Preliminary Findings Supporting Insula Metabolic Activity as a Predictor of Outcome to Psychotherapy and Medication Treatments for Depression
Abstract
A putative right anterior insula metabolism biomarker predictive of treatment outcomes was retrospectively applied to 30 depressed psychotherapy—or escitalopram-treated nonremitters who entered combination treatment. Patients whose added treatment matched the biomarker-indicated treatment remitted more often than biomarker-mismatched patients.
We recently reported a potential imaging biomarker to guide initial treatment selection for major depressive disorder.1 In that randomized trial, pretreatment resting state glucose metabolic activity of 6 brain regions differentially predicted remission to 12 weeks of treatment with escitalopram or cognitive-behavioral therapy (CBT). Of the six regions, the activity of the right anterior insula (rAI) best predicted treatment outcomes: hypometabolism of the rAI predicted remission to CBT and nonresponse to escitalopram (CBT-type), whereas hypermetabolism predicted remission to escitalopram and nonresponse to CBT (escitalopram-type). We concluded that metabolic activity in the rAI represented a possible treatment specific biomarker (TSB), defined as a biological measure that predicts remission to a specific treatment and also predicts nonresponse to an alternative treatment.
Nonremitting patients in the study used to define the TSB1 were eligible to enroll in a 12-week extension phase during which they received combination treatment (escitalopram+CBT). We evaluated the use of the baseline rAI TSB to predict treatment outcomes among these nonremitting patients who received combination treatment. It was hypothesized that patients whose added treatment matched their baseline rAI biomarker-indicated treatment would remit at a higher rate than those whose added treatment was mismatched to the biomarker-indicated treatment.
Methods
Complete descriptions of the study protocol2 and the imaging methods1 have been published previously. Briefly, patients aged 18–60 years who met DSM-IV(TR) criteria for major depressive disorder using information from the Structural Clinical Interview for DSM-IV3 and who had a 17-item Hamilton Depression Rating Scale (HDRS)4 score ≥18 at screening and ≥15 at the baseline randomization visit were eligible for participation. Key exclusion criteria included a primary psychiatric disorder other than major depressive disorder, lifetime bipolar disorder; current obsessive compulsive disorder or psychosis; current substance abuse or dependence; a clinically significant medical condition; use of antidepressants within 14 days of the scanning visit (5 weeks for fluoxetine); current psychotherapy; or previous lifetime nonresponse to either escitalopram (≥10 mg/day for ≥6 weeks) or CBT (≥4 sessions). Written informed consent was obtained from all participants, and the protocol was approved by the Emory Institutional Review Board.
Treatment in the study consisted of two phases. In Phase 1 patients were randomly assigned (1:1 ratio) to 12 weeks of treatment with either escitalopram (10–20 mg/day, based on response and tolerability) or CBT (16 one-hour sessions over 12 weeks). In Phase 2, nonremitters, defined as HDRS total score>7 at either week 10 or 12, were started on a 12-week course of combination treatment. During combination treatment, nonremitters to initial escitalopram treatment received 16 sessions of CBT while continuing on escitalopram, and CBT nonremitters received escitalopram and were offered three CBT booster sessions during the 12 weeks. For each phase, blinded HDRS ratings were performed weekly for the first 6 weeks and then biweekly until week 12. The definition of remission was a HDRS ≤7 at both weeks 10 and 12 of the treatment phase, identical to the Phase 1 definition.
Prior to initial randomization, patients underwent a standard glucose metabolic resting state positron emission tomography (PET) scan and MRI for a T1- weighted image. Each patient’s PET and T1 scans were coregistered and transformed into MNI space using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/). Individual PET scans were then normalized; each voxel was divided by mean whole brain metabolism. To define a TSB type for each patient, mean rAI activity was extracted from each subject’s whole brain (WB) mean activity normalized PET scan using a specified 1.8 mL region-of interest centered on the peak cluster finding (MNI x=+30.0, y=+24.0 z=−13.5) identified in the initial Phase 1 analysis.1 Insula values were then characterized as either CBT-type (hypometabolic, activity <1) or escitalopram-type (hypermetabolic activity >1). We tested the difference in proportions of remitters between those whose added treatment matched their biomarker-indicated treatment versus those for whom it was mismatched using Fisher's exact test.
Results
Ten eligible patients did not enter Phase 2 (four treated with escitalopram in Phase 1; six treated with CBT). Twenty-seven (13 treated in Phase 1 with escitalopram and 14 treated with CBT) of the 30 patients who entered Phase 2 completed the 12 weeks of treatment and, therefore, had remission status. Of the three patients terminating early from Phase 2, two received CBT in Phase 1, one received escitalopram. For the patients entering Phase 2, the mean week 12 HDRS scores for those treated with escitalopram or CBT in Phase 1 did not significantly differ (11.1±3.6 and 13.1±4.9, respectively, t=1.21, p=0.24).
Figure 1 shows the treatment outcomes in the trial. In Phase 2, the overall remission rate for patients whose added treatment matched their baseline TSB was 52.6% (10/19). The remission rate among those mismatched to their TSB was 25% (2/8). Using a risk ratio for remission as our effect size, this difference in outcomes did not reach statistical significance because of low power (risk ratio: 2.11, 95% CI: 0.59–7.52, p=0.24). The difference in remission rates converts to a number needed to treat of 3.6, indicating that four patients would need to undergo pretreatment scanning to achieve one additional remitter. Notably, among patients with a putative escitalopram-type TSB who did not remit with escitalopram in Phase 1, only 1/7 (14.3%) remitted with the addition of CBT in Phase 2. Receiving treatment in Phase 2 matching the baseline TSB type had greater specificity for remission with CBT (4/6, 66.7%) compared with escitalopram (6/13, 46.2%).
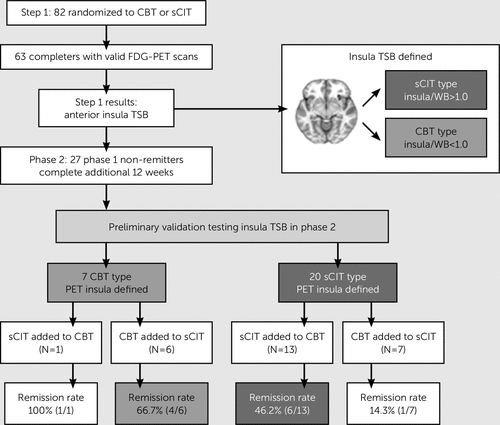
FIGURE 1. Treatment Outcomes by Anterior Insula TSB Typea
a CBT=cognitive-behavioral therapy; PET=positron emission tomography; sCIT=escitalopram antidepressant medication; TSB=treatment selection biomarker; WB=whole brain.
Discussion
In this extension of a larger trial that demonstrated pretreatment regional insula activity could predict the specific treatment that would be efficacious at the individual patient level, we found preliminary evidence that appears to be consistent with the earlier findings. Although the sample size in this cohort is too small to demonstrate statistical significance, the substantial difference in remission rates between biomarker-matched and -mismatched patients provides preliminary support for the insula TSB as meaningful predictor of remission to two of the standard first line major depressive disorder treatments. A formal test of the improvement in remission rates resulting from assigning treatment based on the TSB over existing rates needs to be evaluated in an independent prospective sample.
Another relevant finding from this analysis is that the majority of patients who did not remit after two phases were of the escitalopram type (13/15=86.7%). Further, the addition of CBT was unsuccessful in the majority (86%) of patients with the escitalopram pattern, indicating that the TSB for initial treatment could prove most effective for identifying those patients who are likely to respond to CBT. Taken together, these results suggest that although increased rAI metabolism predicted better response to medication than psychotherapy, high rAI activity is also associated with nonremission to both treatments. This interpretation is consistent with other recent work indicating that greater activity in the anterior insula is associated with poorer response to vagus nerve stimulation5 and that ketamine robustly reduces right insular metabolism.6 Beyond rAI metabolism, nonresponse (less than 50% improvement from baseline) after 24 weeks of treatment (12 weeks of monotherapy followed by 12 weeks of combination) in our sample was associated with higher pretreatment metabolic activity in the subcallosal cingulate cortex and superior temporal sulcus.7 Although a specific activity pattern predictive of poor outcomes to initial treatment remains to be determined, these data suggest that elevated metabolism in key temporolimbic regions will be important contributors to such a biomarker.
The most important limitation to our analysis is that the Phase 2 treatment was a combination treatment and not a switch to the alternative modality. Hence, it is possible that Phase 2 response did not derive specifically from the added treatment, but instead resulted simply from the prolonged treatment duration. Although this brain biomarker did not track with any specific clinical phenotype based on the acquired clinical metrics, there are several potential candidates linked to insula function8,9 that could be tested in future studies and may lead eventually to a more clinically accessible substitute.
1 : Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 2013; 70:821–829Crossref, Medline, Google Scholar
2 : Depression beliefs, treatment preference, and outcomes in a randomized trial for major depressive disorder. J Psychiatr Res 2012; 46:375–381Crossref, Medline, Google Scholar
3 : Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition, (SCID-I/P, version 2.0). New York, Biometrics Research Department, New York State Psychiatric Institute, 1995Google Scholar
4 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
5 : Pretreatment cerebral metabolic activity correlates with antidepressant efficacy of vagus nerve stimulation in treatment-resistant major depression: a potential marker for response? J Affect Disord 2012; 139:283–290Crossref, Medline, Google Scholar
6 : Neural correlates of rapid antidepressant response to ketamine in treatment-resistant unipolar depression: a preliminary positron emission tomography study. Biol Psychiatry 2013; 73:1213–1221Crossref, Medline, Google Scholar
7 : Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry 2014; 76:527–535Crossref, Medline, Google Scholar
8 : Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Crossref, Medline, Google Scholar
9 : Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Soc Cogn Affect Neurosci (Epub ahead of print, April 8, 2014)Google Scholar



