Deficits in Limb Praxis in Patients With Obsessive-Compulsive Disorder
Abstract
There is recent evidence of deficits in praxis in patients with primary dystonia. Obsessive-compulsive disorder (OCD) has been linked to disorders of higher-order motor function, such as dystonia. However, no clear mechanism underlying such a relationship has been found. This pilot study aimed to identify whether patients with OCD might also show deficits in praxis. Patients with OCD were compared with healthy volunteers on a meaningless gesture imitation task. Patients showed significantly lower scores in this task. Further studies are needed to elucidate the nature of patients’ deficits in praxis. This might reveal similar mechanisms underlying OCD and some types of movement disorders.
Recent studies recognize an increased incidence of affective disorders in patients with movement disorders. Of these, several studies report a link between some types of dystonia and obsessive-compulsive disorder (OCD), although the pathophysiology of this association remains unclear.1–4
Dystonia has been hypothesized to arise from deficits in inhibitory control. OCD is a neuropsychiatric disorder characterized by obsessions or compulsions reflecting frontostriatal dysfunction. This raises the possibility of a common mechanism underlying dystonia and OCD.1–3 Limb apraxia is defined as a higher-order motor dysfunction in skillful action despite preservation of coordination, comprehension, and the primary motor and sensory apparatus. Limb apraxia is often observed after a stroke, particularly affecting the parietal lobe, but it has also been described in patients with movement disorders.5
A recent study demonstrated the presence of limb apraxia in a group of patients with primary dystonia.6 The patients tested on the task had cervical dystonia yet showed more generalized deficits, affecting their limbs. Whether limb apraxia is observed in OCD is not yet known.
In this study, we compared rates of limb apraxia between patients with OCD and matched healthy volunteers using a meaningless gesture imitation task, which has been shown to reliably predict deficits in praxis.7,8 We hypothesized patients to be impaired. Our preliminary findings suggested this to be the case.
Our results should encourage further studies of higher-order motor dysfunction in patients with OCD, because they can be readily tested for such deficits in view of their relatively preserved elementary motor function. Identifying a link between OCD and praxis would suggest a common mechanism underlying the two and may predict prognosis for this disorder, particularly in regard to developing dystonia.
Methods
A total of 24 patients with a diagnosis of OCD and 22 healthy volunteers took part in the study. Participants were tested on a 10-item meaningless gesture task, extracted from the Birmingham Cognitive Screen (Figure 1).8
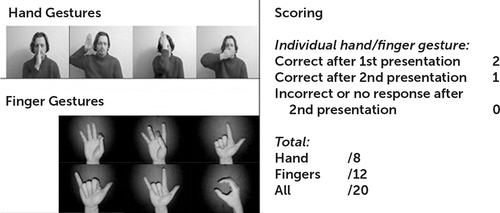
FIGURE 1. Meaningless Gesture Imitation Taska
a The left panel shows hand and finger gestures used for the meaningless gesture imitation task. The right panel describes scoring for each individual gesture (maximum of 2 points per correct gesture production) and the total scores for all. Adapted from Humphreys et al.8 with permission.
Healthy volunteers and patients with OCD were recruited by community advertisements in the East Anglia region. Patients with OCD were also recruited from clinicians and advertisements to local support groups. Healthy participants (mean age=37.4 years [range=21–57 years], male:female ratio=10:12) had no previous history of psychiatric illness and were not taking any medications.
To confirm the diagnosis (DSM-IV-TR criteria) and exclude comorbid psychiatric disorders, patients with OCD (mean age=37.9 years [range=23–67 years], male:female ratio=11:9) were screened by a psychiatrist using the Structured Clinical Interview (Mini-International Neuropsychiatric Inventory). General exclusion criteria for both groups were substance dependence, current major depression of moderate severity, head injury, or serious neurological, medical (in particular, neurological), or psychiatric illness. Notably, patients with OCD in this study had no evidence of neurological disorders such as dystonia.
Patients completed the Yale-Brown Obsessive Compulsive Scale (YBOCS) to assess severity and characteristics of OCD symptoms. Of the 24 patients with OCD, 15 were taking specific selective serotonin reuptake inhibitor medication and four were also taking antipsychotic medications.
Healthy volunteers were recruited to match gender, age, and verbal IQ using the National Adult Reading Test. Healthy volunteers were free from medications and neurological, medical, or psychiatric conditions. All participants completed the Beck Depression Inventory (BDI), the Spielberger State-Anxiety Inventory (SSAI), and the Spielberger Trait-Anxiety Inventory (STAI).
Participants were tested on the meaningless movement imitation task derived from the apraxia testing section of the Birmingham Cognitive Screening tool.8 This cognitive assessment tool has been validated against existing tests of apraxia. Previously, an interrater reliability analysis to determine consistency among raters on this task had a kappa of 1.00 (p<0.001; 95% confidence interval=0.94–1.00; Chapters 6 and 7 in Humphreys et al.8).
The task required imitation of 10 nonsymbolic gestures using the participant’s dominant hand, all of which required holding a static position after demonstration by the experimenter. The test consisted of four gestures involving whole hand movements and six involving independent finger movements. They were performed slowly by the experimenter in front of the participants for them to reproduce immediately afterward. If an item was not reproduced flawlessly on the first presentation, a second trial was given. The participants’ performance was scored 2, 1, or 0, depending on whether it was correct on the first or second presentations or never succeeded. The total possible score was 20 points (with up to eight points for hand gestures and up to 12 points for finger gestures).
The Mann-Whitney test was used to compare between-subject groups. Correlation analyses using Spearman’s rho were performed for patients with OCD to compare between performance on the meaningless gesture tasks and their OCD scores as well as their measures on the National Adult Reading Test, SSAI, and STAI.
Results
The patient demographic and clinical characteristics are shown in Table 1.
| Characteristic | Patients With OCD (N=24) | Healthy Volunteers (N=22) | t | df | p | U | z | r |
|---|---|---|---|---|---|---|---|---|
| Male:female ratio | 14/10 | 12/10 | — | — | — | |||
| Age (years) | 37.9 (14.7) | 37.4 (13.5) | –0.42 | 43 | 0.34 | |||
| Verbal IQ | 115 (5.8) | 117 (3.8) | 1.7 | 34 | 0.26 | |||
| Meaningless gesture imitation score (of 20) | ||||||||
| Mean | 18.3 (1.8) | 19.9 (0.3) | 0.001 | 132.5 | –3.35 | –0.49 | ||
| Median score | 19 | 20 | ||||||
| YBOCS total | 25.8 (6.9) | — | — | — | ||||
| Obsessions | 13.25 (3.6) | — | — | — | ||||
| Compulsions | 12.9 (3.1) | — | — | — | ||||
| Treatment status, N | — | — | — | — | ||||
| None | 9 | |||||||
| SSRI | 15 | |||||||
| Antipsychotics | 4 | |||||||
| BDI | 18.7 (9.6) | 4.4 (4.7) | 5.7 | 34 | <0.001 | |||
| STAI | 35.2 (10.8) | 54.4 (11.7) | 5.5 | 41 | <0.001 | |||
| SSAI | 46.9 (12.2) | 35.5(10.5) | –4.86 | 43 | <0.001 |
TABLE 1. Patient Demographics and Scoresa
The participant male:female ratios between the two groups were not significantly different [χ2(1)=–0.182, p=0.67, and χ2(1)=0.414, p=0.67, for healthy volunteers and patients with OCD, respectively].
Apraxia scores on the meaningless gesture task differed significantly between the two groups. Scores for patients with OCD averaged 18.3 (median=19) compared with those of healthy participants, which averaging 19.9 (median=20; Mann-Whitney U=132.5, z=−3.35, p=0.001, r=−0.49). When looking at the meaningless gesture scores for hand and fingers separately, scores were significantly lower in the OCD group. Mean accuracy for hand gestures in patients with OCD was 7.3 compared with 7.9 in healthy volunteers (Mann-Whitney U=197, z=−2.22, p=0.03, r=−0.33), and mean accuracy for finger gestures in patients was 10.9 compared with 11.9 in healthy volunteers (Mann-Whitney U=170, z=−2.68, p=0.01, r=−0.40). There was no significant difference in the gesture imitation scores between patients with OCD who were taking neuroleptics (N=15) versus patients not taking neuroleptics (N=9; t=1.08, p=0.3).
There were no significant correlations of the apraxia measures on either the global YBOCS score (r=0.082, p=0.73) or the separate obsession (r=−0.050, p=0.83) and compulsion scores (r=−0.004, p=0.99). There was also no correlation between the meaningless gesture imitation task results and age (r=0.308, p=0.20), SSAI (r=−0.217, p=0.32), STAI (r=−0.077, p=0.73), and BDI (r=−0.047, p=0.83) scores.
Discussion
We found that patients with OCD have reduced performance on a meaningless gesture task measuring limb apraxia. Although patients with OCD may not have developed limb apraxia per se, they made more errors than the healthy volunteers did. The task used for this pilot study is designed for detection of limb apraxia in a clinical setting.8 Several studies report meaningless gesture imitation to be the most sensitive method for detecting limb apraxia. A version of this task has previously been used to identify apraxia in patients with mild cognitive impairment and reliably predicted progression to Alzheimer’s disease.7
Two possible mechanisms may underlie our current observations of a deficit in meaningless gesture imitation in OCD. First, this measure of praxis skills likely relates to impairments in action recognition. Meaningless gesture imitation likely demonstrates deficits in perceptual discrimination of human body postures or body motion among individuals with apraxia.9 Along this same line, studies on OCD have shown the condition to be associated with abnormalities in the detection of human motion, as represented by point lights as well as in the discrimination of static human bodily postures.10
Second, apraxia in patients with OCD may be related to abnormalities in motor response inhibition. OCD is associated with deficits in inhibitory processes on several levels. In a recent meta-analysis, OCD was associated with decreased short intracortical inhibition, enhanced intracortical facilitation, and decreased cortical silent period.11 Patients with OCD and their unaffected family members show greater activity of the presupplementary motor area during response inhibition compared with healthy volunteers, suggesting a possible endophenotypic abnormality.11,12 A study supporting the latter reported deficits in the Flanker task in a group of patients with OCD and their unaffected relatives.13,14
To our knowledge, there are no studies to date investigating response inhibition in patients with apraxia. Some authors have suggested that meaningless gesture imitation may involve impairment during action conflict.15,16 However, results from a study using the same cohort of patients with OCD as this one revealed no group differences in tests of response inhibition measured with both the Flanker task and a probabilistic conflict task involving conflict-induced suppression.17
Traditional models of apraxia have implicated two processes for imitating gestures, based on patients’ patterns of error on these tasks: a semantic or “indirect” route, which is more likely involved in imitation of gestures that are meaningful, and a “direct” route to action, which bypasses semantic processing, for the imitation of gestures that are meaningless.18,19 Lesion-mapping studies in patients with limb apraxia, corroborated by functional neuroimaging studies of imitation, report a network of brain regions predominantly within the left hemisphere, which include the superior temporal sulcus, inferior parietal lobe (angular gyrus), and basal ganglia.18,19 The inferior frontal gyrus, including adjacent ventral premotor areas, has been implicated more recently.20,21 The latter regions are also reported in functional neuroimaging studies of response inhibition.22
Performance of a meaningless gesture imitation task could trigger the elicitation of gesture representations, some of which may be meaningful and may require inhibition. The incongruent trials in a Flanker task may elicit similar inappropriate action representations that require inhibition.13,14 However, the group of patients with OCD in this study did not show abnormalities on this task.17 Further studies, perhaps using more traditional tasks of response inhibition (e.g., Stroop22), may unravel the mechanism leading to the deficits observed in this task.
Although these findings are only preliminary, we have identified that patients with OCD may display higher-order motor deficits. Further studies are required to investigate the exact mechanisms underlying this observation.
1 : Psychiatric symptoms associated with focal hand dystonia. Mov Disord 2010; 25:2249–2252Crossref, Medline, Google Scholar
2 : Obsessive compulsive disorder among idiopathic focal dystonia patients: an epidemiological and family study. Biol Psychiatry 2002; 52:356–361Crossref, Medline, Google Scholar
3 : Obsessive-compulsive symptoms in primary focal dystonia: a controlled study. Mov Disord 2011; 26:2274–2278Crossref, Medline, Google Scholar
4 : Myoclonus-dystonia, obsessive-compulsive disorder, and alcohol dependence in SGCE mutation carriers. Neurology 2007; 68:522–524Crossref, Medline, Google Scholar
5 : Limb apraxias: higher-order disorders of sensorimotor integration. Brain 2000; 123:860–879Crossref, Medline, Google Scholar
6 : Patients with primary cervical dystonia have evidence of discrete deficits in praxis. J Neurol Neurosurg Psychiatry 2011; 82:615–619Crossref, Medline, Google Scholar
7 : A novel technique for the quantitative assessment of apraxic deficits: application to individuals with mild cognitive impairment. J Neuropsychol 2007; 1:237–257Crossref, Medline, Google Scholar
8 : The Birmingham Cognitive Screen (BCoS). London, Psychology Press, 2012Google Scholar
9 : Apraxia and the parietal lobes. Neuropsychologia 2009; 47:1449–1459Crossref, Medline, Google Scholar
10 : Selective impairment in visual perception of biological motion in obsessive-compulsive disorder. Depress Anxiety 2008; 25:E15–E25Crossref, Medline, Google Scholar
11 : A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 2013; 124:1309–1320Crossref, Medline, Google Scholar
12 : Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J Psychiatry 2012; 169:1100–1108Crossref, Medline, Google Scholar
13 : Effects of noise letters upon identification of a target letter in a nonsearch task. Percept Psychophys 1974; 16:143–149Crossref, Google Scholar
14 : Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia 2008; 46:1877–1887Crossref, Medline, Google Scholar
15 : The meaning of meaningless gestures: a study of visuo-imitative apraxia. Neuropsychologia 1997; 35:333–341Crossref, Medline, Google Scholar
16 : Goal-directed imitation in patients with ideomotor apraxia. Cogn Neuropsychol 2005; 22:419–432Crossref, Medline, Google Scholar
17 : Evidence accumulation in obsessive-compulsive disorder: the role of uncertainty and monetary reward on perceptual decision-making thresholds. Neuropsychopharmacology 2015; 40:1192–1202Crossref, Medline, Google Scholar
18 : Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain 2007; 130:1111–1126Crossref, Medline, Google Scholar
19 : Neuropsychological perspectives on the mechanisms of imitation. Philos Trans R Soc Lond B Biol Sci 2009; 364:2337–2347Crossref, Medline, Google Scholar
20 : Neural representations of skilled movement. Brain 2000; 123:2306–2313Crossref, Medline, Google Scholar
21 : Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci 2008; 28:3030–3041Crossref, Medline, Google Scholar
22 : Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Hum Brain Mapp 2005; 25:22–34Crossref, Medline, Google Scholar



