Marked Reduction in Serotonergic Activity in a Sexually Aggressive Adolescent Male
Abstract
Serotonergic dysfunctions are implicated in conduct disorder, impulsivity, and aggression. Early adverse experiences increase the risk for these behaviors in adolescents. The authors investigated serotonergic activity in one adolescent male who experienced maternal abandonment and childhood abuse and exhibited severely aggressive sexual offenses. Platelet serotonin (5-HT) concentration, [14C]-5HT uptake kinetics, and plasma prolactin, cortisol response to D,L-fenfluramine (D,L-FEN) were measured. Results showed extremely low 5-HT concentration (2.960.7 ng/108 platelets), [14C]-5HT uptake rate (0.560.04 mM/min/107platelets), undetectable Km and Vmax, and abnormally blunted prolactin, cortisol response to D,L-FEN. These abnormalities in this sexually aggressive adolescent may be a consequence of childhood abuse.
Several behavioral symptoms and syndromes, including impulsive, aggressive, and disruptive behaviors, conduct disorder, and attention deficit hyperactivity disorder (ADHD) are reportedly associated with disturbances in the serotonergic system. The evidence for serotonergic system abnormalities in both the peripheral as well as the CNS in children and adolescents with behavioral problems is derived from studies showing decreased 5-hydroxyindoleacetic acid (5-HIAA) concentrations in CSF, decreased [3H]-imipramine (serotonin transporter) binding sites in platelets, and abnormal neuroendocrine responses to D,L-fenfluramine challenge.1–3 A multitude of recent preclinical and clinical studies have revealed that early life stressors such as lack of nurturing and maternal separation as well as abuse result in persistent alterations in specific neurobiological systems.4,5 For example, maltreated children who develop antisocial behaviors as adults have been reported to exhibit low levels of gene expression of monoamine oxidase-A (MAOA).6,7
Among adolescent males, behavioral disorders, particularly, sexual assault is one of the fastest growing crimes in the United States and accounts for >7% of all violent sex crimes. According to recent reports by law enforcement agencies, 30%‒60% of all child sexual abuse is committed by juvenile sex offenders,8,9 2%‒4% of adolescent males over 12 years of age commit 20% of all rapes and 30%‒50% of all child molestations.10,11 The majority of male adolescent sexual offenders exhibit emotional and behavioral problems as well as a spectrum of personality trait disturbances.12,13 Evidence also suggests that sexually abusive youth mirror the population and generally come from all racial, economic, ethnic, and religious background.14
The etiology of sexual aggression among adolescents is multifactorial and may include sociocultural and poor parental supervision, emotional and developmental problems, disrupted parent-child attachment, parental neglect and/or abuse, exposure to domestic violence, physical and sexual abuse during childhood and adolescence, multiple placements during childhood, and lack of access to social and medical services.15 These adverse sociocultural events during the early developmental years of a child likely contribute to abnormal neural circuits in the CNS,16 including the serotonergic, and HPA axis systems.4–7
Serotonergic system dysfunction has repeatedly been associated with aggressive and violent behaviors in adults. There are multiple reports of decreased CSF 5-HIAAconcentrations in adults with history of aggression, poor impulse control, and violence.17–21 Moreover, altered indices of serotonergic and noradrenergic activity in the periphery and CNS, including decreased 5-HT concentration in whole blood and platelets, decreased 5-HT uptake and [3H]-imipramine binding sites in platelets and brain have been associated with affective disorders, hyperactivity as well as post-traumatic stress disorder among adolescents and adults.22–24 One area for which there is paucity of evidence for neurotransmitters dysfunction, particularly that of the serotonergic system is in sexually aggressive adolescents or even adults. Our group has investigated serotonergic activity in children/adolescents with sexually offending behaviors. One adolescent male in the group who exhibited severely aggressive and sexually abusive behaviors was exposed during infancy and childhood to extreme early life stress, including physical abuse, parental drug and alcohol abuse, domestic violence, and maternal abandonment. In this report, we present our findings of a markedly abnormal profile of peripheral and central serotonergic system in this adolescent male with history of sexually aggressive behaviors.
Methods
Subject
An adolescent white male, age 15 years, with a long history of aggressive sexual offenses and disruptive behavior was referred for a psychiatric evaluation. He fulfilled the DSM-IV diagnostic criteria for conduct disorder and ADHD. His aggressive behaviors included sexual assaults against his two 14- and 16- year-old sisters, and an 11-year-old male cousin. He also had a history of destroying property, aggravated assault against older persons, including a school teacher, and a psychologist. He had a history of substance abuse, including alcohol, marijuana, and had a reported history of school suspension. He had multiple involuntary psychiatric admissions for aggressive conduct. On clinical examination, he was cooperative, surprisingly insightful, and expressed a wish to be able to control his anger. He had a normal physical examination and screening laboratory tests, thyroid function, and urine metabolic tests.
His early family life was characterized by chaos and instability. His parents had separated before he was born, and his biological mother had a history of alcoholism and mental instability. She was living with another man at the time of his birth, and she immediately abandoned him. In his childhood, he was physically abused by his biological father, who had a history of sexually aggressive and violent behaviors, and drug and alcohol abuse. His father was incarcerated for rape of his own 14-year-old daughter.
The patient was admitted to the residential treatment program at the University of Miami Miller School of Medicine/Jackson Mental Health Hospital where he remained for 15 months. He was one of the participants in a study involving a group of adolescent males with conduct disorder who were also sex offenders. All participants were admitted to the UM/Jackson residential treatment program, with no difference in the environment, and were served the same diet prepared in the residential facility. The study was approved by the Institutional Review Board of the University of Miami, and informed consent was obtained from the participants and the guardians of all participants, including our patient. However, our patient was found to be different from other participants with regards to the severity of his sexual assaults, impulsivity, and aggressiveness. He required seclusion and restraints on multiple occasions because of explosive outbursts and physical attacks on others. His disruptive behavior symptoms were partially alleviated by a combination of psychotropic medications, including treatment with carbamazapine (300–600 mg/day), thioridazine (50–100 mg/day), and imipramine (50 mg/day), and a well-designed structured behavior management program, but his sexually aggressive and offending behaviors did not improve. His treatment with psychotropic medication was discontinued, and after the required wash out period (>2 weeks), he was treated with fluoxetine (20 mg/day). Although fluoxetine transiently improved his irritability and impulsivity and he showed interest and motivation in the treatment, he continued to have intense compulsive sexual thoughts and urges, with no improvement in his sexually aggressive behaviors. He was described as “resistant and avoidant”, having great difficulty in modulating his anger, and his treatment with fluoxetine was discontinued.
The neuropsychological evaluation revealed that he had a Full Scale IQ score of 83 (WISC-III), which fell in the below average range. His verbal IQ (VIQ) was 91, and his performance IQ (PIQ) score was 78. His emotional and behavioral functioning was characterized by dysphoria and intense anger. His responses to The Robert's Apperception Test were “violent and aggressive.” He had developmental reading and expressive writing disorders (Wide Range Achievement Test, WRAT) and was diagnosed as having conduct disorder, solitary aggressive type. There was no evidence of psychosis.
After discontinuation of fluoxetine for the recommended washout period (>2 weeks), and before institution of other pharmacological interventions, we studied the patient’s serotonergic, and neuroendocrine systems.
Procedures
Assessment of serotonergic activity in our patient and other adolescent male participants (N=37) was performed after explaining the study protocol to them and to their guardian and obtaining informed consent. Because platelets are considered a peripheral model for CNS serotonergic neurons,25 we determined platelet serotonergic function including measurement of 5-HT concentration and platelet 5-HT uptake kinetics (Km and Vmax) using platelet rich plasma (PRP), freshly prepared by low speed centrifugation of fasting blood samples collected in tubes containing acid citrate dextrose (ACD) as described earlier.26,27 Platelet free plasma (PFP) was prepared by centrifugation of PRP at 3500 rpm. Since we found markedly reduced concentration of 5-HT in platelets of our patient, and also found abnormality in his platelet [14C]-5-HT uptake kinetics, we assessed CNS serotonergic activity only in this patient by measuring the prolactin (PRL) and cortisol (CORT) response to D,L-fenfluramine (D,L-FEN), using the protocol described by Siever et al.28 D,L-FEN stimulates the presynaptic release of 5-HT and inhibits its reuptake by 5-HT transporter, resulting in a net increase in the synaptic concentration of 5-HT which in turn stimulates PRL and CORT release.29,30 An attenuated response reflects pre/and or post-synaptic 5-HT dysfunctions.31,32
Laboratory Procedures
Platelet 5-HT concentration was measured in PRP, and PFP of all participants (N=37) before treatment with any medication, including aspirin, fluoxetine, or other psychotropics. After overnight fasting, blood samples were obtained between 8 and 10 a.m. after resting for 15 minutes. PRP was prepared as described above. Aliquots of PRP and PFP were stored at –70°C until assayed for 5-HT by HPLC-ECD,26 while a 100 µl aliquot of PRP from 17 participants, including one from our patient was used within 2 hours to measure [14C]-5-HT uptake and kinetics, as described earlier.27 Values of Km and Vmax were determined by Lineweaver-Burke plot.33 Because we found deviant values of platelet 5-HT functions in the first blood sample of our patient, we obtained a second sample after 6 months while he was kept free of any psychotropic medications, including fluoxetine. The blood sample was processed for preparation of PRP and PFP, and aliquots were used for assays of 5-HT concentration, 5-HT[14C]-5-HT uptake and kinetics, as described above.
For D, L-FEN challenge in our patient, baseline levels of hormones (PRL, CORT) were measured in two blood samples obtained in the morning at 15 minutes intervals in heparinized tubes, prior to D,L-FEN (1.0 mg/Kg) administration and five more blood samples were obtained, each at 1 hour interval (60–300 minutes) after D,L-FEN administration. All samples were kept in ice until processed for preparation of plasma, and aliquots were stored at –70°C until assayed for PRL and CORT.
Prolactin concentration in plasma was measured by the noncompetitive immune-radiometric (IRMA) assay kits obtained from Diagnostic Systems Laboratory (DSL, Webster, TX). Intra- and inter-assay % CV for PRL are 3.5% and 8%, respectively, and sensitivity is 0.1 ng/ml. Cortisol was measured by the radioimmunoassay (RIA) method using kits from Diagnostic Products Company (DPC, Los Angeles, CA). Intra-and inter-assay coefficients of variance (%CV) for CORT are 8.2% and 9.8%, respectively, and sensitivity is 0.5 µg/dl.
Statistical Analysis
Analysis of variance (ANOVA) was used to determine the mean and SD for 5-HT and hormones levels in PRP and plasma samples obtained at different time points. Student’s t test was used when applicable to examine the significance of difference in means of platelet 5-HT levels and uptake profile between our patient and the other boys of similar age in the study group.
Results
We found markedly reduced 5-HT concentration in both samples of PRP as well as PFP of our patient (obtained within a 6 months interval), compared with that in the other participants in the study who were admitted in the same residential program for treatment of conduct disorder and sex offending behaviors (Table 1, platelet 5-HT, our patient 2.4, 3.4, mean±SD=2.90±0.7 ng/108 platelets, versus other participants, 121.1±47.57 ng/108 platelets, N=36; p<0.0001; and plasma 5-HT, our patient, 0.9 and 2.0 ng/ml, mean±SD=1.45±0.77/ml versus other participants, 2.18±1.83/ml, N=36, p <0.01 (our unpublished data) as well as plasma 5-HT levels reported in other studies 27.68±32.29/ml66), although the number of platelets/ml blood in our patients were not different from the other participants (mean±SD=2.61±0.00 versus 2.68±0.49/ml PRP, N=36, p=NS, our unpublished data). Moreover, kinetics of 5-HT uptake in our patient’s platelets were found to be strikingly abnormal with the rate of total as well as active [14C]-5-HT uptake exhibiting extremely low values (0.5±0.04/107platelets,Table1). At 0.5 µM concentration of [14C]-5-HT, the rate of active uptake (pmoles/minute/107platelets) in two samples of PRP obtained from our patient at 6 months interval, and incubated at 37°C for 4 minutes was essentially the same as passive diffusion of [14C]−5-HT in his PRP incubated at 0°C for 4 minutes (Figure 1); and the uptake kinetics, Km and Vmax could not be determined from the Lineweaver-Burke plot.33 Whereas, in other boys in the study group (N=16), the rate of active uptake of [14C]-5-HT in platelet at 0.5 µM concentration was more than 10 times higher (5.17±1.32 pmoles/min/107 platelets) and uptake kinetics determined by Lineweaver-Burke plot were, Km= 1.12±0.57 µM, and Vmax=15.6±5.62, N=16 (our unpublished data, Table1). Since all participants shared the same residential facility and were served the same diet, there were no environmental and dietary differences between our patient and other adolescent males in the group that could have influenced the observed serotonergic abnormalities in our patient.
| Type of Measure | Our Patient | Adolescent Boys With CD (Participants in This Study) Age, 9–14 years, | Adolescent Boys (Other Studies) | Adults |
|---|---|---|---|---|
| Platelets # ×108/ ml PRP | 2.61±0.00 (N=2) | 2.68±0.49 (N=36) (Our unpublished data) | 1.5–4.5 | 2.58±0.72 (normal) |
| Platelet 5-HT, ng/108 (mean±SD) (values=mean of two blood samples drawn from our patient at 6 months interval) | 2.4, 3.4 | 37.7±23.7 (depressed39; | 60–150 (normal)40 | |
| 2.9±0.7 | 121.1±47.57 ng/108 platelets (N=36) | 250.0±140, N=10 (ADHD,-aggressive)35 | 30–80 (Depressed)40 | |
| (N=2) | (Our unpublished data) | 220.0±110.0, N=15 (ADHD, non-aggressive)34,35 | ||
| Plasma 5-HT, ng/ml (mean±SD) | 0.9, 2.0 | 2.18±1.83, N=36 | 7.76±4.23 (normal controls, 14–19 years (p=0.05)66 | 3.4±2.7 (normal)26 |
| 1.45±0.77 (N=2) | Our unpublished data | 27.68±32.29 (N=10)66 (14–19 years, boys with CD) | ||
| 14C–5-HT, Rate of uptake at 0.5 µM | 0.5±0.04 (N=2) | 1.98–6.91 (5.17±1.32) (N=16) | N/A | 4.07±1.68 (normal)27 |
| Km µM (pmoles/min/107 platelets | ND | 1.12±0.57 (N=16) | N/A | 0.57±0.38 (normal)27 |
| Vmax, pmoles/min/107 platelets | ND | 15.6±5.62 (N=16) (Our unpublished data) | 8.45±4.63 (normal children)67 | 9.30±5.70 (normal)27 |
| 4.27±3.49 (children with CD)67 | ||||
| Prolactin, ng/ml (baseline) | 14.3±3.6 | N/A | 6.4±2.6 (Age, 14.7±1.4)3 | 6.7–8.568 |
| 5.64±1.6634 | ||||
| 5.70±1.4034 | ||||
| Cortisol, µg/dl (baseline) | 26.8±5.2 | N/A | 8.2±1.5 (N=8)3 | 13.3±3.0848 |
| (age 14.7±1.4) | 16.30±3.6246 |

Active 14C-5-HT uptake was determined by subtracting passive diffusion (obtained by incubating platelets at 0°C for 4 minutes) from that of the total 14C-5-HT uptake (obtained by incubating platelets at 37°C for 4 minutes). The concentration range of 14C-5-HT during both incubations was 0.1 to 1.0 μM.
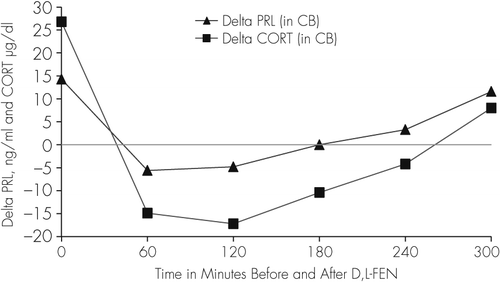
The baseline plasma concentration of both PRL and CORT, as well as the response to D, L-FEN (Table 2) in our patient was abnormal showing higher baseline levels (two baseline PRL levels, T1=16.1, T2=12.5 ng/ml; CORT,T1=29.4, T2=24.2), and an abnormally blunted response to D,L-FEN administration, in contrast to that reported earlier in aggressive children, and normal and depressed adults.3,28 Delta (∆) values and percent (%) change in PRL and CORT levels were determined as the difference between the average of two baseline levels of PRL (14.3 ng/ml) as well as CORT (26.8 µg/dl) and the hormone levels in each of the five samples obtained at 1-hour interval (60–300 minutes) after D, L-FEN administration. We found that at 60–180 minutes post-D,L-FEN administration, levels of both PRL and CORT decreased from the baseline, followed by a rise in PRL levels only at 240 and 300 minutes after D,L-FEN; when CORT levels continued to decrease between 60–240 minutes and increased by approximately 30% only at 300 minutes (Table 2, Figure 2).
| (Post D,L-FEN, time in minutes) | ||||||
|---|---|---|---|---|---|---|
| Baseline | 60' | 120' | 180' | 240' | 300' | |
| PRL ng/ml | 1 4.3 | 8.7 | 9.5 | 14.3 | 17.6 | 25.9 |
| ∆PRL | –5.6 | –4.8 | 0.0 | +3.3 | +11.6 | |
| (% change from baseline) | –39.0 | –33.0 | 0.0 | +23.0 | +81.1 | |
| CORT (µg/dl) | 26.8 | 11.9 | 9.0 | 16.4 | 22.6 | 34.8 |
| ∆CORT | –14.9 | –17.0 | –10.4 | –4.2 | +8.0 | |
| (% change) | –55.6% | –66.4% | –38.8% | –15.6% | +29.8 | |
Discussion
This report documents a markedly abnormal serotonergic system in an adolescent white male with a history of sexual aggression and violent behavior. Serotonergic deficits were evident by very low concentration of 5-HT in his platelet, as well as low rate of platelet [14C]-5-HT uptake, and undetectable levels of uptake kinetics, Km and Vmax, in the face of a normal platelet counts. Deficits were also observed in central serotonergic activity as demonstrated by the high baseline plasma levels of both PRL and CORT and in the clearly abnormal response to D,L-FEN administration.
Low concentrations of platelet 5-HT (2.9 ng/108 platelets) in our patient was an intriguing finding because such low levels have not been reported earlier in normal, depressed, or aggressive children, adolescents, or adults with behavioral problems. Moreover, other boys in the study group with CD and sex offending behavior had higher platelet 5-HT concentration (70–160 ng/108 platelets (121.1±47.57, N=36, our unpublished data, Table 1). Previous studies have also reported higher 5-HT concentration (250±140 and 220±110 ng/ml PRP) in adolescent boys with conduct disorder, ADHD and aggressive and nonaggressive behaviors (Table 1).34 In contrast, higher 5-HT levels in whole blood were reported in children with hyperactivity and autistic disorder (228–309 ng/ml) and in adolescents <16 years old without autistic disorder (200–230 ng/ml).35–38 However, in separate studies carried out in adults and adolescents with depression, we found lower range of platelet 5-HT concentrations (30–80 ng/108 platelets, and 37–65 ng/108 platelets, respectively)39 compared with that in normal adults (60–150 ng/108 platelets).40,41
In addition, platelet [14C]-5HT uptake mechanisms in our patient were found abnormal with values of active uptake too low for even calculation of Km and Vmax.33 The rate of active uptake of [14C]-5-HT in this patient’s platelets incubated at 37°C remained essentially the same as passive diffusion of [14C]-5-HT in his PRP incubated at 0°C (Figure 1), suggesting the absence of platelet 5-HT transporter activity. In fact, the rate of active [14C]-5-HT uptake in our patient’s platelets was even lower than that of passive diffusion of [14C]-5-HT in platelets of a typical sample of an age matched normal adolescent male in the group with significantly higher rate of active [14C]-5-HT uptake than the passive diffusion, as normally expected (Figure 1). These findings suggest that our patient’s platelets were severely dysfunctional with respect to 5-HT transporter activity. However, it is unclear whether the low levels of platelet 5-HT were due to impairment in 5-HT reuptake mechanism(s) or due to a deficiency of serotonin per se. Although our observations support the earlier findings of reduced [3H]-imipramine binding sites on platelets of children with conduct disorder,2 reduced density of platelet 5-HT2A receptors in delinquent adolescents,42 and in boys with adverse rearing and familial psychopathology of antisocial disorder,43 to our knowledge, this report is the first to document such low levels of 5-HT in platelets (2.9 ng/108 platelets) and undetectable platelet [14C]-5HT uptake kinetics (Km and Vmax) in a male adolescent with indiscriminating behaviors of sexual assaults.
Our findings of dysfunctional peripheral 5-HT system were also supported by the abnormalities we observed in the CNS 5-HT activity as inferred from the baseline levels of both PRL (14.3 ng/ml) and CORT (26.8 µg/dl), which were 2–3 times higher than the levels reported in adolescent boys having disruptive and aggressive behaviors (PRL, ng/ml=5.64±1.66, 5.70±1.4; and CORT, µg/dl=7.6±3.6) and in those with adverse rearing conditions3,34,44 and in prepubertal aggressive boys.45,46 Baseline levels of PRL and CORT in these studies ranged between 4 and 8 ng/ml and 8 and 14 µg/dl, respectively. Higher baseline plasma levels of both PRL and CORT suggest in part disturbance in serotonergic regulation of neuroendocrine functions.
Moreover, the PRL and CORT response to D,L-FEN challenge was blunted in our patient. A normal response to D,L-FEN administration is characterized by an increase in PRL and CORT concentrations 60 minutes post D,L-FEN administration with the response peaking at 180 or 240 minutes, with a plateau or initial fall by 300 minutes.31,47 Blunted PRL responses to D,L-FEN have been found in adults with depression,28 and in adolescent males with antisocial and aggressive behaviors.44,46 In this adolescent male, there was a negative response of PRL and CORT to D, L-FEN for 240 minutes, and only a marginal increase occurred at 300 minutes (Figure 2; Table 2). This type of negative and delayed response profile of PRL and CORT after D,L-FEN administration has not been reported in children or adolescent boys with aggressive behaviors or in adults with depression or personality disorders.3,28,31,44–52 This abnormal response of PRL and CORT to D,L-FEN in this patient may be caused by a reduced availability of 5-HT in the CNS. To further delineate the mechanisms associated with 5-HT deficiency and its role in behaviors of adolescent sex offenders, future investigations are warranted in a larger cohort on various indices of 5-HT functions such as availability of circulating tryptophan, genetic characteristics of enzymes and proteins involved in 5-HT synthesis (tryptophan hydroxylase, 5-hydroxytryptophan decarboxylase), 5-HT receptors and their characteristics, 5-HT transporter activity, and monoamine oxidase (MAO). Perhaps more importantly, studies with positron emission tomography (PET) with [18F]-fluoroglucose to assess glucose metabolism or blood flow in response to fenfluramine challenge may help in evaluating dysfunctional serotonergic activity in specific brain regions (orbital frontal and cingulate cortex, raphe nuclei, and amygdala) of adolescent sex offenders, as reported in recent studies in patients with impulsive and aggressive behaviors.53 Also, application of single photon emission computed tomography (SPECT) in human brain imaging using [123]β-CIT (iodine-123- β –carbomethoxy-3 β-(4-iodophenyltropane) as reported in recent studies54,55 to assesses serotonin transporters and 5-HT2 receptor binding in different brain regions would be particularly innovative in this population. These studies may reveal the reduced serotonergic functions in critical brain regions that may be playing a role in modulating aggression and sex offending behavior in adolescent males.
The reduced 5-HT functions observed in this patient may be linked to his early life abuse, neglect, and maternal abandonment. Child abuse and neglect have been implicated in the development of dysfunctional neurobiological systems,16 and children with history of neglect, maltreatment, abuse and poor parent-child emotional bonding have been found to develop abnormal and aggressive behaviors.52–59 Adverse childhood experiences increase the prevalence rate of major psychiatric disorders in adulthood. 60 Furthermore, among the neurobiological modifications found in a cohort of maltreated children is the low levels of monoamine oxidase-A (MAOA) gene expression; these individuals developed to adulthood with antisocial behaviors, compared with the maltreated children who had higher levels of MAOA gene expression.6,7 These findings suggest that antisocial behavior are related to low levels of MAOA gene expression (MAOA metabolizes 5-HT and catecholamines, NE and dopamine).
Studies in nonhuman primates are concordant with the view that early childhood psychosocial environmental stressors produce changes in the development of serotonergic circuits associated with aggressive behavioral patterns, analogous to human aggression.61,62 Experimental studies in rats also reveal that early postnatal handling or non-handling of infants by the mother, and adverse environmental conditions affect the development of different brain regions, including that of the serotonergic system and its regulatory functions in the hippocampus.61–63 It has been suggested that 5-HT modulates aggression by inhibiting behavioral responses to environmental stimuli,64,65 and regulates the HPA axis for adaptability and responses to various stressors.66–68 At present, our knowledge regarding the neurobiological etiology of sexual aggression is limited and warrants further investigations in a larger cohort of children exposed to adverse childhood experiences, particularly the type of rearing environment experienced by our patient.
1 : A 2-year prospective follow-up study of children and adolescents with disruptive behavior disorders. Prediction by cerebrospinal fluid 5-hydroxyindoleacetic acid, homovanillic acid, and autonomic measures? Arch Gen Psychiatry 1992; 49:429–435Crossref, Medline, Google Scholar
2 : Reduction of (3H)-imipramine binding sites on platelets of conduct-disordered children. Neuropsychopharmacology 1987; 1:55–62Crossref, Medline, Google Scholar
3 : Neuroendocrine responses to challenge with dl-fenfluramine and aggression in disruptive behavior disorders of children and adolescents. Psychiatry Res 1992; 43:263–276Crossref, Medline, Google Scholar
4 : Neurobiological consequences of childhood trauma. J Clin Psychiatry 2004l; 65(Suppl 1):18–28Medline, Google Scholar
5 : Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol 2000; 12:5–12Crossref, Medline, Google Scholar
6 : Role of genotype in the cycle of violence in maltreated children. Science 2002; 297:851–854Crossref, Medline, Google Scholar
7 : MAOA, abuse exposure and antisocial behaviour: 30-year longitudinal study. Br J Psychiatry 2011; 198:457–463Crossref, Medline, Google Scholar
8 : Characteristics of youth who sexually offend. J Child Sex Abuse 2004; 13:15–32Crossref, Medline, Google Scholar
9
10 Finkelhor D, Ormrod R, Chaffin M. Office of Juvenile Justice and Delinquency Prevention (OJJDP): Juveniles who commit sex offenses against minors. U.S. Department of Justice, Office of Justice Programs News Bulletin, Dec 2009Google Scholar
11 Barbaree HE, Marshall WL: An introduction to the Juvenile sex offender: terms, concepts and definitions, 2nd ed. New York, Guildford Press 2008Google Scholar
12 Hunter JA, Becker JV: Motivators of adolescent sex offenders and treatment perspectives: Phenomenology and assessment of sexually aggressive behavior and treatment considerations, in Sexual Aggression. Edited by Shaw J. American Psychiatry Press, Inc, Washington, DC, 1999, pp 211–233Google Scholar
13 Shaw JA: Male adolescent sex offenders, in Sexual Aggression. Edited by Shaw J, American Psychiatric Press, Inc, Washington, DC, 1999, pp169–193Google Scholar
14 : Trends in a national sample of sexually abusive youths. J Am Acad Child Adolesc Psychiatry 1996; 35:17–25Crossref, Medline, Google Scholar
15 : Testing a mediational model of sexually aggressive behavior in nonincarcerated perpetrators. Violence Vict 1998; 13:117–130Crossref, Medline, Google Scholar
16 : How trauma and attachment can impact neurodevelopment: Informing our understanding and treatment of sexual behavior problems. J Sex Aggress 2009; 15:261–273Crossref, Google Scholar
17. : Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1979; 1:131–139Crossref, Medline, Google Scholar
18 : Neurotransmitter correlates of impulsive aggression in humans. Ann N Y Acad Sci 1996; 794:82–89Crossref, Medline, Google Scholar
19 : 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry 1976; 33:1193–1197Crossref, Medline, Google Scholar
20 : Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 1983; 33:2609–2614Crossref, Medline, Google Scholar
21 : Cerebrospinal fluid monoamine metabolite levels in male arsonists. Arch Gen Psychiatry 1987; 44:241–247Crossref, Medline, Google Scholar
22 : 5-Hydroxytryptamine (5-HT) in the whole-blood of patients with depressive illness. Postgrad Med J 1976; 52:156–158Crossref, Medline, Google Scholar
23 : Tritiated imipramine binding sites are decreased in platelets of untreated depressed patients. Science 1980; 209:303–305Crossref, Medline, Google Scholar
24 : Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry 1995; 38:174–179Crossref, Medline, Google Scholar
25 : Blood platelets as a model for monoamine-containing neurones. Prog Neurobiol 1973; 1:151–198Crossref, Medline, Google Scholar
26 : A modified HPLC technique for simultaneous measurement of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in cerebrospinal fluid, platelet and plasma. Life Sci 1990; 47:1751–1759Crossref, Medline, Google Scholar
27 : Serotonin uptake and its kinetics in platelets of women with Alzheimer’s disease. Psychiatry Res 1995; 59:145–150Crossref, Medline, Google Scholar
28 : Plasma prolactin changes following fenfluramine in depressed patients compared to controls: an evaluation of central serotonergic responsivity in depression. Life Sci 1984; 34:1029–1039Crossref, Medline, Google Scholar
29 : The mechanism of action of fenfluramine. Postgrad Med J 1975; 51(Suppl 1):27–35Medline, Google Scholar
30 : Prolactin secretion in man: a useful tool to evaluate the activity of drugs on central 5-hydroxytryptaminergic neurones. Studies with fenfluramine. Br J Clin Pharmacol 1983; 16:471–475Crossref, Medline, Google Scholar
31 : Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry 1989; 46:587–599Crossref, Medline, Google Scholar
32. : Neuropsychopharmacological challenge in biological psychiatry. Clin Chem 1994; 40:319–327Google Scholar
33 : The determination of enzyme dissociation constants. J Am Chem Soc 1934; 56:658–666Crossref, Google Scholar
34 : Serotonergic function in aggressive and nonaggressive boys with attention deficit hyperactivity disorder. Am J Psychiatry 1994; 151:243–248Crossref, Medline, Google Scholar
35 : Age-related changes in the association between serotonergic function and aggression in boys with ADHD. Biol Psychiatry 1997; 41:682–689Crossref, Medline, Google Scholar
36 : Plasma free and total tryptophan, blood serotonin, and the hyperactivity syndrome: no evidence for the serotonin deficiency hypothesis. Biol Psychiatry 1981; 16:231–238Medline, Google Scholar
37 : The effect of pyridoxine hydrochloride on blood serotonin and pyridoxal phosphate contents in hyperactive children. Pediatrics 1975; 55:437–441Medline, Google Scholar
38 : Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biol Psychiatry 1999; 45:158–163Crossref, Medline, Google Scholar
39 : Neurochemistry and paroxetine response in major depression. Biol Psychiatry 1995; 37:417–419Crossref, Medline, Google Scholar
40 : Nefazodone treatment of adolescent depression: an open-label study of response and biochemistry. Ann Clin Psychiatry 2000; 12:97–100Crossref, Medline, Google Scholar
41 : Peripheral serotonin levels in women: role of aging and ethnicity. Gerontology 1998; 44:211–216Crossref, Medline, Google Scholar
42 : Reduction in serotonin 5HT2 receptor binding on platelets of delinquent adolescents. Psychopharmacology (Berl) 1995; 118:354–356Crossref, Medline, Google Scholar
43 : Platelet serotonin 2A (5-HT2A) receptor characteristics and parenting factors for boys at risk for delinquency: a preliminary report. Am J Psychiatry 1996; 153:538–544Crossref, Medline, Google Scholar
44 : Neuroendocrine response to fenfluramine challenge in boys. Associations with aggressive behavior and adverse rearing. Arch Gen Psychiatry 1997; 54:839–846Crossref, Medline, Google Scholar
45 : Neurotransmitter-hormonal responses to psychological stress in peripubertal subjects: relationship to aggressive behavior. Life Sci 1998; 62:617–625Crossref, Medline, Google Scholar
46 : Blunted prolactin responses to d-fenfluramine in sociopathy. Evidence for subsensitivity of central serotonergic function. Br J Psychiatry 1992; 160:643–646Crossref, Medline, Google Scholar
47 : Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology 1995; 13:53–64Crossref, Medline, Google Scholar
48 : Adrenocortical response to ovine corticotropin-releasing hormone in young men: cortisol measurement in matched samples of saliva and plasma. Horm Res 2005; 64:55–60Crossref, Medline, Google Scholar
49 : The serotonin system and aggressive personality disorder. Int Clin Psychopharmacol 1993; 8(Suppl 2):33–39Crossref, Medline, Google Scholar
50 : Familial correlates of reduced central serotonergic system function in patients with personality disorders. Arch Gen Psychiatry 1994; 51:318–324Crossref, Medline, Google Scholar
51. : Neurotransmitter-neuroendocrine responses to experimentally induced aggression in humans: influence of personality variable. Psychiatry Res 1997, 66:33–43Google Scholar
52 : A developmental perspective on antisocial behavior. Am Psychol 1989; 44:329–335Crossref, Medline, Google Scholar
53 : d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 1999; 20:413–423Crossref, Medline, Google Scholar
54 : SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT binding kinetics in human brain. J Neural Transm 1993; 94:137–146Crossref, Medline, Google Scholar
55 : Neuropharmacological studies with SPECT in neuropsychiatric disorders. Nucl Med Biol 2000; 27:677–682Crossref, Medline, Google Scholar
56 : From abuse to violence: psychophysiological consequences of maltreatment. J Am Acad Child Adolesc Psychiatry 1992; 31:383–391Crossref, Medline, Google Scholar
57 : Subjective assessment of relationship with parents by sexually aggressive and non-aggressive men. J Interpers Violence 1994; 9(Supp. 3):399–411Crossref, Google Scholar
58 : A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and biogenic amine metabolites in rhesus monkeys. Neuropsychopharmacology 1989; 2:175–189Crossref, Medline, Google Scholar
59 : Adverse early experiences affect noradrenergic and serotonergic functioning in adult primates. Biol Psychiatry 1994; 35:221–227Crossref, Medline, Google Scholar
60 : The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 2006; 256:174–186Crossref, Medline, Google Scholar
61 : The effects of early postnatal handling on the development of hippocampal glucocorticoid receptors: Temporal parameters. Brain Res Dev Brain Res 1985; 22:301–304Crossref, Google Scholar
62 : The role of serotonin in the development and environmental regulation of type II corticosteroid receptor binding in rat hippocampus. Brain Res Dev Brain Res 1990; 55:231–235Crossref, Medline, Google Scholar
63 : Neonatal handling alters serotonin turnover and serotonin type 2 receptor density in selected brain regions: relationship to the handling effect on glucocorticoid receptor expression. Brain Res Dev Brain Res 1994; 80:183–189Crossref, Medline, Google Scholar
64 : Human aggression and the role of central serotonin. Pharmacopsychiatry 1985; 18:218–221Crossref, Medline, Google Scholar
65 : Denosologization of biological psychiatry or the specificity of 5-HT disturbances in psychiatric disorders. J Affect Disord 1987; 13:1–8Crossref, Medline, Google Scholar
66 : Platelet poor plasma serotonin level in delinquent adolescents diagnosed with conduct disorder. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33:1223–1225Crossref, Medline, Google Scholar
67 : Platelet 5-HT uptake in boys with conduct disorder. Neuropsychobiology 2004; 50:244–251Crossref, Medline, Google Scholar
68 : Plasma prolactin, adrenocorticotrophic hormone and cortisol after administration of d-fenfluramine or placebo to healthy subjects. Int Clin Psychopharmacol 1993; 8:123–128Crossref, Medline, Google Scholar



