Normal Pressure Hydrocephalus in a Professional Athlete: a Model of Brain Functional Reserve
To the Editor: Normal pressure hydrocephalus (NPH) is characterized by gait disturbance, dementia, and/or urinary incontinence in addition to dilation of ventricular system because of disturbance of CSF circulation with normal CSF pressure.1 NPH can present in two forms: primary (also known as idiopathic), where there are no causative disorders known; and secondary, where other disorders such as trauma, infection, and hemorrhage are causes for hydrocephalus.1,2 We report a unique case of a patient with remarkable fitness and NPH.
Case Report
Mr. “O.R.” is a 69-year-old man with a long history in racetracks, which he began at the age of 14 years as a physical activity, which later became a life passion. From ages 18 to 25 years, he trained four times a week in athletics. Until the age of 25, he participated in professional athletics, sometimes monthly. He specialized in short races, such as 800 m. In 1983, he even received a silver medal in an official competition (South American Juvenile Championship). From ages 25 to 40 years, he discontinued professional athletics to work as a lawyer, running just for fun. At age 40 years, he restarted intense physical activity. Simultaneously, pain in his left hip made him get an orthopedic evaluation, in which femur osteoarthritis was diagnosed. By medical recommendation, he changed his mode of athletics to athletic march (5 and 10 km). His previous medical files were unremarkable.
One year ago, slow progressive and subjective weakness in both legs began, which was interpreted as vertigo. Skull tomography revealed hydrocephalus without intracranial hypertension signs. Complete neurological examination was normal, and Mini-Mental Status Examination (MMSE) displayed punctuation of 30/30. Additionally, Time UP and GO test was 6 seconds, and Japanese Scale for Idiopathic NPH scored 1 point. CSF pressure in lumbar puncture was 15 cm H20.
Magnetic resonance revealed Evans ratio of 0.42 with communicant hydrocephalus, narrowing of sulci and gyri in vertex, and ballooning of corpus callosum, suggesting idiopathic NPH (Figure 1). Flow study revealed pervious cerebral aqueduct with hyperdynamic flow. No other causes were displayed, such as trauma, infection, and hemorrhage.
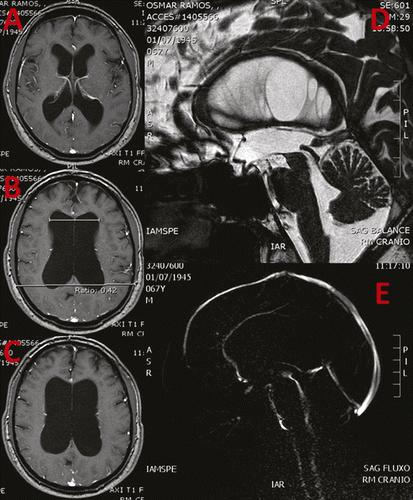
He was referred to a neurosurgical approach because of hydrocephalus and preserved functional activity (Karnofsky performance score of 100; Rankin functional scale of 0). He stated that he wanted to race this year in the São Silvestre Race (15 km); this would be his tenth 15 km race, in addition to the other 12 marathons he already raced in since his youth.
Discussion
We present an elderly man with clinical and image study compatible with NPH. However, because of his previous physical performance, even in context of impressive hydrocephalus, his only complaint was a subjective motor slowing. If image profile was present in another typical elderly man, he would certainly suffer from important motor, urinary incontinence, and cognitive symptoms.
We discuss the concept of motor and functional brain reserve in a professional athlete who, instead of setting up symptoms of NPH, stays with complete functionality. Brain functional reserve, which is also explains interindividual variability in response to a myriad of injuries, such as those caused by traumatic, neoplasic, epileptic, ischemic, and neurodegenerative diseases.5–10 This reserve may be cognitive, such as in a polyglot patient with traumatic or ischemic insult who still persists with language domain above average; hemodynamics, such as the capacity of adjustment of cerebral blood flow after stenosis of intracranial blood vessels. Thus, this reserve generates different profiles of response and reaction to brain damage.
Much evidence points to the that brain has an astounding ability to modulate cognitive and motor skills after acute insults, during insidious neurodegenerative processes, psychological stress, or even in the course of aging. Permanent and transient lesions caused by strokes, tumors, head trauma, and hydrocephalus are good models to understand how compensation process works, following focal or even broader damage.2–5
Brain resilience may be the final result of processes such as redundancy, degeneracy, and pluripotentiality of neural systems.2,4 Neuronal plasticity is a continuous process involving neurogenesis, programed cell death, and activity-dependent synaptic plasticity where the central nervous system learn skills, remembers information, structures neuronal networks in response to environment, and recovers from brain and spinal cord injuries.2 Hydrocephalus, congenital or acquired, represents a model of brain resilience too, once transient or permanent perfusion deficits generate structural and/or functional injuries, being partially or completely compensated by remaining cortical areas.2–4
1 : Role of endoscopic third ventriculostomy and ventriculoperitoneal shunt in idiopathic normal pressure hydrocephalus: preliminary results of a randomized clinical trial. Neurosurgery 2013; 72:845–853, discussion 853–854Crossref, Medline, Google Scholar
2 : Revisiting hydrocephalus as a model to study brain resilience. Front Hum Neurosci 2011; 5:181–184Medline, Google Scholar
3 : Degenerate neuronal systems sustaining cognitive functions. J Anat 2004; 205:433–442Crossref, Medline, Google Scholar
4 : Efficiency and cost of economical brain functional networks. PLOS Comput Biol 2007; 3:e17Crossref, Medline, Google Scholar
5 . Interindividual variability in recovery after traumatic brain injury: Effect of cognitive reserve. Med Clin (Barc) 2013; 140:527–531Crossref, Medline, Google Scholar
6 : The challenging interpretation of genetic and neuroimaging features in basal ganglia calcification. Gen Hosp Psychiatry 2013; 35:210–211Crossref, Medline, Google Scholar
7 : Assessing hippocampal functional reserve in temporal lobe epilepsy: a multi-voxel pattern analysis of fMRI data. Epilepsy Res 2013; 105:140–149Crossref, Medline, Google Scholar
8 : Cognitive and functional resilience despite molecular evidence of Alzheimer's disease pathology. Alzheimers Dement 2012; 9:e89–e95Crossref, Medline, Google Scholar
9 : Increased basal forebrain metabolism in mild cognitive impairment: an evidence for brain reserve in incipient dementia. J Alzheimers Dis 2012; 32:927–938Crossref, Medline, Google Scholar
10 : Severely impaired cerebrovascular reserve in patients with cerebral proliferative angiopathy. J Neurosurg Pediatr 2011; 8:310–315Crossref, Medline, Google Scholar



