Disulfiram-Induced Combined Irreversible Anterior Ischemic Optic Neuropathy and Reversible Peripheral Neuropathy: A Prospective Case Report and Review of the Literature
Abstract
Disulfiram is an alcohol-deterring agent with few rare serious adverse effects, usually dose-related, reversible, and more frequent with comorbid alcohol and nicotine dependence. We report a prospective follow-up of such a case with reversible peripheral neuropathy and irreversible optic neuropathy.
Disulfiram (tetraethylthiuram disulphide), a carbamate derivative used as an alcohol-deterring agent, is a relatively nontoxic substance when administered alone, but it markedly alters the intermediary metabolism of alcohol. It acts by inhibiting aldehyde dehydrogenase, alcohol dehydrogenase, and dopamine beta-hydroxylase along with certain cytochrome P450 isoenzymes (2E1, 2C9, 3A4, 3A5).1 Nicotine also has an inhibitory effect on many cytochrome P450 isoenzymes (1A1, 1A2, 2A6, 2A13, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, 3A4).2 Moreover, common cytochrome P450 isoenzymes (2C9, 2E1, 3A4) involved in both disulfiram and nicotine metabolism may suggest their role in drug-level alteration.1
Disulfiram toxicity may present various clinical aspects, although the mechanism of toxicity (direct or idiosyncratic) remains unclear. Rare reports of psychosis, catatonia, delirium, polyneuritis, peripheral neuropathy, and optic neuritis in conjunction with disulfiram treatment are usually dose-dependent (≥500 mg/day), reversible on withdrawing disulfiram,3–6 and occur more frequently in cases of comorbid alcohol and nicotine dependence.7–10 Peripheral neuropathy presents with various clinical presentations: polyneuritis with sensory, motor, or both deficits, and a few cases of quadriplegia,11 with complete recovery in 1–5 months after stopping treatment.12 Complete recovery of disulfiram-induced severe optic neuritis usually occurs within 2 months of its discontinuation.11 However, irreversible optic neuropathy/neuritis has not been reported in the English literature. We report such a case of disulfiram-induced combined irreversible central (optic) and reversible peripheral (sensory) neuropathy in a patient who was abstinent from alcohol and nicotine.
Case Report
A 43-year-old married adult man from an urban and middle-class socioeconomic background, with a mixed diet, presented with a history of alcohol consumption (42.8% vol. concentration; 270–360 ml/day) for 10 years, with features of insomnia, tremors, anorexia, and anxiety after cessation or reduction of alcohol for the last 2 years. The patient also had history of chewing tobacco, about 15–20 packets per day, for 10 years. He was diagnosed with comorbid alcohol and nicotine dependence syndrome in uncomplicated withdrawal state, as per ICD−10 diagnostic criteria.13
He had no previous medical history of hypertension, diabetes, heavy metal exposure, epilepsy, neurological deficits, or any drug intake. He had received inpatient de-addiction services during his previous three admissions. Family history of alcoholism, and death due to its medical causes (liver problems) were noted in his father and brother. The patient had a cordial family and work atmosphere.
On examination, stable vital parameters and fine tremors of hands were noted. Except for mild hepatomegaly, other systemic examination, including nervous system, showed no abnormalities. Mental status examination revealed preoccupations with alcohol use and anxious mood, with preserved cognitions. His complete blood count and blood glucose levels were within normal limits. Liver function tests revealed elevated liver enzymes (SGOT 145 U/L; SGPT 113 U/L; GGT 765 U/L; alkaline phosphatase 235 U/L). Ultrasonography of abdomen showed mildly enlarged liver, with grade-2 fatty infiltration.
Management of this patient included benzodiazepines; liver cell protectants (silymarin); baclofen 20 mg/day (anti-craving agent); and multivitamin supplementation, including thiamine 100 mg/day. Individual psychotherapy was initiated to educate about the harmful effects of alcohol, coping skills, and relapse-prevention strategies. Considering his frequent relapses and the willingness of both patient and spouse for disulfiram therapy, it was initiated at 500 mg/day after we obtained written informed consent. The patient maintained abstinence from alcohol, with compliance ensured and supervised by his spouse.
Six weeks later, he complained of gradual onset and progressive bilateral joint pains, giddiness, and burning and tingling sensation with numbness in his feet. His nervous system examination revealed well-preserved higher cognition, cranial nerves, motor system, and cerebellar functions. His bilateral ankle reflexes were sluggish, and Babinski’s sign was positive. Sensory abnormalities consisted of impairment of fine touch, pain, and temperature distal to the lower third of the legs. In the lower limbs, joint position and vibration sense were also absent, suggestive of lateral and posterior columnar tract dysfunction. Subsequently, pregabalin (75 mg/day) and methylcobalamin (1,500 mcg/day) were initiated, along with reduction in dose of disulfiram to 250 mg/day. Two weeks later, the patient reported complete improvement in burning sensation in lower limbs, with no clinical signs of peripheral neuropathy.
A month later, he reported diminution of vision in the left eye, of 10 days’ duration. On ophthalmological examination, his right pupil was round, regular, and reactive to light; but the left pupil showed relative afferent pupillary defect (RAPD; Marcus-Gunn pupil) on swinging flashlight test. The Snellen test for distant visual acuity showed 6/6 on right eye and 6/12 on left eye. Jaeger’s chart for near vision revealed N8 for right eye and N18 for left eye. Ishihara’s charts showed normal color vision in the right eye, with red deficiency in the left eye. He had normal bilateral intraocular pressure. Fundoscopy revealed right optic disc temporal pallor and left optic disc pallor with edema (Figure 1, Figure 2). Perimetry revealed right superior and inferior, along with left inferior and temporal, visual field defects. Fundus fluorescein angiography (FFA) revealed left eye sectoral optic nerve head hypoperfusion with leak in the late phase (Figure 3, Figure 4). The patient was diagnosed with non-arteritic anterior ischemic optic neuritis (NAION) and was evaluated for infectious and autoimmune causes of NAION. However, chest roentgenogram, erythrocyte sedimentation rate (ESR), VDRL test for syphilis, and C-reactive protein (CRP) test showed no abnormalities.
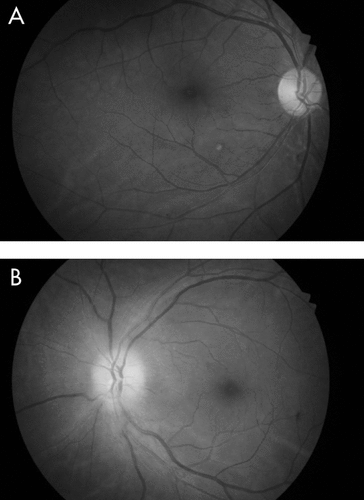
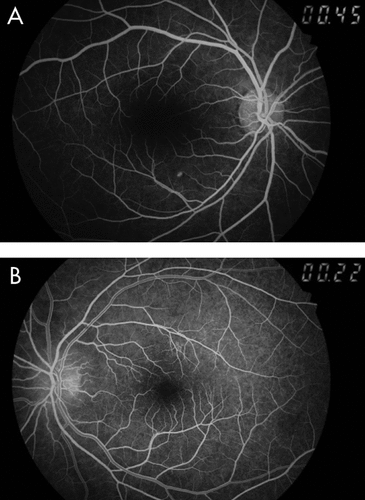
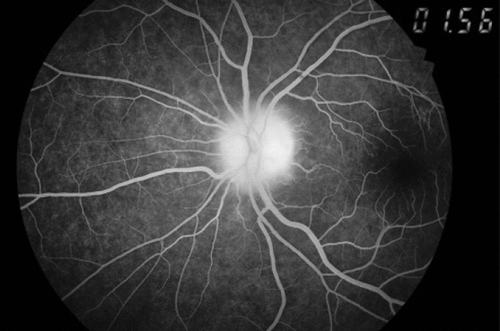
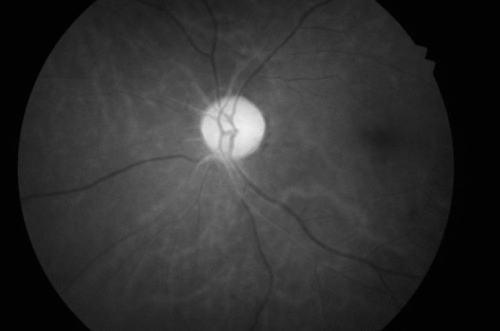
Considering disulfiram as the possible cause for these central (optic) and peripheral (sensory) neuropathic changes, it was discontinued. The patient was treated with aspirin 150 mg/day, antioxidants, multivitamins, and multimineral supplementation. A week later, fundoscopy showed left disc edema, obliteration of cup, and blurred margins. Two weeks later, the patient was symptomatically better and showed improvement in right ocular findings, but had persistent RAPD and pale optic disc in the left eye, even after 2 months. He was advised to continue aspirin 150 mg/day and multivitamin supplementation.
Six months later, the patient maintained abstinence from alcohol, with occasional use of tobacco. He reported difficulties in driving a vehicle, as well as depth and distance perception, especially during twilight hours. His nervous system examination revealed no abnormalities, except for impaired left optic nerve functions such as persistent visual-field defects and red–green deficiency. Nevertheless, he was able to manage his daily routine activities and occupation with less difficulty.
Discussion
Several cases of optic neuritis attributed to disulfiram have been reported, but none have reported irreversible damage. To our knowledge, this is the first case of irreversible NAION related to disulfiram therapy. Alcohol and nicotine have also been implicated in the etiology of amblyopia.14 Our patient, who had comorbid alcohol and nicotine dependence on disulfiram therapy, presented with neuropathic damage during complete alcohol abstinence and significant reduction in nicotine usage. Earlier case reports have noted reversible bilateral optic neuritis with central scotomata as an adverse effect of disulfiram therapy (500 mg daily) to occur as early as a few weeks,8,9 and as late as 18 months of disulfiram therapy.7,10 Improvement of disulfiram-induced optic neuritis was noted as early as 7 weeks8 to 3 months after discontinuation of disulfiram treatment.7
More commonly, patients on disulfiram therapy who have comorbid alcohol and nicotine abuse/dependence pose uncertainty about its attribution to disulfiram or to the comorbid alcohol and nicotine abuse.7–10 In such cases, withdrawing the drug has resulted in improvement to normal vision within 3 months, with no recurrence of such adverse effects upon disulfiram rechallenge in cases of relapse.7 Also, improvement of optic neuritis has been noted in patients who continued smoking despite disulfiram withdrawal.10 These findings suggest the need to address comorbid alcohol and nicotine dependence while planning for disulfiram therapy, in order to avoid possible neuropathic insult in susceptible cases. However, our patient was different from that reported in the literature in having both reversible (right eye) and irreversible (left eye) NAION comorbid with reversible peripheral (sensory) neuropathy. This implies that disulfiram-induced nerve damage may involve various mechanisms: possibly direct toxic damage due to free-acid radicals, due to certain enzyme inhibition, calcium-induced neuronal excitotoxicity, synergistic activity of neurotoxic drug/chemical substances, or a combination of these mechanisms.
Conclusion
Disulfiram is one of the recommended aids that cause few rare serious adverse effects in the management of selected patients with alcohol dependence. Alcohol dependence, for which it can be a helpful treatment, is also associated with high morbidity and mortality.3 Peripheral neuropathy and optic neuritis occurring in such cases are sometimes due to the toxic effects of disulfiram, rather than to alcohol. Unless this is considered in the management of such patients, it is likely that administration of the drug will be continued in the face of adverse effects.15 Possibly, regular monitoring of treated patients by neurological (sensorimotor) and ophthalmological (fundoscopy) examination every 1–2 months for at least the first 6 months of disulfiram therapy may appear prudent to detect neuropathic adverse effects of disulfiram therapy at early stages.11
1 Disulfiram; accessed Dec. 9, 2012; Dec 9; available from http://pubchem.ncbi. nlm.nih.gov/summary/summary.cgi?q=all&cid=3117#ecGoogle Scholar
2 Nicotine; accessed Dec. 9, 2012; available from http://pubchem.ncbi. nlm.nih.gov/summary/summary.cgi?q=all&cid=89594#ecGoogle Scholar
3 : Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf 1999; 20:427–435Crossref, Medline, Google Scholar
4 : Disulfiram-induced transient optic and peripheral neuropathy: a case report. Ir J Med Sci 2007; 176:319–321Crossref, Medline, Google Scholar
5 : [Optic neuropathy while taking disulfiram]. J Fr Ophtalmol 2006; 29:924–928Crossref, Medline, Google Scholar
6 : Reversible optic neuropathy related to disulfiram. J Fr Ophtalmol. 2011; 34(6):382; e1-3; doi: 10.1016/j.jfo.2010.11.019; E-pub April 20, 2011Google Scholar
7 : Névrite rétrobulbaire chronique par Antabuse. Bull Soc Belge Ophtalmol 1953; 104:297–301Medline, Google Scholar
8 : Discussion following paper of Humblet M. Bull Soc Belge Ophtalmol 1953; 104:301Medline, Google Scholar
9 : Nevrite toxique medicamenteuse au cours de la desintoxication ethylique. Bull Soc Ophtalmol Fr 1962; 75:350–357Google Scholar
10 : A propos d’un nouveau cas de névrite optique due au disulfirame. Bull Soc Ophtalmol Fr 1966; 66:159–165Medline, Google Scholar
11 : [Disulfiram (Esperal) toxicity. Apropos of 3 original cases]. Rev Med Interne 1995; 16:67–72Crossref, Medline, Google Scholar
12 : [Optic neuropathy from disulfiram (Antabuse): an observation]. Bull Soc Belge Ophtalmol 1998; 268:161–162Medline, Google Scholar
13
14 Tobacco-alcohol amblyopia: a rare complication of prolonged alcohol abuse. Ind Psychiatry J 2011; 20:66–68Google Scholar
15 : Peripheral neuropathy after disulfiram administration. J Neurol Neurosurg Psychiatry 1971; 34:253–259Crossref, Medline, Google Scholar



