus 2011; 31:E3Crossref, Medline, Google Scholar
16 : Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4:134ra60Google Scholar
17 : The spectrum of disease in chronic traumatic encephalopathy. Brain 2012; (e-pub ahead of print).Medline, Google Scholar
18 : Embracing chaos: the scope and importance of clinical and pathological heterogeneity in mTBI. Brain Imaging Behav 2012; 6:255–282Crossref, Medline, Google Scholar
19 : The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011; 30:33–48, vii–iiiCrossref, Medline, Google Scholar
20 : Biomechanics of concussion. Clin Sports Med 2011; 30:19–31, viiCrossref, Medline, Google Scholar
21 : The pathophysiology of concussion. PM R 2011; 3(Suppl 2):S359–S368; http://www.ncbi.nlm.nih.gov/pubmed/22035678Crossref, Medline, Google Scholar
22 : Micromechanics of diffuse axonal injury: influence of axonal orientation and anisotropy. Biomech Model Mechanobiol 2011; 10:413–422Crossref, Medline, Google Scholar
23 : Multi-scale mechanics of traumatic brain injury: predicting axonal strains from head loads. Biomech Model Mechanobiol 2012; (e-pub ahead of print).Medline, Google Scholar
24 : Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol 2012; 233:364–372Crossref, Medline, Google Scholar
25 : Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J Neurosci 2011; 31:5089–5105Crossref, Medline, Google Scholar
26 : Electrophysiological abnormalities in both axotomized and nonaxotomized pyramidal neurons following mild traumatic brain injury. J Neurosci 2012; 32:6682–6687Crossref, Medline, Google Scholar
27 : Selective vulnerability of non-myelinated axons to stretch injury in an in vitro co-culture system. J Neurotrauma 2011; 28:841–847Crossref, Medline, Google Scholar
28 : Unmyelinated axons show selective rostrocaudal pathology in the corpus callosum after traumatic brain injury. J Neuropathol Exp Neurol 2012; 71:198–210Crossref, Medline, Google Scholar
29 : Transcallosal transfer of information and functional asymmetry of the human brain. Laterality 2011; 16:35–74Crossref, Medline, Google Scholar
30 : Investigating white-matter injury after mild traumatic brain injury. Curr Opin Neurol 2011; 24:558–563Crossref, Medline, Google Scholar
31 : Detection of hemorrhagic and axonal pathology in mild traumatic brain injury using advanced MRI: implications for neurorehabilitation. NeuroRehabilitation 2012; 31:261–279Medline, Google Scholar
32 : Cavum septi pellucidi in boxers. Can Assoc Radiol J 2010; 61:29–32, quiz 1–2Crossref, Medline, Google Scholar
33 : Review: Contact sport-related chronic traumatic encephalopathy in the elderly: clinical expression and structural substrates. Neuropathol Appl Neurobiol 2011; 37:570–584Crossref, Medline, Google Scholar
34 : Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma 2009; 26:1–13Crossref, Medline, Google Scholar
35 : Mild traumatic brain injury: is diffusion imaging ready for primetime in forensic medicine? Top Magn Reson Imaging 2010; 21:379–386Crossref, Medline, Google Scholar
36 : Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav 2012; 6:319–328Crossref, Medline, Google Scholar
37 : Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011; 364:2091–2100Crossref, Medline, Google Scholar
38 : Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain 2012; 135:1281–1292Crossref, Medline, Google Scholar
39 : A case of frontal neuropsychological and neuroimaging signs following multiple primary blast exposure. Neurocase 2012; 18:258–269Crossref, Medline, Google Scholar
40 : Diffuse and spatially variable white-matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage 2012; 59:2017–2024Crossref, Medline, Google Scholar
41 : White-matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry 2012; 169:1284–1291Crossref, Medline, Google Scholar
42 : Diffusion tensor imaging and mild traumatic brain injury in soldiers: abnormal findings, uncertain implications. Am J Psychiatry 2012; 169:1230–1232Crossref, Medline, Google Scholar
43 : Diffusion tensor imaging and beyond. Magn Reson Med 2011; 65:1532–1556Crossref, Medline, Google Scholar
44
45 : Common data elements in radiologic imaging of traumatic brain injury. J Magn Reson Imaging 2010; 32:516–543Crossref, Medline, Google Scholar
46 : Standardizing data collection in traumatic brain injury. J Neurotrauma 2011; 28:177–187Crossref, Medline, Google Scholar



