Proton Magnetic Resonance Spectroscopy in Social Anxiety Disorder
Abstract
In the present study, 24 nonmedicated patients with social anxiety disorder (SAD) were compared with 24 healthy control subjects to assess metabolite levels in the anterior cingulate, insula, caudate, and putamen using proton magnetic resonance spectroscopy. The ratio of N-acetylaspartate (NAA)/creatine (Cr) was significantly higher in patients with SAD than in healthy control subjects in the anterior cingulate and insula. NAA/Cr ratios in the insula correlated positively with the Liebowitz Social Anxiety Scale total scores in patients with SAD. Our results support the significance and biochemical involvement of the anterior cingulate and insula in the pathophysiology of SAD.
Social anxiety disorder (SAD) is a common disorder characterized by marked and persistent fear of one or more social or performance situations in which the individual may be exposed to scrutiny by others, and embarrassment may occur.1
Functional magnetic resonance imaging (MRI) studies have suggested hyperactivation of the limbic and paralimbic regions, encompassing the amygdala, insula, anterior cingulate, and medial temporal lobe, in response to emotional stimuli, particularly to faces in SAD.2,3 Neuroimaging studies using positron emission tomography have reported an increased blood flow in the amygdaloid-hippocampal region, prefrontal, and temporal areas during anticipation of public speaking4 and also in the amygdaloid complex during a stressful speaking task.5 A recent meta-analysis of neuroimaging studies conducted by Brühl et al.6 has also revealed increased activations in medial parietal and occipital brain regions (posterior cingulate, precuneus, and cuneus), which were less functionally and structurally connected in SAD.
Metabolite concentrations in the brains of patients with SAD have been assessed using the neuroimaging technique of proton magnetic resonance spectroscopy (1H-MRS). Several studies have reported abnormal changes in the neural metabolite concentrations among patients with SAD.7–9
It has been reported that the N-acetylaspartate (NAA)/creatine (Cr) ratio was increased in the left dorsolateral prefrontal cortex, due to the decrease of Cr concentration instead of the increase of NAA concentration in patients with SAD compared with healthy control subjects.8 Another study demonstrated an increase in relative NAA metabolite concentrations in the thalamus of patients with SAD compared with healthy control subjects.9 Of the MRS studies that compared patients with SAD with healthy control subjects, one study found increased NAA and glutamate relative to Cr levels,7 whereas another reported decreased relative glutamate concentrations in the anterior cingulate.9
We measured NAA/Cr, choline (Cho)/Cr, and myo-inositol (mI)/Cr ratios in the anterior cingulate, insula, caudate, and putamen in drug-free adult patients with SAD and healthy control subjects to identify possible metabolite abnormalities in these brain regions. To control the possible confounding effect of medications, only drug-naïve or drug-free patients were included in the study. The secondary aim of this study was to assess whether the severity of SAD and the concurrent levels of anxiety were related to the brain metabolite levels. We hypothesized that higher levels of NAA/Cr in the anterior cingulate and insula, but not in the caudate and putamen, would be detected in patients with SAD compared with healthy control subjects because increased activity in the fear processing network, including the anterior cingulate and insula, appears to have an important participation in SAD.
Methods
Participants
A total of 24 consecutive nonmedicated patients (12 women and 12 men) with generalized subtype of SAD, who were seen in the Anxiety Disorders Outpatient Clinic of the Psychiatry Department of Istanbul Faculty of Medicine between September 2011 and December 2012, and fulfilled the DSM-IV1 diagnostic criteria, were included in this study. Twenty-four healthy control subjects were individually matched with the patients for their age ±2 years, gender, and years of education. They were also screened for the absence of psychiatric disorders, as well as any family history of psychiatric disorder, and they were recruited on a voluntary basis. All the patients and control subjects were right-handed.
Inclusion criteria were 1) diagnosis of SAD with use of the Structured Clinical Interview for DSM-IV/Clinical Version (SCID-I/CV),10 2) age between 18 and 50 years, and 3) medication-free status for at least the previous 6 weeks. Exclusion criteria were 1) any current psychiatric disorder other than SAD diagnosed with the SCID-I/CV, 2) a history of alcohol or drug abuse/dependence, 3) any serious concomitant general medical condition or neurologic disease, 4) a history of medical disorders that may have a causal relationship with SAD, and 5) pregnancy or lactation. Exclusion criteria for the patients and control subjects also included any contraindication for MRI and any history of neurodegenerative disease, seizure, central nervous system infection, cerebrovascular disease, diabetes mellitus, and head trauma that caused loss of consciousness lasting more than 30 minutes or that required hospitalization. Although mild traumatic brain injury was not excluded, none of our patients have such a history.
Among our patients, 16 (66.6%) were treatment naïve and 8 (33.3%) had taken different types of antidepressants, with different dosages, before the 6-week drug-free period.
The ethics committee of Istanbul University Istanbul Faculty of Medicine approved the study. Written informed consent was obtained from all participants after the procedures had been fully explained. This study adheres to the Declaration of Helsinki.
Clinical Assessment
Diagnosis was made during the initial interview by trained psychiatrists. The Liebowitz Social Anxiety Scale (LSAS),11 the Hamilton Anxiety Rating Scale (HAM-A),12 the Hamilton Depression Rating Scale (HAM-D),13 and the Sheehan Disability Scale (SDS)14 were conducted in the patients during the second interview. A semistructured interview form prepared by the authors was used to evaluate the demographic features of the participants.
Neuroimaging Procedures
Cranial MRI and 1H-MRS examinations were performed on a 1.5-T superconducting whole-body MRI scanner and spectroscopic system (Philips Medical Systems; The Netherlands) with a 16-channel head coil. Cranial MR images were acquired to position the MRS volume of interest (voxels) and to identify any cerebral pathology defined in the exclusion criteria (Figure 1). MR images included the following: 1) axial fast spin-echo (SE) T2-weighted images (TR=5800 ms, TE=105 ms, number of excitations=2, matrix=368×512, section thickness=5 mm, intersection distance=1.0 mm); 2) coronal SE T1-weighted images (TR=530 ms, TE=30 ms, number of excitations=2, matrix=196×256, section thickness=3 mm, intersection distance=1 mm); and 3) sagittal fast SE T2-weighted images (TR=5800 ms, TE=105 ms, number of excitations=4, matrix=196×256, section thickness=3 mm, intersection distance=1 mm).
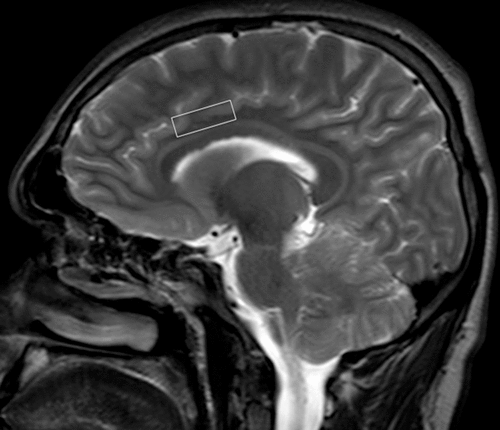
FIGURE 1. Sagittal T2-Weighted Image Showing Voxel Placement Into the Anterior Cingulate Gyrus
Single-voxel 1H-MRS examinations of the patients and control subjects were conducted in the same session with conventional cranial MRI. An experienced neuroradiologist (K.A.) placed the voxels on the left anterior cingulate, insula, putamen, and caudate nuclei using the conventional MR images. The voxels for the insula and cingulate were placed in a manner to maximize the gray matter and minimize white matter and CSF. A 18×12×10-mm–sized voxel on the anterior cingulate gyrus was placed for the anterior cingulate gyrus using the sagittal T2-weighted and coronal T1-weighted images as described by Yücel et al.15 A 10×10×10-mm–sized voxel was positioned for the head of the caudate region. The voxel sizes for the putamen and insula were 14×10×10 and 16×12×10 mm, respectively. The localization of the proton spectra was acquired using the stimulated echo acquisition mode (STEAM; TR=2500 ms, TE=30 ms, 164 acquisitions) sequence. The signals over the voxels were shimmed to within a linewidth of 6–7 Hz. Water suppression was performed with three chemical shift selective saturation pulses at the water resonance. Spectral postprocessing included zero filling to 2048 points, an exponential filter corresponding to 1 Hz of line broadening, Fourier transformation, zero-order phase, and automatic baseline corrections by polynomial interpolation. Spectra with a full width at half maximum smaller than 0.1 ppm and a signal-to-noise ratio above 3 were included in the statistical analysis. The MR system manufacturer’s software package was used for curve fitting and measurement of peak integrals. Major metabolite peaks were assigned to NAA at 2.02 ppm, Cr at 3.02 ppm, Cho at 3.22 ppm, and mI at 3.56 ppm. Cr was used as an internal reference metabolite, and NAA/Cr, Cho/Cr, and mI/Cr ratios were calculated.
Statistical Analysis
The SPSS 11.5 for Windows program was used for statistical analyses. For comparisons between the two groups, Student’s t test was used for continuous variables, and the chi-square test was used for categorical variables. Fisher’s exact test was used when indicated. Pearson’s correlation coefficients were computed between the clinical variables and levels of neural metabolites.
Results
There were no significant differences between the SAD and control groups with respect to age (respectively, 28.50±6.63 and 28.38±5.84 years; t=0.07; not significant) and female ratio (respectively, 50.0% and 50.0%; χ2=00; not significant) (Table 1). The patient and control groups were also not significantly different in terms of the mean number of years of education (respectively, 13.79±2.89 and 13.79±3.22 years, t=0.00; not significant) (Table 1).
| SAD (n=24)[n (%)] | Controls (N=24)[n (%)] | χ2 | p Value | ||
|---|---|---|---|---|---|
| Gender (female) | 12 (50.0) | 12 (50.0) | 0.00 | 1.00 | |
| Mean (SD) | Mean (SD) | df | t | p Value | |
| Age at assessment | 28.50 (6.63) | 28.38 (5.84) | 46 | 0.07 | 0.95 |
| Years of education | 13.79 (2.89) | 13.79 (3.22) | 46 | 0.00 | 1.00 |
| Age of onset | 14.83 (3.82) | — | — | — | — |
| Duration of illness (years) | 14.61 (6.89) | — | — | — | — |
| LSAS-total | 74.04 (27.39) | — | — | — | — |
| LSAS-fear | 39.96 (14.60) | — | — | — | — |
| LSAS-avoidance | 34.09 (13.91) | — | — | — | — |
| HAM-A | 6.86 (5.50) | ||||
| HAM-D | 4.09 (4.32) | — | — | — | — |
TABLE 1. Demographic and Clinical Characteristics of SAD and Control Groupsa
The mean (±SD) age at onset was 14.83 (±3.82) years, and the mean (±SD) duration of illness was 14.61 (±6.89) years. The mean (±SD) LSAS total score was 74.04 (±27.39), the mean (±SD) LSAS fear score was 39.96 (±14.60), and the mean (±SD) LSAS avoidance score was 34.09 (±13.91). The mean (±SD) HAM-A score was 6.86 (±5.50), and the mean (±SD) HAM-D score was 4.09 (±4.32) in the SAD group (Table 1).
Metabolite levels in the four brain regions are given for the SAD group and healthy control subjects in Table 2. The ratios of NAA/Cr were significantly higher in the SAD group than in healthy control subjects in the anterior cingulate and insula (t=4.48, p<0.001 and t=–3.07, p=0.004, respectively). There were no significant differences in regard to the NAA/Cr levels in the anterior cingulate (t=−0.33, p=0.74) and in the insula (t=0.35, p=0.73) between the drug-naïve and drug-free patients with SAD. There were no significant differences in the NAA/Cr ratio between the SAD and healthy control groups in the caudate and putamen (Table 2).
| SAD (n =24) [Mean (SD)] | Controls (N=24) [Mean (SD)] | df | t | p Value | |
|---|---|---|---|---|---|
| Anterior cingulate | |||||
| NAA/Cr | 1.86 (0.05) | 1.80 (0.04) | 42.32 | 4.48 | <0.001 |
| Cho/Cr | 0.83 (0.05) | 0.84 (0.05) | 46 | –0.20 | 0.84 |
| mI/Cr | 0.31 (0.04) | 0.33 (0.05) | 46 | –1.31 | 0.20 |
| Insula | |||||
| NAA/Cr | 1.84 (0.05) | 1.80 (0.03) | 36.62 | 3.07 | 0.004 |
| Cho/Cr | 0.77 (0.03) | 0.77 (0.19) | 41 | 0.02 | 0.99 |
| mI/Cr | 0.26 (0.03) | 0.28 (0.04) | 45 | –1.65 | 0.11 |
| Caudate | |||||
| NAA/Cr | 1.86 (0.03) | 1.85 (0.04) | 46 | 1.34 | 0.19 |
| Cho/Cr | 0.78 (0.05) | 0.77 (0.04) | 46 | 0.50 | 0.62 |
| mI/Cr | 0.35 (0.03) | 0.36 (0.05) | 46 | –1.14 | 0.26 |
| Putamen | |||||
| NAA/Cr | 1.65 (0.20) | 1.64 (0.07) | 46 | 0.08 | 0.94 |
| Cho/Cr | 0.87 (0.03) | 0.86 (0.06) | 34.99 | 0.44 | 0.66 |
| mI/Cr | 0.35 (0.03) | 0.33 (0.04) | 46 | 2.51 | 0.16 |
TABLE 2. Ratios of Metabolite Concentrations for SAD and Control Groupsa
The Cho/Cr ratio showed no difference between the SAD and healthy control groups in the anterior cingulate, insula, caudate, and putamen (Table 2). There were also no significant differences in the mI/Cr ratio between the SAD and healthy control groups in the four brain regions (Table 2).
Table 3 shows the Pearson partial correlation coefficients between neural metabolites and clinical variables. NAA/Cr ratios in the insula correlated positively with the LSAS total scores at the time of MRS scans in patients with SAD (r=0.48, p=0.02). There were no correlations between the LSAS total scores and NAA/Cr ratios in the anterior cingulate, caudate, and putamen. Patients’ LSAS total scores also did not correlate significantly with Cho/Cr and mI/Cr ratios in the four brain regions.
| LSAS Total | HAM-A | HAM-D | |
|---|---|---|---|
| Anterior cingulate | |||
| NAA/Cr | 0.09 (23) | 0.08 (21) | –0.23 (22) |
| Cho/Cr | 0.23 (23) | 0.05 (21) | –0.25 (22) |
| mI/Cr | –0.16 (23) | 0.33 (21) | 0.28 (22) |
| Insula | |||
| NAA/Cr | 0.48 (23) | 0.18 (21) | 0.18 (22) |
| Cho/Cr | 0.09 (23) | –0.20 (21) | –0.05 (22) |
| mI/Cr | 0.41 (22) | 0.24 (20) | 0.08 (21) |
| Caudate | |||
| NAA/Cr | 0.03 (23) | –0.12 (21) | –0.31 (22) |
| Cho/Cr | 0.27 (23) | 0.32 (21) | 0.36 (22) |
| mI/Cr | –0.16 (23) | 0.33 (21) | 0.28 (22) |
| Putamen | |||
| NAA/Cr | –0.29 (23) | 0.15 (21) | 0.12 (22) |
| Cho/Cr | –0.15 (23) | –0.14 (21) | –0.10 (22) |
| mI/Cr | 0.05 (23) | 0.18 (21) | –0.11 (22) |
TABLE 3. Correlations Between Neurochemical Levels and Symptomatology Within the SAD Samplea
There were no significant correlations between the HAM-A scores of the patients with SAD and the NAA, Cho, or mI concentrations in the insula, anterior cingulate, caudate, and putamen (Table 3). There was also no significant correlation found between the HAM-D scores and the NAA, Cho, or mI concentrations in the four brain regions in patients with SAD (Table 3).
Discussion
The present study revealed a higher NAA/Cr ratio in the anterior cingulate and insula in patients with SAD compared with healthy control subjects. Similar to our findings, Phan et al.7 reported significantly higher NAA levels in the anterior cingulate of patients with SAD compared with those of control subjects. We found a significant correlation between the severity of social anxiety symptoms and the NAA/Cr levels in the insula in patients with SAD, whereas the results of the study by Phan et al.7 showed that the NAA/Cr in the anterior cingulate cortex (ACC) correlated positively with intensity of social anxiety symptoms. The same authors also found that the Cho/Cr ratio was reduced in the ACC of patients with SAD.7 Our study failed to find significant differences in Cho/Cr or mI/Cr ratios between the SAD and healthy control groups in any of the brain regions studied.
It has been reported that the NAA/Cr ratio of patients with SAD was significantly higher than that of control subjects in the left dorsolateral prefrontal cortex.8 According to the authors, this finding was caused by the decrease in Cr concentration instead of the increase in NAA concentration.8 In another study, NAA metabolite concentration was found to be increased in the thalamus of patients with SAD.9
Different from the study that found decreased NAA concentrations in the subcortical regions,16 the findings of the study by Howells et al.9 and of our study showed that no significant difference in the NAA/Cr ratio in the caudate and putamen between the SAD and healthy control groups. In another study, no difference was also found in NAA/Cr ratio in the subcortical regions between individuals with social phobias and controls.17
Functional MRI studies have suggested increased activity in the fear processing network, including the amygdala, hippocampus, insula, ACC, and medial prefrontal cortex, in SAD.3,6,18 There is plenty of evidence for the role of the ACC in the processing of emotional information and fear-related responding to social interaction.19 On the other hand, reviews and meta-analysis of neuroimaging studies in SAD revealed that the insular cortex is hyperactive in response to emotional stimuli compared with healthy control subjects.2,18
It has been indicated that participants with SAD might experience a stronger sensation of disgust than participants without SAD when observing disgusted faces.19 Phillips et al.20 demonstrated a neural substrate for perception of facial expressions of disgust, involving primarily the anterior insula. Besides the insula, the ACC was also shown to be activated but to a lesser extent by observing emotional facial expressions of disgust.21
Craig22 proposed that primary interoceptive activity is produced in the anterior insula, which seems to provide the basis for emotional awareness. Phillips et al.23 showed that there is a relationship between activation of the anterior insula and ACC and the emotional anxiety and discomfort produced by nonpainful visceral stimulation while viewing fearful faces. A hypothetical link between the insula and ACC that interacts when processing threat has been suggested.24–26
A recent study by Klumpp et al.27 showed that patients with SAD exhibit exaggerated insula reactivity and deficient ACC recruitment during direct and indirect processing of emotional faces.
NAA is a marker for neuronal health and viability and thereby provides functional and structural integrity of neurons.28,29 Recent experimental studies reported that NAA reflects the functional role of neurochemical alterations, primarily as a metabolically linked system to provide for the basic needs of neurons.30 Given that NAA is a marker of neuronal integrity and functionality, increased levels of NAA in the anterior cingulate and insula of patients with SAD may reflect increased activity of an emotional processing network.
The following limitations must be considered when evaluating the results of our study. First, because this study was based on a small sample population, our results must be treated with caution. Another possible limitation in the current study was that we used the ratios of NAA, Cho, and mI to Cr instead of absolute concentrations of the metabolites. Third, two-thirds of our patients with SAD were drug naïve, whereas others were drug free for at least 6 weeks. However, we compared drug-naïve and drug-free patients with SAD in regard to the NAA/Cr levels in the anterior cingulate and insula to eliminate this limitation and found no differences between the two groups. An additional limitation of our study was that we obtained 1H-MRS data at a relatively low field strength of 1.5 T, which could have limited the signal-to-noise ratio of our measures.
In conclusion, we found that the ratio of NAA/Cr was significantly higher in the anterior cingulate and insula in patients with SAD than in healthy control subjects. In addition, we found a positive correlation between the NAA levels in the insula and SAD symptom severity, implicating the significance of the insula as an important region in SAD. Our results support the significance and biochemical involvement of the anterior cingulate and insula in the pathophysiology of SAD.
1 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, American Psychiatric Association, 1994.Google Scholar
2 : Neuroimaging in social anxiety disorder: a systematic review of the literature. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:565–580Crossref, Medline, Google Scholar
3 : Functional magnetic resonance imaging during emotion recognition in social anxiety disorder: an activation likelihood meta-analysis. Front Hum Neurosci 2012; 6:347.Medline, Google Scholar
4 : Cerebral blood flow during anticipation of public speaking in social phobia: a PET study. Biol Psychiatry 2002; 52:1113–1119Crossref, Medline, Google Scholar
5 : Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001; 158:1220–1226Crossref, Medline, Google Scholar
6 : Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev 2014; 47:260–280Crossref, Medline, Google Scholar
7 : Anterior cingulate neurochemistry in social anxiety disorder: 1H-MRS at 4 Tesla. Neuroreport 2005; 16:183–186Crossref, Medline, Google Scholar
8 : Quantitative 3.0T MR spectroscopy reveals decreased creatine concentration in the dorsolateral prefrontal cortex of patients with social anxiety disorder. PLoS One 2012; 7:e48105.Crossref, Medline, Google Scholar
9 : (1)H-magnetic resonance spectroscopy in social anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry 2015; 58:97–104Crossref, Medline, Google Scholar
10 : Structured Clinical Interview for DSM-IV Clinician Version (SCID-I/CV). Washington, DC, American Psychiatric Publishing, 1997Google Scholar
11 : Social phobia. Mod Probl Pharmacopsychiatry 1987; 22:141–173Crossref, Medline, Google Scholar
12 : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
13 : Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
14 : The Anxiety Disease. New York, Scribner, 1984, pp 148–149Google Scholar
15 : Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust N Z J Psychiatry 2008; 42:467–477Crossref, Medline, Google Scholar
16 : Magnetic resonance spectroscopy in social phobia: preliminary findings. J Clin Psychiatry 1993; 54(Suppl):19–25Medline, Google Scholar
17 : A repeat proton magnetic resonance spectroscopy study in social phobia. Biol Psychiatry 1997; 42:419–424Crossref, Medline, Google Scholar
18 : Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164:1476–1488Crossref, Medline, Google Scholar
19 : Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry 2005; 57:975–981Crossref, Medline, Google Scholar
20 : A specific neural substrate for perceiving facial expressions of disgust. Nature 1997; 389:495–498Crossref, Medline, Google Scholar
21 : Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron 2003; 40:655–664Crossref, Medline, Google Scholar
22 : Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol 2003; 13:500–505Crossref, Medline, Google Scholar
23 : The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain 2003; 126:669–684Crossref, Medline, Google Scholar
24 : How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70Crossref, Medline, Google Scholar
25 : Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Crossref, Medline, Google Scholar
26 : Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 2007; 104:11073–11078Crossref, Medline, Google Scholar
27 : Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biol Mood Anxiety Disord 2013; 3:7.Crossref, Medline, Google Scholar
28 : N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol 1995; 46:531–540Crossref, Medline, Google Scholar
29 : N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007; 81:89–131Crossref, Medline, Google Scholar
30 : Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain’s “operating system”: how NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids 2010; 39:1139–1145Crossref, Medline, Google Scholar



