Default Mode Network Functional Reorganization During Early Abstinence in Polysubstance-Using Emerging Adults Treated for Opioid Dependence
Abstract
This study examined default mode network connectivity within the first 30 days of abstinence in emerging adults entering treatment for opioid dependence. There were significant associations between abstinence duration and coupling strength with brain regions within and outside of the network.
Emerging adulthood describes the period from the late teens through the 20s, during which brain neurodevelopment is ongoing.1 Compared with other age cohorts, emerging adults have the highest rates of opioid and other illicit substance use,2 are more likely to use opioids in combination with other substances,3 and have worse treatment outcomes.4 Functional brain organizational differences among emerging adults are posited to play a role in their greater vulnerability to drug addiction and its negative cognitive neurological consequences, including lifetime executive function deficits.5
Among opioid-dependent patients, reduced regional coupling strength within the default mode network (DMN) has been observed in association with greater impulsivity,6 greater reported craving,7 and a longer lifetime duration of heroin use.8 These international studies, however, combined developmentally different age groups and excluded polydrug users, who comprise approximately 85% of persons entering opioid detoxification treatment in the United States.9 To our knowledge, no studies have examined the association between DMN connectivity and early abstinence (within the first 30 days), which is the timeframe when deficits in higher-order cognitive function are observed to peak in opioid dependence.10
On the basis of evidence that reduced regional coupling strength within the DMN is associated with more lifetime opioid use and greater cognitive and clinical severity measures, we hypothesized that a longer duration of abstinence from all substances would be associated with stronger regional within-network DMN connectivity in a sample of emerging adults entering treatment for opioid dependence.
Methods
Participants
Participants were recruited from the partial hospitalization program at McLean Hospital after completion of a 3- to 4-day inpatient detoxification program. Exclusion criteria included neurologic illness or injury, MRI contraindications, any psychiatric condition that would interfere with provision of consent or valid self-report, acute intoxication, substance withdrawal, pregnancy, or safety concerns. The McLean Hospital Institutional Review Board approved this study. Informed consent was obtained from participants after the study procedures had been fully explained.
Data Collection and Analyses
Substance use data in the past 30 days were collected using the Addiction Severity Index (ASI). The ASI was modified to include the question, “How many days has it been since you used [substance].” Data regarding DSM-IV substance use and co-occurring disorders were collected through the Structured Clinical Interview.
MRI scans were conducted using a Siemens Trio 3T scanner and 32-channel head coil. Structural MRI images were acquired for co-registration with the functional data using the following parameters: resolution, 1.0×1.0×1.33 mm; TR, 2.1 seconds; TE, 3.3 milliseconds; slices, 128; matrix, 256×256; and flip angle, 7 degrees. Six-minute, eyes-open, resting-state gradient echo-planar functional magnetic resonance imaging (fMRI) images were acquired with the following parameters: TR, 2.5 seconds; TE, 30 milliseconds; flip angle, 90 degrees; slices, 42; and voxel size, 3.5 mm isotropic.
All data analyses were performed using the fMRI Software Library (FSL). Motion correction, brain extraction, slice timing correction, spatial smoothing with a Gaussian kernel of full width at the half maximum (6 mm), a high-pass temporal filter with Gaussian-weighted least-squares, and straight-line fitting (100 seconds) preprocessing steps were conducted. Subject-specific data were registered to the Montreal Neurological Institute MNI-152 standard space template at 2 mm3. fMRI data were transformed using 2×2×2-mm resolution. The FSL MELODIC was used to remove sources of noise for each individual’s data.
A group-level independent component analysis using FSL MELODIC was then performed to define the DMN for the participant sample. Consistent with our previous work,11,12 dimensionality was fixed to 35 components to examine large-scale resting-state networks. The DMN was identified visually, and its spatial distribution matched previous work.13 To calculate participant-specific time courses and spatial maps for the DMN, we used the dual regression approach implemented with FSL.
To assess whether DMN coupling varies relative to abstinence duration, we used the nonparametric permutation method (FSL Randomize) to correlate days since last substance use with DMN coupling (5,000 permutations). Cluster-based thresholding was corrected for multiple comparisons by using the null distribution of the max cluster size across the image (cluster-corrected z=2.3, p<0.05).
Results
A total of 13 Caucasian emerging adults (eight men and five women) aged between 18 and 27 years completed an fMRI brain scan within 24 hours of partial hospital admission for treatment of opioid dependence. Equal numbers (46%) presented for heroin or prescription opioid dependence, and 8% presented for both. An estimated 15% of participants were also receiving opioid agonist therapy, which was continued during and after detoxification treatment. Participants reported a mean (± standard deviation) of 1±1 year of lifetime opioid use and 5±6 days since using any psychoactive drug (including opioids, cannabis, cocaine, sedatives, and alcohol and excluding prescribed opioid agonist medication and nicotine). There were no significant relationships between the number of days since last substance use (range=1–23 days) and age (r=−0.20, p=0.50), sex (r=−0.16, p=0.61), or education level (r=0.41, p=0.19). Participants met DSM-IV dependence criteria for additional substances (54% cannabis, 23% sedatives, 15% cocaine, 8% alcohol) and met criteria for co-occurring disorders (23% substance-induced mood or anxiety disorder, 15% depression, 7% panic disorder). Spontaneous resting-state blood-oxygen-level–dependent signal fluctuations ascribable to the DMN were detected in our sample (Figure 1 [A]), and there was a significant association between abstinence duration and strength of connectivity between the DMN and brain regions identified in Figure 1 [B].
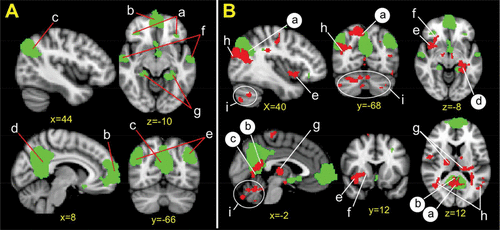
FIGURE 1. [A] DMN and [B] DMN Regional Coupling and Abstinencea
a[A]The DMN encompasses a set of distributed brain regions including the (a) dorsomedial prefrontal cortex, (b) ventromedial prefrontal cortex, (c) ventral precuneus cortex, (d) posterior cingulate cortex, (e) inferior parietal cortex, (f) lateral temporal cortex, and (g) hippocampal formation. Using the fMRI Software Library, we conducted an independent component analysis to identify the DMN. Spontaneous resting-state blood-oxygen-level–dependent signal fluctuations ascribable to the DMN were detected in our sample. These data were visually inspected to ensure component singularity and spatial consistency with previously published work.13 [B] Red overlay represents brain regions with significant coupling with the DMN (green underlay) relative to increased abstinence duration. (a–d) Brain regions with significant within-network DMN coupling are identified in black lettering and include the (a) right precuneus; (b) posterior cingulate cortex, which extends into (c) retrosplenial cortex (forming a posterior cingulate/retrosplenium cluster); and (d) hippocampal formation. (e–i) Brain regions with significant outside-of-network coupling are identified in white lettering and include the (e) right insula, which extends into (f) putamen (forming a putamen/insula cluster); (g) thalamus; (h) bilateral lateral occipital cortex; and (i) bilateral cerebellum. Cluster-corrected z=2.3, p<0.05, 5,000 permutations. Coordinates are in millimeters in the Montreal Neurological Institute MNI-152 standard space. DMN, default mode network.
Discussion
Consistent with our hypothesis, there was a significant positive relationship between abstinence duration and DMN strength of within-network coupling. Our findings are similar to those from the only other study to examine the association between strength of DMN coupling and drug use patterns in patients seeking treatment for opioid dependence.8 In that study, within-network DMN coupling was associated with duration of lifetime opioid use; in our study, it was associated with recent (past 30-day) substance use. Other authors have speculated that a longer duration of alcohol sobriety is associated with increased within-network DMN coupling because it provides time for recovery and repair.14 This finding may be relevant to our findings, in that DMN connectivity changes within the first 30 days of abstinence may also reflect recovery and repair.
In addition to our hypothesized within-network changes, we also found that strength of diffuse outside-of-network connectivity was positively associated with abstinence duration. We cannot draw conclusions about this incidental finding within the constraints of our small pilot study, but other studies may help identify relevant variables and questions for future studies. For example, in a study that examined resting-state fMRI data through topographical analysis, polysubstance users receiving treatment for cocaine dependence had stronger and more diffuse but less efficient interregional resting-state network connections compared with control participants.15 These authors speculated that connection strength might increase to compensate for a “hyperconnected” and less efficient resting-state network in the addicted brain, but it is unknown how abstinence duration may affect these relationships.
This pilot study had several limitations, including a small sample size, absence of data from a second time point, reliance on self-report for substance use data, absence of data regarding recent nicotine use and its unknown effects on the DMN, and inability to control for multiple substances. However, this study also has a high degree of ecological validity and is highly relevant to the management of persons entering treatment for opioid dependence who have the highest rates of opioid and co-occurring substance use and the poorest opioid dependence treatment outcomes. Future studies should examine DMN coupling as a repeated measure and with a comparison group and larger sample size to better understand how time and abstinence duration affect within-network and outside-of-network coupling.
1 : Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000; 55:469–480Crossref, Medline, Google Scholar
2 : Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2014Google Scholar
3 : Illicit use of pharmaceutical opioids among young polydrug users in Ohio. Addict Behav 2009; 34:649–653Crossref, Medline, Google Scholar
4 : Emerging adult age status predicts poor buprenorphine treatment retention. J Subst Abuse Treat 2014; 47:202–212Crossref, Medline, Google Scholar
5 : The influence of substance use on adolescent brain development. Clin EEG Neurosci 2009; 40:31–38Crossref, Medline, Google Scholar
6 : Reduced ventral medial prefrontal cortex (vmPFC) volume and impaired vmPFC-default mode network integration in codeine-containing cough syrups users. Drug Alcohol Depend 2014; 134:314–321Crossref, Medline, Google Scholar
7 : Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals. PLoS One 2011; 6:e23098Crossref, Medline, Google Scholar
8 : Combining spatial and temporal information to explore resting-state networks changes in abstinent heroin-dependent individuals. Neurosci Lett 2010; 475:20–24Crossref, Medline, Google Scholar
9 : Heroin detoxification with buprenorphine on an inpatient psychiatric unit. J Subst Abuse Treat 2002; 23:163–169Crossref, Medline, Google Scholar
10 : Cognitive function during early abstinence from opioid dependence: a comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry 2006; 6:9.Crossref, Medline, Google Scholar
11 : Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend 2012; 125:252–259Crossref, Medline, Google Scholar
12 : An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PLoS One 2014; 9:e88228Crossref, Medline, Google Scholar
13 : Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 2011; 23:4022–4037Crossref, Medline, Google Scholar
14 : Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex 2011; 21:2272–2281Crossref, Medline, Google Scholar
15 : Default mode network activity in male adolescents with conduct and substance use disorder. Drug Alcohol Depend 2014; 134:242–250Crossref, Medline, Google Scholar



