A Look Inside the Mirror Neuron System
Abstract
Over 20 years ago, the world was introduced to a fascinating new class of cortical neurons that responded similarly when a goal-directed action was performed and when performance of the same action by another was observed.6–8 The authors of these studies called them mirror neurons (MNs) and noted the potential importance of such neurons for learning by imitation and for understanding the actions of others. Since that time, MNs have been identified in many species, including humans. Considered by some a cornerstone of human empathy, language, and other vital processes, MNs are the subject of lively ongoing debates and controversies.9–12
Defining Mirror Neurons
The identification of MNs came from observing neuronal activity in inferior premotor cortex (Area F5; Figure 1) in macaque monkeys while they engaged in behavioral activities.6–8 It was known that neurons in Area F5 were active during hand and mouth movements that were goal-directed (transitive), such as grasping, tearing, manipulating, or holding objects, but not during similar movements that were not goal-specific (intransitive), such as waving or moving the arms. Using surgically implanted microelectrodes, the activity of single neurons in Area F5 was recorded as the monkeys reached and grasped objects, as well as when they observed the examiner performing various actions. Overall, about 17% of the neurons (92/532) were activated both during observation and execution of similar goal-directed actions (Figure 1). Thus, individual neurons displayed similar activity when observed actions mimicked (or mirrored) performed actions. The study authors proposed that this indicated generation of a similar internal representation during action execution and action observation by MNs. They speculated that this matching of activity provides a basis for implicit recognition and, therefore, understanding of observed motor events. Noting that Area F5 does not receive direct visual input, the authors suggested that this information might be provided by inferior parietal areas that project to F5.
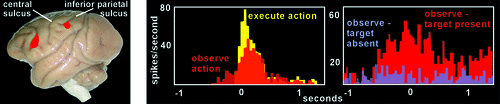
This seminal study sparked this group and others to further characterize MNs within the premotor cortex in monkeys and to seek them in other regions.1,13–16 Of particular importance, studies have shown that the context in which an action occurs can have a profound impact on the firing of MNs. One study compared Area F5 MN activity in monkeys observing humans reaching and grasping objects or humans reaching behind a screen.1,14,16 They found that MNs did indeed fire during the hidden condition portion of the experiment, but only if the monkey saw the object that was behind the screen before viewing the reaching movement (Figure 1). Conversely, MNs did not fire when the monkey was aware that there was no object behind the screen. This demonstrated that the monkey was able to understand or predict the outcome of the observed reaching movement, despite not being able to view the last portion of the movement. Other studies have shown that Area F5 MNs are also responsive to sound that is closely linked to the goal of an action that is familiar to the monkey (e.g., cracking peanuts, ripping paper).1,13–16 This can be interpreted as evidence that MNs are specialized for understanding action goals. The finding that there are MNs in Area F5 responsive to mouth-related movements (e.g., lip-smacking, lip or tongue protrusion) that are more related to communication than consumption led to the interesting proposal that communicative actions may have derived from other goal-directed actions.1 MNs have also been found in the inferior parietal lobule (IPL; Figure 1), an area that receives input from auditory, visual, and somatosensory cortices. Studies of these multimodal IPL MNs have reported that the majority are sensitive to the end-goal of an observed action sequence (e.g., eating or placing target), rather than the intermediate movements (e.g., grasping, arm movement).1,13–15 Thus, a major functional role of parietofrontal MNs may be to automatically understand the goals of motor acts performed by others, through a process of matching the observed acts to internal motor representations already stored in the individual’s motor repertoire.13 In addition to primates, MN-like responses have also been demonstrated in rodents and birds.14
The Mirror Neuron Sustem (MNS) in Humans
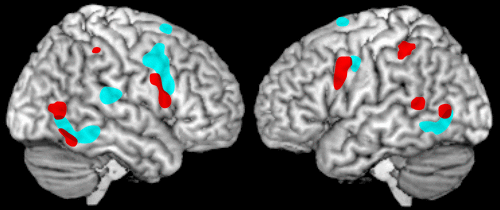
Although MNs were identified and characterized by direct measurement of the responses of individual neurons, such studies are rare in human research. There is, to date, a single electrophysiological study in patients confirming the existence of MNs in several brain regions.17 The non-invasive techniques commonly utilized in human research measure changes on a larger scale (i.e., not single neurons). Since only a portion of the neurons in any area are MNs, such studies necessarily are capturing the responses of many different types of neurons in addition to MNs. Thus, what is actually studied is whether an area responds in a mirror fashion, defined as reacting similarly to execution and observation of the same action. Such areas are considered to be part of the mirror neuron system (MNS), although the actual participation of MNs in the evoked responses cannot be confirmed by these techniques. Specifics vary across studies, but areas most commonly activated (in functional magnetic resonance imaging [fMRI] or positron emission tomography [PET]) by both action observation and action execution are generally similar to the parietofrontal areas identified in the monkey (Figure 2).2,18 Of concern, although comparison of matched action observation and action execution conditions is required for identification of areas with mirror properties, a recent metaanalysis of fMRI studies noted that only about one-third of the studies they reviewed included both conditions.18
From the beginning of MNS research in nonhuman primates, speculations were made about how this new-found class of neurons might play a role in the human brain and human interactions.7,8,13–16 The hypothesized function of the MNS is that it provides a mechanism for implicit understanding of the observed actions of others based on matching to one’s own motor repertoire. Thus, it would be predicted that actions that observers have stored in their own repertoire will more strongly activate the MNS, and that MNS activation should be altered by the observer’s experiences. In studies of humans observing actions of animals, MNS activation (fMRI) occurred in response to actions that humans also routinely perform (e.g., biting to eat) but not to species-specific actions (e.g., barking).13,15 Studies in several domains of expertise (e.g., pianists, dancers) have shown heightened MNS activations (fMRI) in skilled subjects as compared with unskilled subjects during observation of skilled movements.13,16,19 Similarly, skilled subjects demonstrated greater activations when observing familiar movements than with unfamiliar. The MNS is also activated during imitative learning.13,20 Longitudinal studies have demonstrated training-related changes, including a positive correlation between the level of skill in action execution and amount of MNS activation during action observation.16,19 Overall, these studies are compatible with the view that the experiences of an individual interacting with the external world form the basis for internal mental representations (e.g., embodied cognition), although not all investigators agree.9,11,12,16,21
In this view, the MNS supports implicit understanding of the actions of others, based on rapid, automatic matching of the observed actions to internally-stored action representations. This leads, in turn, to the proposal that the MNS might also support aspects of understanding of the emotional state of others and thus contribute to social cognition, particularly empathy.22,23 Human beings are social creatures, existing in a complex society, and observational learning plays a vital role in guiding individuals to conduct themselves appropriately.22,23 A key aspect of social interaction is recognition of the emotional aspects of another person’s actions (e.g., facial expressions, body language).15 There is growing evidence from functional imaging (fMRI, PET) that the motor (action) MNS plays a role in coding for experienced, observed, and imagined emotional states and behaviors (Figure 2).2,3,24,25 Also, neuroimaging studies (fMRI) have reported mirror-like activation in structures involved in emotional regulation, suggesting existence of an emotional (limbic) MNS (Figure 3).4,5,13,26
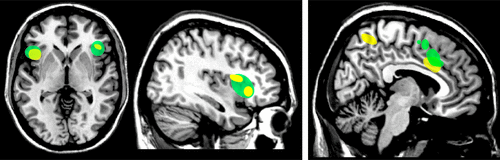
Empathy plays a major role in socially-appropriate emotional responses and behaviors. Although definitions for empathy vary, important components include a feeling of commonality (typically based on socially-shared emotional experiences) and cognitive mechanisms such as perspective-taking and understanding the difference between self and others during interpersonal interactions. Several studies (fMRI, PET) have used observation and imitation of emotional facial expressions (e.g., happy, sad, angry, surprised, disgusted, afraid) in order to identify areas of the brain that activate to both conditions, the minimum criterion for MNS involvement.2,3,24,25 A common finding across studies is that both conditions evoke statistically significant activation (greater for imitation than observation) within the premotor cortex. This finding supports the participation of at least the frontal portion of the motor MNS in automatic understanding of emotional facial expressions (Figure 2). MNS activations have also been reported to socially expressive body movement.27 Studies have varied in whether limbic-related areas (e.g., insula, amygdala) activated during imitation were also activated during observation. It has been postulated that the insula may be the conduit connecting the action MNS to areas important for emotion (e.g., the amygdala).24 As noted in one study, imitation of emotional facial expressions likely differs in important ways from spontaneous emotions.3 The neural correlates of empathic understanding can also be studied, using emotions that can be directly induced (e.g., pain, disgust). The areas consistently activated (fMRI) by both experiencing and observing another person experiencing one of these states are middle cingulate cortex and anterior insula/inferior frontal cortex (Figure 3).4,5,26 Evidence that limbic-related regions also have mirror properties supports the proposal that the MNS goes beyond just physical actions, but also plays a role in how individuals respond to emotion.
Clinical Implications
If the MNS is not functioning properly, it is plausible that individuals could have deficits in imitation and observational learning with regard to both physical actions and appropriate emotional responses, such as understanding facial expressions.28–30 The Autism Spectrum Disorders (ASD) have been characterized by such problems, including impaired social and emotional skills, which limit proper social interaction with others. It has been hypothesized that disorders on this spectrum are a result of an impaired MNS. Given the evidence for the MNS’s participation in learning through imitation, failure in the development of this system during early childhood could easily lead to development of social-cognitive deficits. Some studies have reported less MNS activity in individuals with ASD as compared with healthy-control groups, but contrary results have also been reported.28–30
It has been postulated that the MNS may be responsible for the pain relief experienced by many amputees after Mirror Therapy for phantom limb pain.31 The etiology of phantom limb pain is not entirely understood, but both peripheral and central mechanisms have been implicated.32,33 In the majority of cases, the abnormal sensations manifest immediately after waking from the procedure. However, phantom limb pain may also have late onset, months-to-years after the amputation. In as many as 70% of cases, phantom limb pain can persist for years or decades. Mirror Therapy has been shown to be an effective treatment for phantom limb pain for some patients.32 The individual performs simple movements while viewing a reflected image of their intact limb in a mirror positioned to give the illusion that they are viewing the amputated limb move. It is hypothesized that this visual input activates the MNS, correcting a central sensory mismatch caused by loss of sensation from the amputated limb.31–33 This approach has recently been extended by the successful use of sensory Mirror Therapy (illusory touch) in a case series of amputees who did not experience pain relief with movement-based Mirror Therapy (illusory movement).34
Knowledge of the MNS and how the observation of actions can recruit the same motor representations has sparked interest in using the MNS to enhance rehabilitative techniques.35,36 Motor deficiency is known to be a leading cause of disability after stroke. It has been proposed that activation of the MNS might be utilized to enhance restoration of function after stroke. Several preliminary studies support the efficacy of action-observation therapy, in which observation of video sequences containing hand and arm actions similar to those that would be used in daily life is followed by repetitive practice of the observed actions.35,36 In addition to improved motor function, fMRI performed before and soon after the end of the treatment indicated increased movement-related activations in the treatment group that were not found in the control group.37 Action-observation therapy is also being tested in other conditions, such as recovery from aphasia after stroke, to reduce motoric deficits in patients with Parkinson’s disease, and in rehabilitation after orthopedic surgery.35,36 Movement-based therapy designed to modulate affective state has also been proposed.38 Although still quite limited, these studies suggest that activation of the MNS could be beneficial in multiple clinical conditions.
Conclusions
There are many questions still to be answered about the MNS, and a wide range of opinions about its functional role(s).11,12,16,21,39 As noted in a recent editorial, publications related to these debates (reviews and position papers) considerably outnumber basic research reports.11 In the interplay between genetic and developmental influences, for example, some theories support primacy of genetics in determining the capacity of MNs to recognize and match observed actions (e.g., adaptation hypothesis), whereas others support the importance of exposure to sensory and motor experiences throughout the course of development (e.g., associative sequence learning hypothesis).40 There is increasing interest in understanding what role(s) the MNS may play in how humans connect with one another emotionally and how they understand the actions and intentions of others. Further research on the MNS may well provide insights into some aspects of psychiatric illness and have an impact on the treatment of certain medical conditions.
1 : Mirror neurons (and beyond) in the macaque brain: an overview of 20 years of research. Neurosci Lett 2013; 540:3–14Crossref, Medline, Google Scholar
2 : ALE meta-analysis of action observation and imitation in the human brain. Neuroimage 2010; 50:1148–1167Crossref, Medline, Google Scholar
3 : Functional imaging of face and hand imitation: towards a motor theory of empathy. Neuroimage 2004; 21:601–607Crossref, Medline, Google Scholar
4 : Is there a core neural network in empathy? An fMRI-based quantitative meta-analysis. Neurosci Biobehav Rev 2011; 35:903–911Crossref, Medline, Google Scholar
5 : Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 2011; 54:2492–2502Crossref, Medline, Google Scholar
6 : Understanding motor events: a neurophysiological study. Exp Brain Res 1992; 91:176–180Crossref, Medline, Google Scholar
7 : Action recognition in the premotor cortex. Brain 1996; 119:593–609Crossref, Medline, Google Scholar
8 : Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res 1996; 3:131–141Crossref, Medline, Google Scholar
9 : Critical review of the research leading to the mirror neuron paradigm, Biomed 2010. Biomed Sci Instrum 2010; 46:422–427Medline, Google Scholar
10 : Mirror neurons: enigma of the metaphysical modular brain. J Nat Sci Biol Med 2012; 3:118–124Crossref, Medline, Google Scholar
11 : Editorial: Mirror neurons are a class of neurons discovered by Rizzolatti and colleagues. Neurosci Lett 2013; 540:1–2Crossref, Medline, Google Scholar
12 : Mirroring mirror neurons in an interdisciplinary debate. Conscious Cogn 2013; (E-pub ahead of print)Crossref, Google Scholar
13 : The mirror neuron system. Arch Neurol 2009; 66:557–560Crossref, Medline, Google Scholar
14 : Evolution of mirror systems: a simple mechanism for complex cognitive functions. Ann N Y Acad Sci 2011; 1225:166–175Crossref, Medline, Google Scholar
15 : Interpreting actions: the goal behind mirror neuron function. Brain Res Brain Res Rev 2011; 67:260–267Crossref, Google Scholar
16 : A word in the hand: action, gesture, and mental representation in humans and non-human primates. Philos Trans R Soc Lond B Biol Sci 2012; 367:129–143Crossref, Medline, Google Scholar
17 : Single-neuron responses in humans during execution and observation of actions. Curr Biol 2010; 20:750–756Crossref, Medline, Google Scholar
18 : Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev 2012; 36:341–349Crossref, Medline, Google Scholar
19 : Sensorimotor learning and the ontogeny of the mirror neuron system. Neurosci Lett 2013; 540:21–27Crossref, Medline, Google Scholar
20 : Imitation and observational learning of hand actions: prefrontal involvement and connectivity. Neuroimage 2012; 59:1668–1683Crossref, Medline, Google Scholar
21 : What is so special about embodied simulation? Trends Cogn Sci 2011; 15:512–519Crossref, Medline, Google Scholar
22 : The “shared manifold” hypothesis: from mirror neurons to empathy. J Conscious Stud 2001; 8:33–50Google Scholar
23 : The manifold nature of interpersonal relations: the quest for a common mechanism. Philos Trans R Soc Lond B Biol Sci 2003; 358:517–528Crossref, Medline, Google Scholar
24 : Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A 2003; 100:5497–5502Crossref, Medline, Google Scholar
25 : Imitating expressions: emotion-specific neural substrates in facial mimicry. Soc Cogn Affect Neurosci 2006; 1:122–135Crossref, Medline, Google Scholar
26 : The neural basis of empathy. Annu Rev Neurosci 2012; 35:1–23Crossref, Medline, Google Scholar
27 : Social grasping: from mirroring to mentalizing. Neuroimage 2012; 61:240–248Crossref, Medline, Google Scholar
28 : Empathy: shared circuits and their dysfunctions. Dialogues Clin Neurosci 2010; 12:546–552Medline, Google Scholar
29 : The mirror mechanism and its potential role in autism spectrum disorder. Dev Med Child Neurol 2013; 55:15–22Crossref, Medline, Google Scholar
30 : Reflecting on the mirror neuron system in autism: a systematic review of current theories. Dev Cogn Neurosci 2013; 3:91–105Crossref, Medline, Google Scholar
31 : Sensations referred to a patient’s phantom arm from another subject’s intact arm: perceptual correlates of mirror neurons. Med Hypotheses 2008; 70:1233–1234Crossref, Medline, Google Scholar
32 : Phantom limb pain: theories and therapies. Neurologist 2010; 16:277–286Crossref, Medline, Google Scholar
33 : Phantom limb pain: mechanisms and treatment approaches. Pain Res Treat 2011; 2011:864605-Google Scholar
34 : An alternative to traditional mirror therapy: illusory touch can reduce phantom pain when illusory movement does not. Clin J Pain 2013; (E-pub ahead of print)Crossref, Medline, Google Scholar
35 : The mirror neuron system and treatment of stroke. Dev Psychobiol 2012; 54:293–310Crossref, Medline, Google Scholar
36 : Action observation versus motor imagery in learning a complex motor task: a short review of literature and a kinematics study. Neurosci Lett 2013; 540:37–42Crossref, Medline, Google Scholar
37 : Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage 2007; 36(Suppl 2):T164–T173Crossref, Medline, Google Scholar
38 : Emotion regulation through execution, observation, and imagery of emotional movements. Brain Cogn 2013; 82:219–227Crossref, Medline, Google Scholar
39 : Clarifying the role of the mirror system: interviewed by Gregory Hickok. Neurosci Lett 2013; 540:62–66Crossref, Medline, Google Scholar
40 : Where do mirror neurons come from? Neurosci Biobehav Rev 2010; 34:575–583Crossref, Medline, Google Scholar



