A Rare Association of Fahr’s Disease With an Autoimmune Triad
To the Editor: Fahr’s disease is a rare neurodegenerative disorder characterized by idiopathic bilateral basal ganglia calcifications associated with neuropsychiatric and cognitive impairment. It was described in 1930 by Fahr in a 55-year-old patient who died after a series of tetanic seizures. However, Delacour was reported to describe calcification of basal ganglia as early as 1850.1 The disease can manifest by a variety of movement disorders such as dystonia, ataxia, and parkinsonism, along with cognitive impairment and behavioral changes.1 Most cases display autosomal dominant transmission; however, the exact etiology still remains unclear. The typical age of onset of clinical symptoms can range between 30 and 60 years. We report an unusual association of this disease in a patient with three autoimmune diseases.
Case Report
A 47-year-old woman presented with a 2-year history of progressive dysphagia, dysphonia, and bilateral weakness of the upper and lower extremities. Her medical history was significant for insulin-dependent diabetes mellitus, myasthenia gravis, and idiopathic thrombocytopenia. She was treated for myasthenia gravis with oral prednisone and pyridostigmine. Unfortunately, her symptoms continued to progress and eventually she developed cognitive impairment. A CT scan of the brain was performed, which revealed extensive bilateral calcifications of basal ganglia, thalamus, cerebral white matter, brain stem, and cerebellum (Figure 1). The patient was diagnosed as having Fahr’s disease. Her serum calcium and alkaline phosphatase levels were normal. No infectious, toxic, or traumatic cause was identified. There was no known family history of Fahr’s disease; however, her daughter was diagnosed with rheumatoid arthritis. Interestingly, the patient’s idiopathic thrombocytopenia over the years became relatively refractory to therapies, requiring multiple inpatient hospitalizations for her worsening thrombocytopenia. She was treated for idiopathic thrombocytopenia with steroids, intravenous immunoglobulin, and a splenectomy. Fortunately, the patient never had any episode of major bleeding. Her most recent treatment for idiopathic thrombocytopenia was with romiplostim, a fusion protein analog of thrombopoietin, to which she had a favorable response.
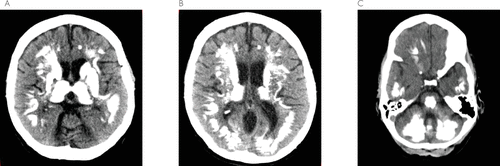
FIGURE 1. A CT Brain Scan Showed Extensive Bilateral Calcifications of Basal Ganglia
The figure shows [A]: the thalamus, [B]: cerebral white matter, and [C]: brain stem and cerebellum.
Discussion
Fahr’s disease is a rare neurodegenerative disorder characterized by idiopathic bilateral basal ganglia calcifications, with a possible higher incidence in men.1 The most common presentation in symptomatic patients is movement disorders, of which parkinsonism is predominant.1 Other neurologic symptoms include cognitive impairment, cerebellar signs, speech disorder, pyramidal signs, psychiatric features, sensory changes, and gait disorders.1 The transmission is most often autosomal dominant.2 For years there has been inquisitiveness to comprehend the disease process, and researchers have extensively investigated images from autopsy specimens. Immunohistological studies of brain lesions in Fahr-type calcifications were performed by Fujita et al3 for 19 patients and showed variable findings including diffuse neurofibrillary tangles with calcification, Alzheimer's disease, Pick's disease, progressive supranuclear palsy, and Parkinson's disease. Moreover, three different patterns of calcium deposition were observed: diffuse deposition within the tunica media of small- and medium-sized vessels, free spherical or lobulated concretions in the parenchyma, and rows of small calcospherites lying along capillaries.3 Some radiological studies showed that, at times, the intensity of calcification may not correlate with neurological impairment.4
Certain gene mutations have been implicated for its possible etiology, and mutations in SLC20A2 [solute carrier family 20 (phosphate transporter), member 2] are considered one of the major causes of familial idiopathic basal ganglia calcification.5,6 Other genetic mutations reported are PDGFRB [platelet-derived growth factor receptor, beta polypeptide] (especially seen in families with an absent SLC20A2 mutation) and chromosome 14q, 2q37 loci variations.7–9 In another study, mutations in pantothenate kinase 2-related neurodegeneration were described and associated with idiopathic basal ganglia calcifications.10
The role of autoimmunity in Fahr’s disease is described very little in the literature. Sava et.al described a case of intracerebral symmetrical calcifications secondary to hypoparathyroidism. The patient’s autopsy of the parathyroid glands showed fibroadipose tissue, suggesting a remote autoimmune pathology of the parathyroid glands.11 Morgante et al. hypothesized that gliovascular changes caused by cerebral inflammation may facilitate calcifications within the striopallidodentate system when there is a disturbance in calcium metabolism.12 Another clinical case of a patient who presented with loss of consciousness and convulsions was reported by Arranz Perez et al., who described calcifications of the basal ganglia, hypocalcemia, and myocardiopathy of hypoparathyroidism, and attributed these findings to autoimmune polyendocrinopathy.13 Fahr’s disease has also been described in association with idiopathic pulmonary hemosiderosis and primary hypoparathyroidism, which were considered related because of their autoimmune nature.14 Similarly, the syndrome has been seen with pseudohypoparathyroidism and autoimmune hypothyroidism.15
Conclusion
It is not clear whether central nervous calcification in Fahr’s disease is a metastatic deposition, secondary to local disruption of the blood–brain barrier, or is due to a neuronal calcium metabolism disorder.16 The gliovascular changes caused by cerebral inflammation may be secondary to autoimmune invasion and thus facilitate calcifications within the striopallidodentate system. Our case, to the best of our knowledge, is the first reported in the literature describing an autoimmune triad. One may argue that this is simply a coincidence; however, exploring more cases of Fahr’s disease and its occurrence with autoimmune diseases could be an area of contemplation. There is no definitive treatment for Fahr’s disease available except for symptomatic relief. The prognosis of these patients remains variable and difficult to predict.
1 : Bilateral striopallidodentate calcinosis: clinical characteristics of patients seen in a registry. Mov Disord 2001; 16:258–264Crossref, Medline, Google Scholar
2 : Familial idiopathic striopallidodentate calcifications. Neurology 1989; 39:381–385Crossref, Medline, Google Scholar
3 : Immunohistochemical examination on intracranial calcification in neurodegenerative diseases. Acta Neuropathol 2003; 105:259–264Medline, Google Scholar
4 : [Calcification of the basal ganglia and Fahr disease. Report of two clinical cases and review of the literature]. Recenti Prog Med 1993; 84:192–198Medline, Google Scholar
5 : Mutations in SLC20A2 link familial idiopathic basal ganglia calcification with phosphate homeostasis. Nat Genet 2012; 44:254–256Crossref, Medline, Google Scholar
6 : Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification. Neurogenetics 2013; 14:11–22Crossref, Medline, Google Scholar
7 : Mutation of the PDGFRB gene as a cause of idiopathic basal ganglia calcification. Neurology 2013; 80:181–187Crossref, Medline, Google Scholar
8 : Identification of a locus on chromosome 14q for idiopathic basal ganglia calcification (Fahr disease). Am J Hum Genet 1999; 65:764–772Crossref, Medline, Google Scholar
9 : 2q37 as a susceptibility locus for idiopathic basal ganglia calcification (IBGC) in a large South Tyrolean family. J Mol Neurosci 2009; 39:346–353Crossref, Medline, Google Scholar
10 : Idiopathic basal ganglia calcifications: an atypical presentation of PKAN. Pediatr Neurol 2013; 49:351–354Crossref, Medline, Google Scholar
11 : The Fahr syndrome and the chronic lymphocytic thyroiditis. Rom J Morphol Embryol 2013; 54:195–200Medline, Google Scholar
12 : Fahr’s syndrome: local inflammatory factors in the pathogenesis of calcification. J Neurol 1986; 233:19–22Crossref, Medline, Google Scholar
13 . (Fahr's disease and hypocalcemic syndromes. Presentation of a clinical case) Med Interna 1992; 9:495–497Google Scholar
14 : [Fahr disease and idiopathic pulmonary hemosiderosis in a 10 year old patient (author’s transl)]. An Esp Pediatr 1980; 13:599–604Medline, Google Scholar
15 : Intracranial hemorrhage revealing pseudohypoparathyroidism as a cause of fahr syndrome. Case Rep Neurol Med 2011; 2011:407567Medline, Google Scholar
16 : P.V., Naik D, Fahr disease: a rare neurodegenerative disorder. Neuroradiology 2004; 14:383–384Google Scholar



