A Novel Anxiety and Affective Spectrum Disorder of Mind and Body—The ALPIM (Anxiety-Laxity-Pain-Immune-Mood) Syndrome: A Preliminary Report
Abstract
The authors describe a spectrum disorder comprising a core anxiety (A) disorder and four domains: joint laxity (L), chronic pain syndromes (P), immune disorders (I), and mood disorders (M)—dubbed the ALPIM syndrome. This study examined 76 consecutive outpatients with an anxiety disorder plus at least one somatic condition from three domains. More than 80% of the patients had panic attacks, fibromyalgia, and major depressive episodes. Associations were found between joint laxity and bipolar III, headache with bipolar II, and bipolar II with chronic fatigue syndrome. Significant relationships were demonstrated within and between domains, validating ALPIM as a syndrome.
The co-occurrence of mental and physical illnesses, particularly those with a paucity of physical signs, has long evoked curiosity. In many cases, these disorders remain medically unexplained or functional, such as fibromyalgia, irritable bowel syndrome, chronic fatigue syndrome (CFS), interstitial cystitis,1 and chronic prostatitis.2 Extensive comorbidity with psychiatric disorders has been described.2–5
Hudson and Pope6 described the “affective spectrum disorder,” which represents a group of 14 psychiatric and medical disorders. The 10 psychiatric conditions include attention deficit hyperactivity disorder, bulimia nervosa, dysthymic disorder, generalized anxiety disorder, major depressive disorder, obsessive-compulsive disorder, panic disorder, posttraumatic stress disorder, premenstrual dysphoric disorder, and social phobia. The four medical conditions include fibromyalgia, irritable bowel syndrome, migraine, and cataplexy. Aggregations of these disorders were observed in family members of patients with fibromyalgia.7
Replicated publications by Bulbena et al.8–10, García Campayo et al.11, and Martín-Santos et al.12 brought attention to the coexistence of panic disorder and joint hypermobility syndrome. Joint hypermobility syndrome is an inherited connective tissue disorder that consists of joint laxity, as defined by Walker et al.,13 and includes specific somatic conditions such as easy bruising, scoliosis, fibromyalgia, and double jointedness.14
Genes on chromosome 13q,15 and possibly on chromosome 22,4 influence the susceptibility toward a pleiotropic syndrome (the panic disorder syndrome) described by Weissman et al.,15 which includes panic disorder, bladder problems, severe headaches, mitral valve prolapse, and thyroid conditions. A national survey compared 313 control subjects with 313 women with interstitial cystitis; the results showed that interstitial cystitis had significant associations with irritable bowel syndrome, CFS, fibromyalgia, migraine, depression, and allergies.16 Despite the absence of bipolar spectrum disorders from the affective disorder spectrum, significant comorbidity between panic disorder and bipolarity was previously described.17,18 Moreover, genetic links between bipolar disorder and panic disorder have been noted.19,20 Notably, replicated studies have established linkage of bipolar disorder to chromosome 18q.21,22 A higher occurrence of panic disorder in bipolar disorder linked with 18q suggests a genetic subtype of bipolar disorder identified by comorbid panic disorder.23 Moreover, recurrent major depressive disorder and anxiety show links to 18q, supporting overlapping genetic etiologies.24 In a recent meta-analysis, single nucleotide polymorphisms in chromosome 3p21.1 showed a significant association with mood disorders and possibly a shared genetic susceptibility locus for bipolar disorder and major depressive disorder.25 Thus, we contend that a spectrum disorder consisting of high rates of anxiety disorder would predict high levels of comorbidity with bipolar disorder.
Extending previous observations, we not only noted a relationship between ligamentous laxity and panic disorder, but we also saw comorbidity with other anxiety disorders, chronic pain disorders, immune disturbances, and mood disturbances. Clinical observation prompted the formulation of a domain-defined clinical syndrome, in which putative comorbidities exist along a spectrum and a patient may exhibit anywhere from one disorder under one domain to multiple disorders under multiple overlapping domains. In contrast with earlier spectrum disorder studies, we sought to integrate unipolar and bipolar mood disorders.
We identified five domains that captured the most commonly occurring comorbidities: anxiety, joint laxity, pain disorders, immune disorders, and mood disorders. This revised version of the previous spectrum disorder was therefore termed the ALPIM syndrome, forming an acronym representing the abovementioned domains. A cross-sectional naturalistic study was performed using a novel questionnaire with patients who were diagnosed with a core anxiety disorder and at least one index physical disorder. The first null hypothesis maintained that the comorbid conditions identified would not differ compared with rates from the general population. The first hypothesis stated that the rates of comorbid conditions that we observed in this cohort would exceed those observed in the general population, implying an enriched sample. The second null hypothesis stated that comorbidity within one ALPIM domain does not significantly increase the likelihood of a second comorbid condition, either within or between domains. We endeavored to provide statistical evidence for significant associations between conditions both within and between domains to refute the second null hypothesis.
A recent large-scale study demonstrated that common single nucleotide polymorphisms are associated with a range of psychiatric disorders—namely, autism spectrum disorder, attention deficit hyperactivity disorder, bipolar disorder, major depressive disorder, and schizophrenia.26 The authors concluded that widespread pleiotropy exists across the currently described arbitrary psychiatric diagnoses. We extend this distinction beyond psychiatric disorders and propose to include certain medical conditions whose physical diagnosis may be similarly arbitrary. Plausible spectrum disorders may provide the critical impetus to support a potential common etiologic pathway toward the development of psychiatric and physical conditions that were previously considered unrelated.
The authors hypothesize that the ALPIM syndrome warrants preliminary consideration as a spectrum disorder, with predictable psychiatric and medical comorbidities, and this syndrome has potential relevance to our conceptualizations of boundaries within and between psychiatric and certain medical disorders.
Methods
On the basis of clinical experience and the extant literature, a number of conditions were selected for each ALPIM domain to test for comorbidity in our cohort of patients. The anxiety domain includes panic disorder, generalized anxiety disorder, and social anxiety disorder. The laxity domain includes joint laxity, mitral valve prolapse, scoliosis, double jointedness, and easy bruising. The pain domain includes fibromyalgia, chronic daily headaches, interstitial cystitis, and prostatitis. The immune domain includes asthma, hypothyroidism, CFS, and allergic rhinitis. Finally, the mood domain comprises bipolar I, bipolar II, bipolar III, major depressive episodes, and antidepressant medication tachyphylaxis. We developed the ALPIM Inventory Questionnaire (Figure 1), and each subject was nonblindly evaluated with this instrument. This inventory was fashioned to detect the various conditions falling within the ALPIM spectrum. On the basis of clinical observation, disorders were not required to be contemporaneously comorbid; rather, lifetime occurrence was used. A combination of history taking, direct examination, and review of medical records was used to gather data. A total of 76 patients were recruited. In terms of ethnic background, all patients were white, except for one Latino patient. The mean age of the sample was 43.19 years (SD 11.03), with a minimum of 19 years and a maximum of 73 years. Of the 76 patients, 58 (76%) were women. Because of the location of the outpatient facility, patients were deemed to have middle to upper middle socioeconomic status. All participants had at least one DSM-IV current anxiety disorder and one index disorder from the laxity, chronic pain, and/or immune domains (i.e., at least one somatic condition was present). The sample comprised outpatients followed either at the New York State Psychiatric Institute/Columbia Presbyterian Medical Center or a private practice (J.C.). Only nine of 76 patients were recruited from the academic setting (11.8%). We do not have the denominator of patients from which the 76 patients with ALPIM syndrome were selected, but all patients were identified for this study within an 18-month period. Patients were recruited consecutively at each setting, provided that they presented with a comorbid physical condition in addition to a specified anxiety disorder. The low number of subjects recruited from the academic setting would appear to contradict the view that these were patients with a more complex set of problems. The breakdown of the sample predominantly implies a typical patient with anxiety that was treated in the community. Appropriate institutional review board approval was obtained.
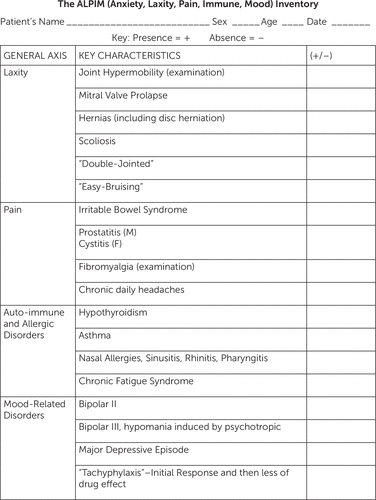
FIGURE 1. The ALPIM Inventorya
aThe ALPIM inventory was administered to patients presenting with a core anxiety disorder and a single comorbid physical condition in order to detect presence of comorbid conditions falling under the ALPIM domains. ALPIM, anxiety, laxity, pain, immune, mood.
Diagnostic criteria for clinical conditions included in the five ALPIM domains are as follows. For inclusion in the study, patients were required to have a primary DSM-IV diagnosis of panic disorder, social anxiety disorder, or generalized anxiety disorder. Joint examinations were performed to detect joint laxity using Beighton’s criteria14 (Figure 2 and Figure 3). Scores of ≥4 in men and ≥5 in women are considered positive for joint laxity. J.C. was first trained in this examination by Bulbena et al.,27 and he subsequently achieved an interrater reliability of 0.96 with an independent blinded rater (Dr. Jane Fried).

FIGURE 2. The Beighton Scale for Joint Laxitya
aJoint examinations were performed to detect joint laxity using Beighton’s criteria. Scores of ≥4 in men and ≥5 in women were considered positive for increased joint laxity. L, left; R, right. (Reproduced from Bulbena et al. [see reference 9].)
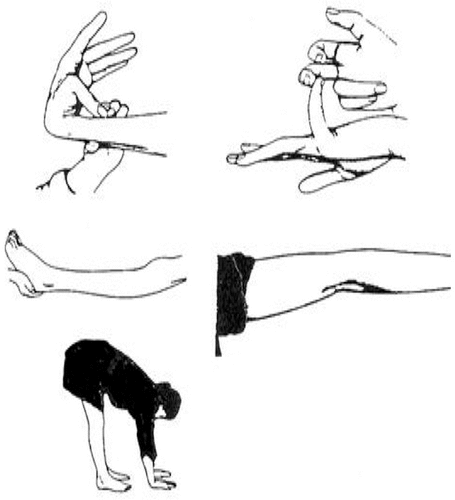
FIGURE 3. Assessment of Joint Laxitya
aThis illustration demonstrates the maneuvers used to score the Beighton scale (see Figure 2 for criteria) for ligamentous laxity. The joint examination looked for the following (clockwise from the top): ability to oppose thumb tip to forearm, finger hyperextension (>90°), elbow extension (>180°), ability to place palms on floor while standing with knee extended, and knee hyperextension (>180°). Scores of ≥4 in men and ≥5 in women were considered positive for joint laxity.
Joint hypermobility syndrome includes mitral valve prolapse, scoliosis, double jointedness, and easy bruising.14 Patients were required to have echocardiographic evidence of mitral valve prolapse and radiographic evidence of scoliosis. Double jointedness and easy bruising were subjective reports by patients. Double jointedness was recorded as positive when the patient responded yes to being asked “Have you ever considered yourself to be double jointed?” and could demonstrate exaggerated flexibility at one or more joint.28 Easy bruising was recorded as positive if the patient gave a history of frequent bruising with minimal trauma. The diagnoses of mitral valve prolapse and scoliosis were not independently confirmed by radiologic evidence. The patient’s self-report of echocardiographic or radiologic evidence was considered sufficient.
Although hernias are included in joint hypermobility syndrome and were initially included in the ALPIM questionnaire, we do not include them in our tabulation of prevalence because many subjects endorsed hiatal and lumbar disc herniation, which made the diagnosis unclear. Therefore, hernia diagnosis was omitted from our analyses.
Our criteria for diagnosis of fibromyalgia were based on the 1990 American College of Rheumatology29 classification criteria. However, subsequent studies30,31 questioned the validity of these criteria. In addition, data from a study by Arnold et al.32 imply that if one tender point is positive, the likelihood of other tender points being positive is very high. Because it was impractical to conduct an examination of 18 trigger points in an outpatient psychiatric setting, we conducted a mini tender point examination entailing six easily accessible tender points (Figure 4). An affirmative diagnosis required four of the six tender points to be positive and endorsement of complaints of diffuse bodily pain, symptoms of fatigue, waking unrefreshed, and cognitive symptoms.
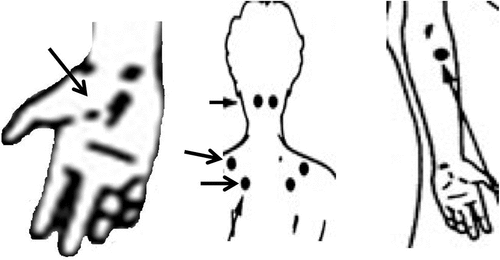
FIGURE 4. The Mini Tender Point Examinationa
aA mini tender point examination was conducted, entailing six tender points (five of which are shown here) as follows (from left to right): base of thenar eminence, suboccipital nuchal point, midpoint of the superior edge of the trapezius, a point superior to the tip of the scapula, and the elbow insertion of the brachioradialis.
For interstitial cystitis and prostatitis, no specific diagnostic tests for either diagnosis are available and each is defined as chronic dysuria and pelvic pain with no known infectious etiology.33,34 Patients were classified as having interstitial cystitis/abacterial prostatitis if they gave a history of chronic pelvic pain and dysuria that did not respond to antibiotics and associated with abnormal urinalysis and urine culture results. Interstitial cystitis and abacterial prostatitis were included as a single entity because of mounting evidence for significant overlap in epidemiology, pathophysiology, and even therapy.35
The Manning criteria were used for diagnosis of irritable bowel syndrome.36 The criteria include onset of abdominal pain linked to more frequent bowel movements, looser stools associated with onset of pain, noticeable abdominal bloating, sensation of incomplete evacuation >25% of the time, and diarrhea with mucus >25% of the time. The presence of more than three of the listed six criteria was considered diagnostic. Irritable bowel syndrome was only diagnosed if the patient reported a history of a negative colonoscopy.
Chronic daily headache37 is a descriptive term that encompasses several different specific headache diagnoses characterized by frequent headaches, which are arbitrarily defined as headaches occurring >15 days per month or 180 days per year in the absence of an underlying structural or systemic disease.37 We did not distinguish between chronic migraines and nonmigrainous headaches. Other disorders causing secondary headache were ruled out on the basis of the patient providing a history of a negative comprehensive physical, neurologic examination, and brain scan. Chronic daily headache was considered positive on our questionnaire only if the patient provided a history of being prescribed ongoing medication for headache relief.37
CFS was defined based on the revised Centers for Disease Control and Prevention criteria.38 CFS comprises clinically evaluated, unexplained, persistent, or relapsing fatigue that is of new or definite onset, is not the result of ongoing exertion, is not alleviated by rest, and results in substantial reduction in previous levels of occupational, educational, social, or personal activities, along with four or more of the following: self-reported impairment in short-term memory or concentration, sore throat, tender cervical or axillary nodes, muscle pain, multijoint pain without redness or swelling, or unrefreshing sleep or postexertional malaise lasting ≥24 hours.
All patients who gave a history of either active or past asthma, including exercise-induced asthma, were included in the analysis. In addition, the patients had to provide a history of receiving current or past treatment with medications for asthma prescribed by their pulmonologists, internists, or primary care providers.
Primary hypothyroidism—commonly known as Hashimoto’s thyroiditis—was the first condition recognized as an autoimmune disease.39 The patients included in our study were considered to have hypothyroidism once they provided a history of hypothyroidism diagnosed by blood testing. These participants may or may not have received a current thyroid replacement regimen. Of note, none of the patients had ever been treated with lithium.
Allergic rhinosinusitis40 entails chronic anterior and/or posterior mucopurulent drainage, along with two or more of the following: nasal obstruction, facial pain, pressure and/or fullness, and decreased sense of smell.
The presence or history of a major depressive episode was established using DSM-IV criteria. The major depressive episode could be current or past, and a distinction was not made regarding whether the episode was part of an underlying unipolar versus bipolar disorder. This was assessed using a semistructured interview based on the Structured Clinical Interview for DSM-III-R.41
Patients underwent a semistructured clinical interview and comprehensive psychiatric assessment to diagnose bipolar disorder. Patients were diagnosed with either bipolar I or II disorder based on the fulfillment of DSM-IV criteria. Bipolar III disorder was diagnosed based on the presence of hypomania or mania resulting from the use of antidepressant medications.42
Tachyphylaxis, or tolerance to antidepressant drug therapy, generally refers to the loss of antidepressant efficacy during long-term use.43–47 A significant decrease in clinical response was defined as a change in symptomatology that necessitated an adjustment in a stable pharmacotherapeutic regimen. Studies of tachyphylaxis showed that this phenomenon occurs in 25%−50% of patients during long-term antidepressant drug therapy.44,46,47
Patients with any unstable medical condition (e.g., hypertension, diabetes, cardiac disease, or seizure disorders) were excluded. One patient with stable myasthenia gravis was included. Treatment with statins for dyslipidemia was not an exclusion criterion.
Statistical Analysis
Data for 76 patients were available for analysis. On the basis of the analysis, we calculated and compared the frequencies, p values, odds ratios, and confidence intervals (CIs). SPSS (version 18; SPSS Inc., Chicago, IL) was used to run logistic regressions to demonstrate associations within and between the ALPIM domains. The same data set was also used to run a cluster analysis to determine whether there were within- and between-domain groupings of the comorbidities under the ALPIM categories.
Results
Rates of Comorbid Conditions
The comorbid conditions had a consistently higher prevalence in the study sample compared with prevalence studies performed in the general population (Table 1). Panic disorder occurred in 86.8% of our study subjects; generalized anxiety disorder and/or social anxiety disorder comprised the remainder of the core anxiety disorder diagnosis. Table 1 provides rates of disorders in the study sample compared with the general population. With respect to the remaining ALPIM domains, the following frequencies were noted.
| ALPIM Spectrum | Comorbidity | Observed Incidence (%) | Population Prevalence (%) |
|---|---|---|---|
| Laxity (L) | Joint laxity | 59.3 | 10–15 (12) |
| Mitral valve prolapse | 32.9 | 2.4 (48) | |
| Easy bruising | 76.3 | 43 (49) | |
| Double jointedness | 57.9 | 4–13 (14) | |
| Scoliosis | 28.9 | 8.3 (50) | |
| Pain (P) | Fibromyalgia | 80.3 | 2.1–5.7 (51) |
| Irritable bowel syndrome | 76.3 | 17 (52) | |
| Chronic daily headaches | 63.2 | 11–22 (53) | |
| Prostatitis/cystitis | 38.2 | 0.5 (54) | |
| Immune (I) | Allergic rhinitis | 71.1 | 20 (55) |
| Chronic fatigue syndrome | 42.1 | 2.6 (56) | |
| Hypothyroidism | 39.5 | 4.6 (57) | |
| Asthma | 32.9 | 8.2 (58) | |
| Mood (M) | Major depressive episode | 92.1 | 16.6 (59) |
| Bipolar II disorder | 71.1 | 3.9 (59) | |
| Bipolar III disorder | 67.1 | 0.56 (61) | |
| Tachyphylaxis | 92.1 | 9–57 (60) |
TABLE 1. Comparative Overview of Incidences of Individual ALPIM Comorbiditiesa
For the ligamentous laxity domain, joint laxity was observed in 59.3% of subjects compared with a prevalence of approximately 10%−15% in the general population.12 Mitral valve prolapse was noted in 32.9% of subjects, whereas its prevalence is about 2.4% in the general population.48 Easy bruising was reported in 57.9% of our cohort. We could not find any studies that examined the prevalence of easy bruising in the general population. However, in a study conducted in children, 43% described a history of easy bruising among those who presented to a pediatric rheumatology and joint hypermobility clinic.49 Double jointedness was observed in 57.9% of our cohort, whereas hypermobility that is not associated with systemic disease occurs in 4%–13% of the general population.14 Scoliosis was noted in 28.9% of subjects, whereas scoliosis is prevalent in approximately 8.3% of the U.S. population.50
With respect to the pain domain, fibromyalgia was observed in 80.3% of our subjects compared with approximately 2.1%−5.7% in the general population.51 Irritable bowel syndrome was observed in 76.3% of our cohort compared with about 17% in the general population.52 Chronic daily headache was reported in 63.2% of subjects compared with about 11% and 22% of men and women, respectively, in the general population.53 Prostatitis/cystitis was noted in 38.2% of our cohort, whereas its prevalence is 0.5% in the general population.54
Within the autoimmune domain, allergic rhinitis was observed in 71.1% of subjects, whereas its prevalence is approximately 20% in the general population.55 CFS was noted in 42.1% of subjects, with a prevalence of approximately 2.6% in the general population.56 Hypothyroidism was noted in 39.5% of subjects, which is seen in about 4.6% of the general population.57 Asthma was noted in 32.9% of patients, with a prevalence of about 8.2% in the general population.58
In the mood disorder domain, major depressive episode and bipolar II disorder were noted in 92.1% and 71.1%, respectively, of our cohort compared with 16.6% and 3.9% in the general population.59 Tachyphylaxis was observed in 92.1% of subjects, whereas the return of depressive symptoms during maintenance antidepressant treatment occurred in 9%–57% of patients in published trials.60 Bipolar III was observed in 67.1% in our sample, whereas switching to bipolarity in patients with major depressive disorder treated with antidepressants was reported as 0.56% over a 3-month period in a recent prospective study.61
Logistic Regression Analysis
More than 80% of patients had a lifetime history of panic disorder, fibromyalgia, major depressive episode, and tachyphylaxis. Because these disorders were observed to occur at a high prevalence (>80%), this combination of disorders was included as a core phenotype of the ALPIM syndrome. Therefore, we elected not to include these disorders in the logistic regression analysis. Careful review of the data revealed that all patients with a major depressive episode experienced our criteria for tachyphylaxis.
Logistic regression (Figure 5 and Table 2) revealed multiple significant associations. Joint laxity was significantly comorbid with bipolar III (odds ratio=4.3, CI=1.5–11.7, p=0.005) but was not significantly comorbid with tachyphylaxis (odds ratio=8.5, CI=0.9–76.4, p=0.57). Interestingly, there was a near significant association between mitral valve prolapse and bipolar III (odds ratio=0.4, CI=0.14–1.0, p=0.05). Scoliosis was significantly comorbid with asthma (odds ratio=5.1, CI=1.7–14.7, p=0.003). Double jointedness was significantly comorbid with easy bruising (odds ratio=3.4, CI=1.3–8.9, p=0.02). Headache was significantly comorbid with bipolar II (odds ratio=6.8, CI=2.3–20.2, p=0.001), asthma (odds ratio=7.1, CI=1.9–26.5, p=0.004), and rhinitis (odds ratio=6.8, CI=2.3–20.2, p=0.001). Asthma was significantly comorbid with allergic rhinitis (odds ratio=4.4, CI=1.1–10.5, p=0.031). CFS was significantly comorbid with bipolar II disorder (odds ratio=3.4, CI=1.1–10.5, p=0.03). Of note, none of the subjects met DSM-IV criteria for bipolar I disorder. Bipolar II was significantly comorbid with bipolar III (odds ratio=3.8, CI=1.3–10.7, p=0.01).
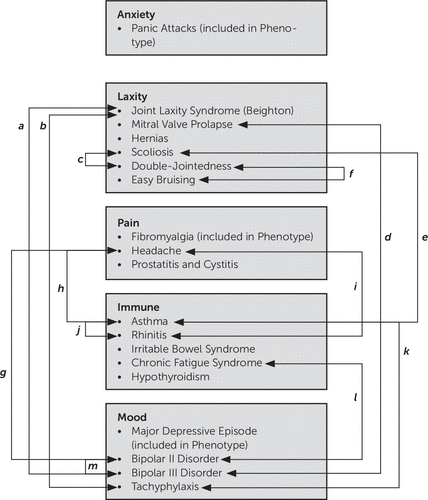
FIGURE 5. A Schema Demonstrating Significant Associations Within and Between ALPIM Domainsa
aThis schematic diagram depicts, via line connections, significant associations within and between the ALPIM domains (see the Results for a description). Table 2 reports corresponding significant probability levels, odds ratios, confidence intervals, and Wald statistics. ALPIM, anxiety, laxity, pain, immune, mood.
| Association | p Value | Odds Ratio (Confidence Interval) | Wald Statistic |
|---|---|---|---|
| Joint laxity with bipolar III | 0.005 | 4.3 (1.5–11.7) | 7.9 |
| Joint laxity with tachyphylaxis | 0.57 | 8.5 (0.9–76.4) | 3.6 |
| Scoliosis with double jointedness | 0.04 | 3.0 (1.1–8.3) | 4.3 |
| Mitral valve prolapse with bipolar III | 0.05 | 0.4 (0.14–1.0) | 3.7 |
| Scoliosis with asthma | 0.003 | 5.1 (1.7–14.7) | 8.9 |
| Double jointedness with easy bruising | 0.02 | 3.4 (1.3–8.9) | 5.9 |
| Headache with bipolar II | 0.001 | 6.8 (2.3–20.2) | 11.7 |
| Headache with asthma | 0.004 | 7.1 (1.9–26.5) | 8.3 |
| Headache with rhinitis | 0.001 | 6.8 (2.3–20.2) | 11.7 |
| Asthma with rhinitis | 0.03 | 4.4 (1.1–16.5) | 4.7 |
| Asthma with tachyphylaxis | 0.03 | 0.1 (0.01–0.7) | 5.0 |
| Chronic fatigue syndrome with bipolar II | 0.03 | 3.4 (1.1–10.5) | 4.5 |
| Bipolar II with bipolar III | 0.01 | 3.8 (1.3–10.7) | 6.2 |
TABLE 2. Significant Associations Within and Between ALPIM Domainsa
Cluster Analysis
The cluster analysis (Figure 6) revealed Euclidean distances within and between ALPIM domains. Disorders from the core phenotype are included in the cluster analysis. Two clear clusters are observed in Figure 6. In addition, the disorders seem to co-occur within domains, to some degree (e.g., the pain domain). The results revealed that all of the mood disorders and tachyphylaxis cluster together. Additional clusters include the following: headache and fibromyalgia (pain domains), along with allergic rhinitis; double jointedness and easy bruising; and hypothyroidism and CFS (immune domains). Interestingly, scoliosis (laxity domain) and asthma (immune domain) also clustered together.
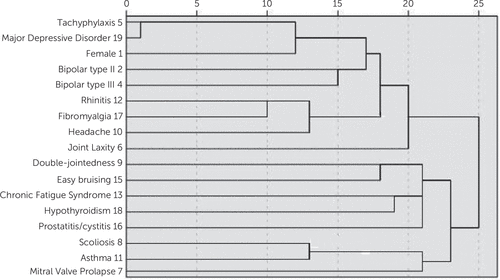
FIGURE 6. Cluster Analysis of the ALPIM Comorbiditiesa
aThis graph represents the rendering of cluster analysis conducted on data collected from all study subjects (N=76). The Euclidean distances are demonstrated on the x-axis, whereas the comorbidities are listed on the y-axis. Smaller Euclidean distances signify higher co-occurrence (see the Results for a description). Interestingly, major depressive disorder and tachyphylaxis are closest in Euclidean distances. The results were concordant with ALPIM syndrome and showed appreciable clustering both within and between domains. ALPIM, anxiety, laxity, pain, immune, mood; ASTHMA, asthma; BIPOL2, bipolar II disorder; BIPOL3, bipolar III disorder; CFS, chronic fatigue syndrome; DJ, double jointedness; EB, easy bruising; FIBROMYA, fibromyalgia; HA, headache; HYPOTH, hypothyroidism; JLS, joint laxity; MDD, major depressive episode; MVP, mitral valve prolapse; NASAL, rhinitis; P_C, prostatitis/cystitis; SCOLIOS, scoliosis; SEX, female sex; TACHY: tachyphylaxis.
Discussion
We aimed to define a refined and extended spectrum disorder based on previous studies conducted by Hudson and Pope,6 Bulbena et al.,8 and Weissman et al.,15 as well as the National Institute of Diabetes and Digestive and Kidney Diseases interstitial cystitis study16 and a study on joint hypermobility syndrome.62 This study, which was conducted with patients with at least one anxiety disorder and one physical disorder, tested our first null hypothesis that the comorbid conditions identified would not differ in rates from the general population. We refute the first null hypothesis because the rates of comorbid conditions we observed in this cohort consistently exceeded those observed in the general population (Table 1). The second null hypothesis stated that comorbidity within one ALPIM domain would not significantly increase the likelihood of a second comorbid condition, either within or between domains. We refute the second null hypothesis, noting that the logistic regression analyses revealed further evidence for significant comorbidity within and across domains and thus validates the underlying hypothesis of a spectrum disorder. We therefore argue that a statistically significant relationship exists between five potentially pathophysiologically linked domains: anxiety disorders, joint laxity, chronic pain disorders, immune dysfunction, and mood disorders (Figure 7). The cluster analysis showed “soft clustering” of disorders within their putative domains. In addition, the Euclidean distances between clusters also suggested a close relationship across domains.
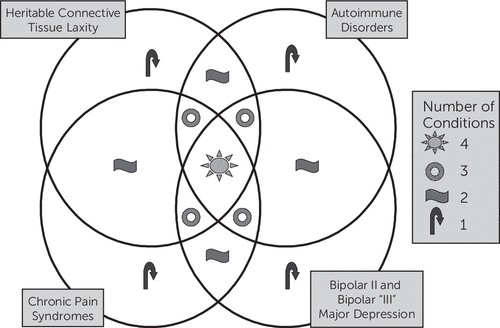
FIGURE 7. The ALPIM Syndrome: A Neuropsychosomatic Spectrum Disordera
aSchematic Venn diagram showing the hypothesized spectrum of comorbidity in patients having a core anxiety disorder with laxity, pain, immune, and mood disorders. The overlapping circles demonstrate that comorbidities exist along a spectrum, in which a patient might have anywhere from just one disorder under one domain to multiple disorders under multiple overlapping domains. ALPIM, anxiety, laxity, pain, immune, mood.
Our refined spectrum disorder systematically builds on the published descriptions of five prior spectrum disorders (Table 3), such that our results are additive to the extant literature. In fact, the only novel condition we introduce to the prior literature, once integrated, is the high rate of bipolar disorder (and its attendant bipolar III and tachyphylaxis). Multiple overlaps between the individual spectrum disorders can be identified (Table 3). Warren et al.16 and the National Institute of Diabetes and Kidney Disease clinical trials group recently described a syndrome that includes interstitial cystitis, irritable bowel syndrome, CFS, fibromyalgia, migraine, depression, and allergies, which is perhaps most similar to ALPIM syndrome. However, our addition of bipolar II disorder, tachyphylaxis, and joint hypermobility to the syndrome identified by Warren et al.16 is notable. On the basis of our literature review, the inclusion of bipolar II disorder seems justified by studies showing a higher incidence of anxiety among patients with bipolar disorder.63
| Spectrum Disorder Studies | Psychiatric Disorders | Medical/Physical Disorders | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anxiety (A) | Mood (M) | Laxity (L) | Pain (P) | Immune (I) | |||||||||
| Anxiety | Mood | Bipolar | Joint Hypermobility | Mitral Valve Prolapse | Scoliosis | Fibromyalgia | Irritable Bowel Syndrome | Headache | Interstitial Cystitis/Bladder Problems | Chronic Fatigue Syndrome | Allergies/Asthma | Thyroid | |
| Affective spectrum disorders6,7,b | + | + | + | + | + | + | |||||||
| Panic disorder and joint hypermobility syndrome8–10 | + | + | + | ||||||||||
| Joint hypermobility syndrome13,14,c | + | + | |||||||||||
| Panic disorder syndrome15 | + | + | + | + | + | ||||||||
| NIDDK study16,d | + | + | + | + | + | + | + | + | |||||
| ALPIM | + | + | + | + | + | + | + | + | + | + | + | + | + |
TABLE 3. Comparison of Five Spectrum Disorders in Relation to the Features of the Proposed ALPIM Syndromea
We endeavor to demonstrate the difference between symptoms and disorders. Interestingly, DSM-564 refers to the “somatic symptom disorder,” which involves anxiety and pain, in certain instances but is otherwise nonspecific. However, we now point out that disorders that were previously viewed purely as somatic symptoms by the psychiatric field have since been recognized as bona fide disorders in other fields of medicine. CFS, which has fought to constitute its own disorder, is one example. Our group previously reported that individuals with CFS have elevated levels of lactate in the CSF, and we used magnetic resonance spectroscopic imaging to assess healthy volunteers and patients with generalized anxiety disorder65 compared with healthy volunteers and patients with major depressive disorders.66 These studies serve to delineate CFS as being biologically distinct from anxiety and mood disorders. Fibromyalgia, a disorder that was frequently viewed as comprising nonspecific somatic symptoms, is another example. Several serotonin/norepinephrine reuptake inhibitors are now approved by the Food and Drug Administration for treatment of fibromyalgia. However, the efficacy of pregabalin,67 an anticonvulsant that has no overt antidepressant properties but possesses anxiolytic effects, contradicts the view that antifibromyalgia effects are merely an alternate presentation of a mood disorder.68 Compared with healthy volunteers, patients with irritable bowel syndrome have rectal distention that produces disproportionate pain and they have excessive activation of the anterior cingulate gyrus. Amitriptyline, an effective drug used to treat irritable bowel syndrome, attenuates pain from rectal distention, but only under stress conditions, suggesting a CNS site of action.69 We therefore argue that a set of symptoms should not be relegated to somatic complaints reiterated by patients. Rather, patients are entitled to receive a medical diagnosis that has a biological basis and facilitates effective and, in certain instances, Food and Drug Administration–approved treatments.
This study has several important limitations that must be considered. Our study lacks a control group; therefore, the hypotheses must be regarded as preliminary. In future studies, it would be useful to examine psychiatric patients without the physical comorbidities of the ALPIM syndrome. The subjects did not necessarily suffer from the disease entities at the time of administration of the ALPIM questionnaire; rather, a lifetime history of the entities was considered. A Hawthorne effect, or an observer bias,70 must be considered because the patient may have been motivated to nonselectively endorse the inquiries of the physician, leading to higher prevalence. Assessment of tender points is a standard component of the rheumatology examination. In this study, we performed an abbreviated form of the tender point examination. However, the diagnosis of fibromyalgia is moving toward nonreliance on tender point examination.71 An additional concern is that patients who have many psychosomatic complaints tend to have more medical tests and thus are more likely to be able to report an abnormality. It appears plausible that patients with somatic cardiac symptoms are more likely to be diagnosed with mitral valve prolapse. However, the case for scoliosis appears less clear-cut. Scoliosis is usually revealed on routine examination or because of physical deformity, especially in a younger population.
Diagnoses were established using a semistructured psychiatric assessment in addition to the ALPIM questionnaire. The interrater reliability of the ALPIM questionnaire has not been established because this is a novel instrument examining these specific comorbidities and the rater was not blinded to the status of the subjects, leading to a potential bias of endorsement and false positive results. Perhaps the most convincing evidence for the diagnosis of the disorder, in addition to fulfilling diagnostic criteria, was that the patient had pursued medical treatment for that disorder (e.g., for irritable bowel syndrome, subjects were required to have abnormal colonoscopy results). Although this protected the likelihood of bias, we still acknowledge that future blinded studies are required. Of note, men were relatively underrepresented in the sample, which could be related to either sampling bias or a lower incidence of ALPIM in men. We acknowledge the vulnerability of this study to bias and we thus refer to this article as a preliminary report, encouraging the field to replicate our findings under more rigorous nonbiased conditions.
We conclude that patients with ALPIM syndrome possess a probable genetic propensity that underlies a biological diathesis for the development of the spectrum of disorders. Viewing patients as sharing a psychological propensity toward somatizing behavior essentially denies patients access to care for the diagnosable medical conditions with which they present. We emphasize that the proposal of ALPIM syndrome is not an entirely “newly recognized syndrome,” in that ALPIM syndrome contains significant elements of past syndromes. Our primary contribution is to add novel elements and groupings to previously described spectrum syndromes. We endeavored to capture these novel contributions (Table 3) and document features either present or absent from previous syndromes. Our results provide further evidence to support a possible common pathophysiologic pathway toward the development of a related set of psychiatric and physical conditions, which were previously considered and, in certain instances, were unrelated.
1 : Functional somatic syndromes: one or many? Lancet 1999; 354:936–939Crossref, Medline, Google Scholar
2 : Functional and chronic anorectal and pelvic pain disorders. Gastroenterol Clin North Am 2008; 37:685–696, ixCrossref, Medline, Google Scholar
3 : Cognitions, behaviours and co-morbid psychiatric diagnoses in patients with chronic fatigue syndrome. Psychol Med 2013; 43:375–380Crossref, Medline, Google Scholar
4 : Further genetic evidence for a panic disorder syndrome mapping to chromosome 13q. Proc Natl Acad Sci USA 2003; 100:2550–2555Crossref, Medline, Google Scholar
5 : Diagnosis, comorbidities, and management of irritable bowel syndrome in patients in a large health maintenance organization. Clin Gastroenterol Hepatol 2012; 10:37–45Crossref, Medline, Google Scholar
6 : The concept of affective spectrum disorder: relationship to fibromyalgia and other syndromes of chronic fatigue and chronic muscle pain. Baillieres Clin Rheumatol 1994; 8:839–856Crossref, Medline, Google Scholar
7 : Family study of fibromyalgia and affective spectrum disorder. Biol Psychiatry 2004; 56:884–891Crossref, Medline, Google Scholar
8 : Joint hypermobility syndrome and anxiety disorders. Lancet 1988; 2:694Crossref, Medline, Google Scholar
9 : Anxiety disorders in the joint hypermobility syndrome. Psychiatry Res 1993; 46:59–68Crossref, Medline, Google Scholar
10 : Joint hypermobility syndrome is a risk factor trait for anxiety disorders: a 15-year follow-up cohort study. Gen Hosp Psychiatry 2011; 33:363–370Crossref, Medline, Google Scholar
11 : Association between joint hypermobility syndrome and panic disorder: a case-control study. Psychosomatics 2010; 51:55–61Crossref, Medline, Google Scholar
12 : Association between joint hypermobility syndrome and panic disorder. Am J Psychiatry 1998; 155:1578–1583Crossref, Medline, Google Scholar
13 : The marfanoid hypermobility syndrome. Ann Intern Med 1969; 71:349–352Crossref, Medline, Google Scholar
14 : Benign joint hypermobility syndrome: evaluation, diagnosis, and management. J Am Osteopath Assoc 2006; 106:531–536Medline, Google Scholar
15 : Potential panic disorder syndrome: clinical and genetic linkage evidence. Am J Med Genet 2000; 96:24–35Crossref, Medline, Google Scholar
16 : Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology 2009; 73:52–57Crossref, Medline, Google Scholar
17 : Familial bipolar disorder: preliminary results from the Otago Familial Bipolar Genetic Study. Aust N Z J Psychiatry 1998; 32:823–829Crossref, Medline, Google Scholar
18 : Prevalence and impact of comorbid anxiety and bipolar disorder. J Clin Psychiatry 2006; 67(Suppl 1):5–7Crossref, Medline, Google Scholar
19 :
20 : Panic disorder with familial bipolar disorder. Biol Psychiatry 1997; 42:90–95Crossref, Medline, Google Scholar
21 : Evidence for linkage of bipolar disorder to chromosome 18 with a parent-of-origin effect. Am J Hum Genet 1995; 57:1384–1394Medline, Google Scholar
22 : Isolation of chromosome 18-specific brain transcripts as positional candidates for bipolar disorder. Am J Med Genet 1997; 74:140–149Crossref, Medline, Google Scholar
23 : Bipolar disorder and panic disorder in families: an analysis of chromosome 18 data. Am J Psychiatry 1998; 155:829–831Crossref, Medline, Google Scholar
24 : Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet 2005; 135B:85–93Crossref, Medline, Google Scholar
25 :
26
27 : Clinical assessment of hypermobility of joints: assembling criteria. J Rheumatol 1992; 19:115–122Medline, Google Scholar
28 : The association of soft-tissue rheumatism and hypermobility. Br J Rheumatol 1998; 37:382–386Crossref, Medline, Google Scholar
29 :
30 : Fibromyalgia: a rheumatologic diagnosis? Rheumatol Int 2007; 27:999–1004Crossref, Medline, Google Scholar
31 : Stop using the American College of Rheumatology criteria in the clinic. J Rheumatol 2003; 30:1671–1672Medline, Google Scholar
32 : A 14-week, randomized, double-blinded, placebo-controlled monotherapy trial of pregabalin in patients with fibromyalgia. J Pain 2008; 9:792–805Crossref, Medline, Google Scholar
33 :
34 : Epidemiology and evaluation of chronic pelvic pain syndrome in men. Int J Antimicrob Agents 2008; 31(Suppl 1):S108–S111Crossref, Medline, Google Scholar
35 : Similarities between interstitial cystitis and male chronic pelvic pain syndrome. Curr Urol Rep 2002; 3:313–318Crossref, Medline, Google Scholar
36 : Towards positive diagnosis of the irritable bowel. BMJ 1978; 2:653–654Crossref, Medline, Google Scholar
37 : Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology 1996; 47:871–875Crossref, Medline, Google Scholar
38 :
39 : The Autoimmune Epidemic. New York, Touchstone, 2009, p 352Google Scholar
40 : Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg 2003; 129(3 Suppl):S1–S32Crossref, Medline, Google Scholar
41
42 : Validating antidepressant-associated hypomania (bipolar III): a systematic comparison with spontaneous hypomania (bipolar II). J Affect Disord 2003; 73:65–74Crossref, Medline, Google Scholar
43 : Can long-term treatment with antidepressant drugs worsen the course of depression? J Clin Psychiatry 2003; 64:123–133Crossref, Medline, Google Scholar
44 : Dual reuptake inhibitors incur lower rates of tachyphylaxis than selective serotonin reuptake inhibitors: a retrospective study. J Clin Psychiatry 2005; 66:705–707Crossref, Medline, Google Scholar
45 : Loss of response to antidepressants and subsequent refractoriness: diagnostic issues in a retrospective case series. J Affect Disord 2001; 64:99–106Crossref, Medline, Google Scholar
46 : Tachyphylaxis in unipolar major depressive disorder. J Clin Psychiatry 2005; 66:283–290Crossref, Medline, Google Scholar
47 : Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr 2006; 11(Suppl 15):12–21Crossref, Medline, Google Scholar
48 : Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999; 341:1–7Crossref, Medline, Google Scholar
49 : Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford) 2005; 44:744–750Crossref, Medline, Google Scholar
50 : Prevalence rates for scoliosis in US adults: results from the first National Health and Nutrition Examination Survey. Int J Epidemiol 1987; 16:537–544Crossref, Medline, Google Scholar
51 : The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38:19–28Crossref, Medline, Google Scholar
52 : Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology 1991; 101:927–934Crossref, Medline, Google Scholar
53 : Epidemiology of headache in a general population—a prevalence study. J Clin Epidemiol 1991; 44:1147–1157Crossref, Medline, Google Scholar
54 : Epidemiology of interstitial cystitis. Urology 1997; 49(5A Suppl):2–9Crossref, Google Scholar
55 Epidemiology of rhinitis and asthma. Clin Experimental Allergy1998; 28(Suppl 2):3–10Medline, Google Scholar
56 : The prevalence and morbidity of chronic fatigue and chronic fatigue syndrome: a prospective primary care study. Am J Public Health 1997; 87:1449–1455Crossref, Medline, Google Scholar
57 : The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160:526–534Crossref, Medline, Google Scholar
58 : Asthma incidence among children and adults: findings from the Behavioral Risk Factor Surveillance system asthma call-back survey—United States, 2006-2008. J Asthma 2012; 49:16–22Crossref, Medline, Google Scholar
59 : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
60 : Loss of antidepressant efficacy during maintenance therapy: possible mechanisms and treatments. J Clin Psychiatry 1998; 59:279–288Crossref, Medline, Google Scholar
61 : Clinical responses to antidepressants among 1036 acutely depressed patients with bipolar or unipolar major affective disorders. Acta Psychiatr Scand 2013; 127:355–364Crossref, Medline, Google Scholar
62 : Articular mobility in an African population. Ann Rheum Dis 1973; 32:413–418Crossref, Medline, Google Scholar
63 : The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord 1998; 50:143–151Crossref, Medline, Google Scholar
64
65 : Ventricular cerebrospinal fluid lactate is increased in chronic fatigue syndrome compared with generalized anxiety disorder: an in vivo 3.0 T (1)H MRS imaging study. NMR Biomed 2009; 22:251–258Crossref, Medline, Google Scholar
66 : Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: comparison with major depressive disorder. NMR Biomed 2010; 23:643–650Crossref, Medline, Google Scholar
67 : Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. J Pain 2010; 11:505–521Crossref, Medline, Google Scholar
68 : The effect of anxiety and depression on improvements in pain in a randomized, controlled trial of pregabalin for treatment of fibromyalgia. Pain Med 2007; 8:633–638Crossref, Medline, Google Scholar
69 : Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005; 54:601–607Crossref, Medline, Google Scholar
70 : Management and the Worker: An Account of a Research Program Conducted by the Western Electric Company. Cambridge, MA, Harvard University Press, 1939Google Scholar
71 : A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum 2004; 50:2974–2984Crossref, Medline, Google Scholar



