Pilot Study of Neurological Soft Signs and Depressive and Postconcussive Symptoms During Recovery From Mild Traumatic Brain Injury (mTBI)
Abstract
Neurological soft signs (NSSs) tap into a variety of perceptual, motor, and cognitive functions. The authors administered a battery of NSSs serially to a group of 14 pilot patients recruited from an emergency room after they experienced a mild traumatic brain injury. Patients were seen within 96 hours after injury, and again 30 and 90 days later. Measures of balance, mood, and postconcussive symptoms and impairment were also obtained. NSSs and balance improved across visits. Across visits, NSSs and balance were not significantly associated with any postconcussive outcome measures, although depressive symptoms were. Initial neurological impairment appeared to predict subsequent residual postconcussive symptoms and impairment, but this result requires replication.
Mild traumatic brain injury (mTBI) resulting from physical forces applied to the head or neck is a common condition. Although most patients recover without the benefit of medical intervention over the course of days to weeks, a subset of individuals exhibit a persistent postconcussive syndrome (PCS), with reported functional incapacity.1,2 In the civilian population, the incidence of all-cause TBI has been estimated at 1.7 million new cases annually in the US,3with about two-thirds in the mild range.4 In military combatants, mTBI has been reported in an estimated 237,000 service members during the years 2000–2013.5 Failure to detect, monitor, and/or treat at-risk patients increases the likelihood of long-term morbidity,6 whereas treating patients who are destined to recover on their own wastes clinical and financial resources. Misidentifying recovered mTBI patients as still impaired also expends resources, unnecessarily curtails activities, risks iatrogenic effects, and may lead to unwarranted compensation seeking.7 Silverberg and Iverson have concluded that the literature does not support more than 3 days of enforced total rest following acute mTBI, and that a longer period of inactivity may be associated with adverse outcomes.8 In contrast, Brown et al. noted positive associations among self-reported levels of cognitive activity, postconcussive symptom scores, and time to recovery.9
Presently available means for assessing prognosis after mTBI are inadequate. Postconcussive symptoms comprise an indistinct pool of mostly subjective, somatic, emotional, and cognitive manifestations (e.g., headache, irritability, poor concentration), with individual differences in symptom profiles across patients.2 Some mTBI symptoms overlap with those seen in other conditions such as depression10 and fibromyalgia.11 Factors such as sleep duration can impact performance and thus influence perception of deficits.12 Patients’ self-reports can be confounded by both under- and over-reporting biases.13
The traditional neurological examination is typically unrevealing in mTBI.14 Standardized neuropsychological instruments have been unsuccessful either in defining a consistent pattern of deficits associated with mTBI, or in predicting a given individual's trajectory of recovery and ultimate functional outcome.15,16
Neurological soft signs (NSSs) are objective performance measures of sensorimotor, reflexive, perceptual, and cognitive capabilities. Impaired execution yields subtle indicators of brain dysfunction. Examination of NSSs entails low-tech, inexpensive, relatively brief, and readily administered clinical maneuvers. NSSs have been found to be elevated in a variety of mental disorders, with more than 100 studies of NSSs in the psychiatric literature. Our group has examined the incidence of NSSs among various groups of post-traumatic stress disorder (PTSD) patients.17–20 Three of these four studies found a small but consistent elevation in NSSs among PTSD survivors.
In contrast, there have been almost no studies of NSSs in TBI or PCS. One study demonstrated that an NSS examination administered to an acquired brain injury population could be reliably rated by even nonexpert clinicians. The authors concluded from their findings that, “a standardized battery of tests for assessing neurologic soft signs could be a useful adjunct in determining impairment in acquired brain injury patients.”21 Several studies have incorporated solitary or isolated elements of an NSS battery, with promising results. One such study reported that impaired tandem gait was increased almost threefold in patients with a history of mTBI.6 Other studies have found postconcussive impairments in balance.22–25 Additional isolated abnormal soft signs found to be present in mTBI include occulomotor and visuomotor tracking dysfunction,26 dysdiadochokinesia,27 and bradykinesia.28
The aim of the present research was to measure NSSs in the acute aftermath of an mTBI and evaluate their ability to track recovery of function and to predict subsequent postconcussive symptoms. A clinical examination battery comprised of 45 NSSs was administered to a small sample of mTBI patients in the days following injury and again at 1 and 3 months postinjury. The following hypotheses were tested: 1) NSSs would be elevated at Visit 1 and trend downward at Visit 2 and Visit 3 as patients recovered; 2) at each visit, NSSs would be positively associated with self-reported postconcussive symptoms; 3) Visit 1 NSSs would predict a) Visits 2 and 3 postconcussive symptoms, b) Visits 2 and 3 self-reported functional impairment, and c) Visit 3 objective impairment on neuropsychological testing. We also included another soft neurological measure, the Modified Balance Error Scoring System (BESS) because it has previously been found to be associated with postconcussive symptoms.29 Because of evidence that the consequences of mTBI are potentially explained by depression,30 we also included measurements of this affective dimension using a validated self-report instrument.
Methods
Patients
All patients were recruited according to a Spaulding Rehabilitation Hospital Institutional Review Board-approved research protocol. Study candidates were drawn from patients presenting with an acute mTBI to the Massachusetts General Hospital Emergency Department (ED), screened by a dedicated ED-based research team, and formally recruited in a subsequent telephone conversation initiated by the first author. At the initial visit, which was mandated to take place within 96 hours of injury occurrence, patients were provided with a full explanation of study procedures and the potential risks and benefits of participation. Written informed consent was obtained using an Institutional Review Board-approved form. Inclusion criteria were the occurrence within the past 96 hours of an mTBI as defined by the Defense and Veterans Brain Injury Center (DVBIC) Conference on the Acute Management of Concussion/Mild Traumatic Brain Injury (mTBI) in the Deployed Setting,5 viz., “Anyone who was dazed, confused, ‘saw stars,’ lost consciousness even momentarily, or ha[d] memory loss as a result of an explosion/blast, fall, motor vehicle crash, or other event involving abrupt head movement, a direct blow to the head or other head injury.”
Any patient with a TBI that was more than mild, as evidenced by skull fracture, reconstructed Glasgow Coma Scale score <13, loss of consciousness >30 minutes, or posttraumatic amnesia >24 hours, was excluded. Additional exclusion criteria were any of the following: age <18 or >65 years; left or mixed handedness; localizable abnormality on neurological examination; structural brain lesion on imaging; hospitalization >1 day; surgical intervention; history of serious neurological condition; current major depressive or dysthymic disorder; current or past psychotic, bipolar, melancholic, substance dependence or abuse disorder, or mental disorder due to a general medical condition; use within the month prior to enrollment of psychoactive medications; a urine specimen containing drugs of abuse; a physical injury or condition that prevented examination of one or more of the 45 NSSs in the battery (e.g., prosthetic limb or paraplegia); or inability to understand the study's procedures, risks, and side effects or to otherwise give informed consent for participation.
Out of a total of 99 patients contacted by telephone following their ED visit, 78 refused to be consented. Obtaining the reason for refusal, or demographic or clinical data, from these individuals was not covered under the Institutional Review Board authorization and, hence, was not carried out. Of the 21 consented subjects, two failed the toxic screen (tetrahydrocannibinol, benzodiazepine); one was excluded for active alcohol dependence; three were excluded for current use of prescribed psychoactive medications for depressive disorder; and one was lost because of attrition. In the remaining 14 patients, mechanisms of injury included fall N=5, motor vehicle or bicycle accident N=3, head striking (other) object N=3, and (other) object striking head N=3.
Measures/Procedure
The primary predictive measure was a battery of 45 NSSs administered and scored according to a technique described in detail elsewhere in the literature.18–20 This battery included measures of balance, gait, and somatosensory, visuospatial, simple, and complex motor function. A description of each NSS appears in Table S1 of the data supplement accompanying the online edition of this article. All NSS examinations were performed by the first author (M.S.G.), a senior neuropsychologist who had been trained by the NSS instrument’s creator (T.V.G.). The 1-hour NSS examinations were video-recorded in high definition by a research assistant. They were then encrypted and sent for scoring to a single expert rater (T.V.G.) who was blind to all other clinical information and measures. The NSS battery comprised 45 signs (NSS-45), each scored as 0 - no deficit, 1- mild deficit, 2 - moderate deficit, 3 - marked deficit. An average overall NSS (avgNSS-45) score was calculated by dividing the sum of the scores for all items by 45. This measure has been previously shown to have an inter-rater reliability of 0.74 (intraclass correlation coefficient) for two raters independently scoring blindly from videotapes.18 Because patients’ scores on the great majority of individual NSSs were below the level of mild deficit, we also selected the subset of 7 NSSs (NSS-7) for which the Visit 1 group mean was>1 (i.e., more than mild) for separate analyses (see under Results).
Visit 1 was conducted within 96 hours postinjury. It required approximately 150 minutes and consisted of obtaining informed consent, screening for exclusion criteria, and administration of the following: toxic urine screen; handedness determination (Edinburgh Handedness Inventory)31; Michigan Alcoholism Screening Test (MAST)32; TBI severity determination consisting of an inquiry regarding the circumstances of the injury, the extent of loss of consciousness, and a retrospective approximation of the Glasgow Coma Scale33in the immediate aftermath of the injury; clinical evaluation for current and past psychiatric illness applying DSM-IV-TR criteria; and medication use determination. If the patient passed the screening, the following study measures were then collected: NSS battery, Modified Balance Error Scoring System (BESS),29 Rivermead Post-Concussion Symptoms Questionnaire (RPQ),34 and Beck Depression Inventory (BDI-II).35
Visit 2 was undertaken approximately one month postinjury, required approximately 120 minutes, and repeated the NSS Examination, BESS, RPQ, and BDI-II. A functional adaptation survey, the Mayo-Portland Adaptability Inventory Index-Fourth Edition (MPAI-4),36 was also administered.
Visit 3 was undertaken approximately 3 months postinjury, required approximately 180 minutes, and repeated the NSS Examination, BESS, RPQ, BDI-II, and MPAI-4. In addition, a brief battery of neurocognitive performance measures was administered, consisting of the Trailmaking Test37; Symbol Digit Modalities Test38; California Verbal Learning Test II - Short Form39; Benton Visual Retention Test40; Weschler Memory Scale-Third Edition: Digit Span, Visual Memory Span, Mental Control41; Grooved Pegboard Test42; Wisconsin Card Sorting Test −64 Version43; Controlled Oral Word Association Test44; and Letter Cancellation Test.45 These neurocognitive measures were scored according to standards provided in their respective test manuals, correcting for age, gender, and education as appropriate, generating z-scores that reflected performance levels relative to population norms. To prevent any instrument from disproportionally contributing to overall level of impairment, each test was represented by one or at most two parameters. These z-scores were then averaged into an overall measure of neurocognitive impairment (NCI).
The key predictor variables were avgNSS-45, avgNSS-7, BESS, and BDI-II scores. The key predicted (outcome) variables were the RPQ, MPAI-4, and NCI.
Design
This study’s design was a prospective cohort pilot study in which a single clinical group (patients who had suffered an acute mTBI) was assessed repeatedly for 3 months following their injury.
Statistical Analysis
Data were analyzed according to a general linear mixed effects model (Statistical Analysis System PROC MIXED). There were no missing data. The VISIT variable was arbitrarily assigned a value of 1 (96 hours), 2 (30 days), or 3 (90 days). In order to obtain a measure of effect size, data were standardized prior to analysis, so that the estimates yielded by PROC MIXED represented standardized regression coefficients (β). The ability of the Visit 1 predictor measures to predict the Visits 1, 2, and 3 outcome measures was analyzed by means of Pearson product-moment correlations. Because the hypotheses were directional, one-tailed p values were calculated; significance levels are indicated with asterisks as follows: *p<0.10, **p<0.05, ***p<0.01, ****p<0.001.
Results
Patient Characteristics
Table 1 displays the sample’s demographic profile. Only 2/14 patients reported a discrete period of loss of consciousness related to their injury
| Characteristic | Visit 1 (96 Hours) | Visit 2 (30 Days) | Visit 3 (90 Days) |
|---|---|---|---|
| Age (years) | 32.2 (11.5) | ||
| Educational level (grades completed) | 16.1 (1.8) | ||
| Gender | 6M, 8F | ||
| AvgNSS–45 (0–3)** | 0.5±0.0 | 0.4±0.0 | 0.4±0.0 |
| AvgNSS–7 (0–3)**** | 1.7±0.36 | 1.2±0.36 | 1.0±0.36 |
| BESS (0–60)*** | 20.1±10.1 | 16.9±5.4 | 16.4±7.2 |
| BDI-II (0–63)**** | 7.6±2.9 | 5.6±5.8 | 1.7±3.6 |
| Rivermead Post-Concussion Symptoms Questionnaire (RPQ) (0–64)*** | 19.6±7.2 | 7.7±10.1 | 3.7±6.1 |
| Mayo-Portland (0–111)** | 14.1±13.3 | 6.1±9.7 | |
| NCI (z-score) | 0.15±0.54 |
TABLE 1. Group Demographic, Psychometric, and Neurological Soft Sign Means by Visita
Neurological and affective markers of acute concussion and recovery.
Of the 45 individual NSSs, the group mean score was >1 for only seven NSSs. These included, in descending order: walking on heels (M=2.6), optic agnosia (M=2.1); astereognosis-left (M=2.1), astereognosis-right (M=1.6), Luria's motor sequence task (fist-palm-side)-right (M=1.4), Money Road-Map Test of Direction Sense task (M=1.4), and walking on tiptoes (M=1.1). The average of the scores on these seven items formed the avgNSS-7 measure.
Across visits, avgNSS-45 score was significantly associated with avgNSS-7 score: β=0.85****, and with BESS score: β=0.59****. Additionally, avgNSS-7 score was significantly associated with BESS score: β=0.43****. Neither avgNSS-45 score, avgNSS-7 score, nor BESS score was significantly associated with BDI-II score across visits.
Table 1 presents group mean scores for selected measures by Visit. It will be seen that group mean avgNSS-45, avgNSS-7, and BESS scores declined from Visit 1 to Visit 2 and tended to stabilize between Visits 2 and 3. Group mean BDI-II scores also showed a significant decline over visits, although the effect was more pronounced between Visits 2 and 3.
Neurological and affective associations with postconcussive symptoms and impairment
Across visits, avgNSS-45 score, was not significantly associated with either RPQ score, MPAI-4 score (Visits 2 and 3 only), or NCI score (Visit 3 only). Neither were avgNSS-7 score nor BESS score significantly associated with these latter measures. In contrast, BDI-II score was significantly associated with RPQ score: β=0.16*** and especially with MPAI-4 score: β=1.1****, but not with NCI score. At Visit 3, NCI score was also not significantly associated with RPQ or MPAI-4 score.
In order to control for the possibility that the association between depression and postconcussive symptoms was eclipsing associations between NSSs and the latter, we reperformed the above analyses entering BDI-II score as a covariate. Even after this adjustment, no significant associations emerged across visits between avgNSS-45, avgNSS-7, and BESS scores on the one hand, and RPQ, MPAI-4, and NCI scores on the other.
Subacute neurological and psychological predictors of chronic postconcussive symptoms and impairment.
Pearson product-moment correlations between various Visit 1 predictors and various Visit 1, 2, and 3 outcome measures appear in Table 2. It will be seen that although Visit 1 avgNSS-45 score initially did not significantly predict Visit 1 RPQ score, it went on to significantly predict Visit 2 and Visit 3 RPQ scores. Scatterplots for these associations appear in Figure 1. Visit 1 avgNSS-45 score also significantly predicted Visit 2 and Visit 3 MPAI-4 scores but not Visit 3 NCI score. Parallel but weaker results were obtained with Visit 1 avgNSS-7 score as predictor. Visit 1 BESS score only significantly predicted Visit 2 MPAI-4 score. Visit 1 BDI-II score only significantly predicted Visit 3 NCI score.
| Visit 1 Predictors (<96 hours) | ||||
|---|---|---|---|---|
| Outcome Measures | AvgNSS–45 | AvgNSS–7 | BESS | BDI-II |
| Rivermead Post-Concussion Symptoms | ||||
| Questionnaire | ||||
| Visit 1 | 0.12 | 0.08 | 0.29 | 0.17 |
| Visit 2 | 0.58** | 0.37* | 0.46* | 0.31 |
| Visit 3 | 0.58** | 0.39* | 0.36 | 0.29 |
| Mayo-Portland Adaptability Inventory | ||||
| Index | ||||
| Visit 2 | 0.53** | 0.36 | 0.53*** | 0.44* |
| Visit 3 | 0.53** | 0.38* | 0.35 | 0.31 |
| Neurocognitive Impairment | ||||
| Visit 3 | –0.30 | –0.24 | –0.20 | 0.56* |
TABLE 2. Correlations Between Visit 1 Neurological and Affective Predictors and Visit 1, 2, and 3 Postconcussive Symptoms and Impairment Outcome Measuresa
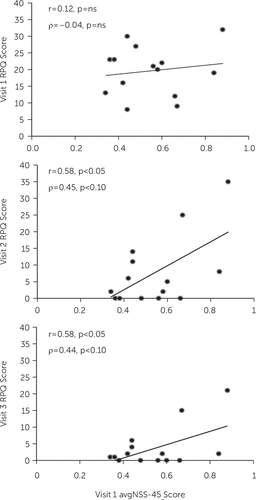
FIGURE 1. Pearson (r) and Spearman (ρ) Correlations Between Average Score of the 45 Neurological Soft Signs at Visit 1 and Rivermead Post-Concussion Symptoms Questionnaire Score at Visits 1, 2, and 3a
a p values are one-tailed
Discussion
The high correlations between the NSS measures and scores on the Balance Error Scoring System suggest that to some degree these measured the same thing. However, the NSS examination goes far beyond balance to measure other aspects of subtle neurological dysfunction. Of the seven most elevated NSSs during Visit 1, only two involved balance (actually gait). The observation that the NSS and BESS scores were significantly higher when first measured within 96 hours of concussion than when reassessed at 1 and 3 months later supports our first hypothesis and suggests that these measures were sensitive to acute manifestations of mTBI. Although this normalizing trend over visits can be seen as validating their use in detecting acute mTBI-related dysfunction, the absence of a control group mandates caution in this interpretation; it is also possible that the reduction in impairment in this measure over time was due to regression to the mean or practice effects. The latter explanation was rendered less likely by the use of alternate forms for the NSSs for which the potential for practice effects was higher due to an obvious recall component, such as tongue twisters and astereognosis. Moreover, no diminution in NSS performance was observed between Visits 2 and 3.
Although as noted above, the NSSs and BESS measures may have been sensitive to mTBI and recovery, they were not sensitive to postconcussive symptoms or impairment, providing no support for our second hypothesis. These negative results echo previous failures to find hard neurological and neuroimaging abnormalities that are directly related to postconcussive symptoms.14,22 One potential explanation for the failure of subtle neurological impairment to explain postconcussive symptoms or impairment in this study is that the majority of our sample only suffered mTBIs that were at the low end of the severity spectrum, with only 2/14 having experienced any discrete loss of consciousness.
Despite the absence of association between the neurological and postconcussive measures across visits, the results provided some evidence supporting our third hypothesis, that subacute neurological compromise (at Visit 1) would predict subsequent postconcussive symptoms, and self-reported functional impairment (at Visits 2 and 3). Although the average Visit 1 score of the 45 NSSs and the initially highest seven NSSs did not predict Visit 1 total Rivermead Post Concussion Symptoms Questionnaire score, as scores on the latter declined across subsequent visits, the Visit 1 NSS scores came to significantly predict them, and the Mayo-Portland Adaptability Inventory Index scores as well, at Visits 2 and 3. These results suggest that initial neurological compromise, either pre-existing or acquired along with the mTBI, may negatively impact recovery from the subacute postconcussive symptoms and impairment that a majority of mTBI patients initially experience, and it may increase the likelihood of their eventually belonging to the subgroup with persistent postconcussive manifestations.
The reliability of the above inferences is reduced by the liberal threshold for statistical significance that was employed, including the use of a one-tailed p value, no Bonferroni-type corrections for multiple comparisons, and the post hoc identification of more severely affected NSSs at Visit 1. We deemed reduction of Type II error to be more important than containing Type I error in this pilot study. Hence, the predictive value of initial subacute neurological impairment for subsequent chronic postconcussive cognitive symptoms suggested in the results is in need of replication and cross-validation in a larger sample.
In contrast to the neurological measures, the presence of self-reported depressive symptoms following mTBI was significantly associated with postconcussive symptoms and functional impairment across visits. This finding comports well with the existing literature on the role of mood dysfunction in mTBI.10 The instrument used to assess depression in the present study, viz., the BDI-ll, was not designed to distinguish symptoms of idiopathic depression from those arising in the setting of brain injury. Moreover, the design of this pilot effort did not allow for the acquisition of pre-mTBI depression or other psychometric data. Thus, our results cannot resolve the origin of the comorbid depression found in this study, specifically whether depressive symptoms (short of a diagnosable depressive disorder, which was an exclusion criterion) represent: 1) a pre-existing risk factor for the development of postconcussive symptoms; 2) an acquired “organic” result of the mTBI, 3) a psychological consequence of having experienced a disturbing head injury, or 4) a combination of the foregoing.
The absence of associations between neurological variables and objective neurocognitive impairment (NCI) is consistent with the prevailing literature, which reports that group data on psychometric tests of cognition are not sensitive to the effects of mTBI following acute recovery.15,16 In contrast, the obtained association between the BDI-II and NCI is congruent with the studies that demonstrate an effect of both depressed mood46 and low effort47 on formal cognitive measures.
1 : Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995; 45:1253–1260Crossref, Medline, Google Scholar
2 : Outcome from mild traumatic brain injury. Curr Opin Psychiatry 2005; 18:301–317Crossref, Medline, Google Scholar
3 : Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Atlanta, GA, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2010Crossref, Google Scholar
4 : Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Atlanta, GA, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2006Google Scholar
5 Bazarian J, Boerman H, Bolenbacher R, et al: Defense and Veterans Brain Injury Center Consensus Conference on the Acute Management of Concussion/Mild Traumatic Brain Injury (mTBI) in the Deployed Setting. Washington, DC, July 31 and Aug 1, 2008Google Scholar
6 : Long-term morbidities following self-reported mild traumatic brain injury. J Clin Exp Neuropsychol 2007; 29:585–598Crossref, Medline, Google Scholar
7 : A longitudinal study of the relationship between financial compensation and symptoms after treated mild traumatic brain injury. J Clin Exp Neuropsychol 2002; 24:187–193Crossref, Medline, Google Scholar
8 : Is rest after concussion “the best medicine?”: recommendations for activity resumption following concussion in athletes, civilians, and military service members. J Head Trauma Rehabil 2013; 28:250–259Crossref, Medline, Google Scholar
9 : Effect of cognitive activity level on duration of post-concussion symptoms. Pediatrics 2014; 133:e299–e304Crossref, Medline, Google Scholar
10 : Misdiagnosis of the persistent postconcussion syndrome in patients with depression. Arch Clin Neuropsychol 2006; 21:303–310Crossref, Medline, Google Scholar
11. : A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001; 134:868–881Google Scholar
12 : Baseline neurocognitive testing in sports-related concussions: the importance of a prior night’s sleep. Am J Sports Med 2014; 42:472–478Crossref, Medline, Google Scholar
13 : Early predictors of post-concussion symptoms in patients with mild head injury. Eur J Neurol 2003; 10:175–181Crossref, Medline, Google Scholar
14 : Abnormal neurological exam findings in individuals with mild traumatic brain injury (mTBI) versus psychiatric and healthy controls. Clin Neuropsychol 2012; 26:1102–1116Crossref, Medline, Google Scholar
15 : Is neuropsychological testing useful in the management of sport-related concussion? J Athl Train 2005; 40:139–152Medline, Google Scholar
16. : Neuropsychological outcomes in OEF/OIF veterans with self-report of blast exposure: associations with mental health, but not mTBI. Neuropsychology 2013; 28:337–346Google Scholar
17. Absence of selected neurological soft signs in Vietnam nurse veterans with post-traumatic stress disorder. Psychiatry Res 2002; 110:81‒85Google Scholar
18 : Neurologic soft signs in chronic posttraumatic stress disorder. Arch Gen Psychiatry 2000; 57:181–186Crossref, Medline, Google Scholar
19 : Neurological status of Vietnam veterans with chronic posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci 1993; 5:183–188Link, Google Scholar
20 : Subtle neurologic compromise as a vulnerability factor for combat-related posttraumatic stress disorder: results of a twin study. Arch Gen Psychiatry 2006; 63:571–576Crossref, Medline, Google Scholar
21 : Interrater reliability of neurologic soft signs in an acquired brain injury population. Arch Phys Med Rehabil 1998; 79:811–815Crossref, Medline, Google Scholar
22. : Contributions of neuroimaging, balance testing, electrophysiology and blood markers to the assessment of sport-related concussion. Br J Sports Med 2009; 43(suppl 1):i36‒i45Google Scholar
23 : Emergency department assessment of mild traumatic brain injury and prediction of post-concussion symptoms at one month post injury. J Clin Exp Neuropsychol 2006; 28:755–772Crossref, Medline, Google Scholar
24 : Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA 2003; 290:2549–2555Crossref, Medline, Google Scholar
25 : Gait stability following concussion. Med Sci Sports Exerc 2006; 38:1032–1040Crossref, Medline, Google Scholar
26. : Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain 2009; 132(pt 10): 2850–2870Google Scholar
27. : Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009; 32(pt 3): 695–708Google Scholar
28 : Bradykinesia and bradyphrenia revisited: patterns of subclinical deficit in motor speed and cognitive functioning in head-injured patients with good recovery. Brain Inj 1998; 12:429–441Crossref, Medline, Google Scholar
29 : Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train 2001; 36:263–273Medline, Google Scholar
30. : Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 2008; 358:453‒463Google Scholar
31 : The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971; 9:97–113Crossref, Medline, Google Scholar
32 : The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry 1971; 127:1653–1658Crossref, Medline, Google Scholar
33. : Assessment of coma and impaired consciousness: a practical scale. Lancet 1974; 304:81–84Google Scholar
34 : The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol 1995; 242:587–592Crossref, Medline, Google Scholar
35 : Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996; 67:588–597Crossref, Medline, Google Scholar
36 : Factor analysis of the Mayo-Portland Adaptability Inventory: structure and validity. Brain Inj 1997; 11:469–482Crossref, Medline, Google Scholar
37 : Neuropsychological impairment scale (NIS): initial validation study using trailmaking test (A & B) and WAIS digit symbol (scaled score) in a mixed grouping of psychiatric, neurological, and normal patients. J Clin Psychol 1983; 39:746–748Crossref, Medline, Google Scholar
38 : Screening for cognitive impairment in multiple sclerosis using the Symbol digit Modalities Test. Mult Scler 2007; 13:52–57Crossref, Medline, Google Scholar
39 : Criterion validity of the California Verbal Learning Test-Second Edition (CVLT-II) after traumatic brain injury. Arch Clin Neuropsychol 2007; 22:143–149Crossref, Medline, Google Scholar
40 : Benton Visual Retention Test performance in normal adults and acute stroke patients: demographic considerations, discriminant validity, and test-retest reliability. Clin Neuropsychol 2009; 23:962–977Crossref, Medline, Google Scholar
41 : Education-stratified base-rate information on discrepancy scores within and between the Wechsler Adult Intelligence Scale—Third Edition and the Wechsler Memory Scale—Third Edition. Psychol Assess 2004; 16:146–154Crossref, Medline, Google Scholar
42 : Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills 1993; 76:1219–1230Crossref, Medline, Google Scholar
43 : The WCST-64: a standardized short-form of the Wisconsin Card Sorting Test. Clin Neuropsychol 2001; 15:228–234Crossref, Medline, Google Scholar
44 : Normative comparisons for the controlled oral word association test following acute traumatic brain injury. Clin Neuropsychol 1999; 13:437–441Crossref, Medline, Google Scholar
45 : Verbal and Nonverbal Cancellation Tasks. Principles of Behavioral Neurology. Philadelphia, F.A. Davis, 1985Google Scholar
46 : Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 2013; 139:81–132Crossref, Medline, Google Scholar
47 : Cognitive performance after mild traumatic brain injury: the impact of poor effort on test results and its relation to distress, personality and litigation. Brain Inj 2007; 21:309–318Crossref, Medline, Google Scholar



