Transcranial Magnetic Stimulation Modulates Resting EEG Functional Connectivity Between the Left Dorsolateral Prefrontal Cortex and Limbic Regions in Medicated Patients With Treatment-Resistant Depression
Abstract
High-frequency left prefrontal repetitive transcranial magnetic stimulation (rTMS) has been shown to have efficacy in treatment-resistant depression. However, the effects of rTMS on functional connectivity are still not clear. To examine changes in functional connectivity before and after rTMS, resting EEG of 14 patients with treatment-resistant depression was recorded twice at baseline and at week 4, respectively. The EEG data were analyzed using the standardized low-resolution brain electromagnetic tomography (sLORETA). The results reveal that high-frequency left prefrontal rTMS modulates resting EEG functional connectivity between the left dorsolateral prefrontal cortex and limbic regions, including the subgenual cingulate cortex and parahippocampal gyrus.
Depression is a common, severe psychiatric disorder with an individual and social burden, and characterized by the dysregulation of mood and cognition.1,2 Although treatments for depression are available and generally effective, not all patients respond to them. A considerable number of patients with depression are deemed treatment-resistant depression,3 which is defined as unresponsiveness to one or more standard antidepressant treatment trials.4
Neuromodulation therapy such as repetitive transcranial magnetic stimulation (rTMS) have taken on an increasingly primary role in the treatment of these patients, providing evidence that these innovative therapeutic interventions can modulate specific neural networks involved in the pathophysiology of neuropsychiatric disorders.5,6 TMS is a noninvasive neuromodulation technique that can change cortical and subcortical activities via neural circuits.7–9 High-frequency left prefrontal rTMS has been shown to have efficacy in treatment-resistant depression.10–12
Multiple lines of evidence have indicated that the dysregulation of the prefrontal and limbic network is implicated in the pathogenesis of depression,1,6,13–15 with prefrontal hypoactivity13,16–18 and limbic hyperactivity.1,6,14,15,19–21 The left dorsolateral prefrontal cortex (DLPFC), a cognition-related region, is a stimulation target by a noninvasive neuromodulation technique such as rTMS for treating depression.9,12 Our previous study showed that high-frequency rTMS increased regional cerebral blood flow (CBF) in the left DLPFC with clinical improvement.8 Meanwhile the limbic regions, including the subgenual cingulate cortex, and amygdala,1,21 show abnormally increased activity, and neurosurgery such as DBS for treating severe refractory depression has targeted the subgenual cingulate cortex, which plays a critical role in regulating emotions.6,19,20 However, the effects of rTMS on intrinsic functional connectivity between the left DLPFC and limbic regions are still not clear.
We sought to examine changes in resting EEG functional connectivity before and after high-frequency left prefrontal rTMS in treatment-resistant depression using the lagged nonlinear connectivity analysis in the standardized low-resolution brain electromagnetic tomography (sLORETA) in this study.22,23 This lagged nonlinear connectivity analysis called functional wire connectivity analysis (fWCA) can detect functional connectivity between two brain regions, which are placed as regions of interest (ROI). Subsequently, we hypothesized that high-frequency left prefrontal rTMS could modulate resting EEG functional connectivity between the left DLPFC, subgenual cingulate cortex, and amygdala, and also could entrain specific oscillations in the prefrontal and limbic network.
Methods
Fourteen patients with a DSM-IV-TR diagnosis of major depressive disorder participated in this study at Kyorin University Hospital and National Center Hospital, National Center of Neurology and Psychiatry. Inclusion criteria were a total score of greater than 18 on the 17-item Hamilton Depression Rating Scale (HAM-D), aged 20–70, the current depressive episode duration of 3 years or less, and insufficient clinical benefit to 1–4 adequate medication trials in the current depressive episode. Exclusion criteria for this study included psychotic episode, bipolar disorder, obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and eating disorder; previous treatment with brain stimulation therapy such as electroconvulsive therapy (ECT), rTMS, and vagus nerve stimulation (VNS); pregnancy; a personal or close family history of a seizure disorder; neurologic disorder and organic brain disease; substance or alcohol dependence; presence of ferromagnetic material in body or close to head; severe metabolic disease and endocrine disease; active suicide idea; taking medications known to lower seizure threshold. Medical treatments given to the patients were not allowed to have changed in the 6 weeks before the start of the first rTMS treatment or during the trial. All patients provided written informed consent for participation in this study after receiving a full explanation of the procedures. This study was respectively approved by the ethics committee of Kyorin University School of Medicine and National Center Hospital.
rTMS was administered using the NeuroStar TMS Therapy System (Neuronetics, Inc., Malvern, Pennsylvania). Treatment was standardized at 120% motor threshold (MT) with 10 Hz for a total of 3,000 pulses per day, 5 days per week, for 4 weeks. The standardized stimulation site was over the left prefrontal cortex and was determined by movement of the rTMS coil 5 cm anterior to the MT location. The severity of depression was rated with HAM-D every 2 weeks. Remission was defined as a total score of 7 or less on the HAM-D. Response was defined as a 50% or greater reduction in the HAM-D score. Changes in the mean HAM-D score were analyzed using one-way repeated-measures analysis of variance (ANOVA) and Bonferroni correction to estimate the time of effect. Statistical analysis was conducted using IBM SPSS Statistics 20.0 (IBM Corporation, Armonk, New York), with the level of statistical significance set at p<0.05.
Resting EEG was recorded using Geodesic EEG System 300 (Electrical Geodesics, Inc., Eugene, Oregon) with 65 electrodes including the reference Cz. The EEG with resting eye-closed state was recorded for approximately 5 minutes before the first rTMS session (at baseline) and at week 4, respectively. Subsequently, the EEG data for 53 electrodes after eliminating nonscalp electrodes were analyzed with sLORETA.22,23 For each subject, about more than 60 artifact-free epochs excluding blinking, muscle and electrode artifacts were extracted using the EEG edit control, sLORETA, and secondly, were computed at a sampling rate of 128 Hz for nine frequency bands (1–45 Hz): delta (δ, 1–3.5 Hz), low theta (θ1, 4–5.5 Hz), high theta (θ2, 6–7.5 Hz), low alpha (α1, 8–10 Hz), high alpha (α2, 10.5–12.5 Hz), low beta (β1, 13–18.5 Hz), middle beta (β2, 19–24.5 Hz), high beta (β3, 25–30.5 Hz), gamma (γ, 31–45 Hz). For functional connectivity, we used the lagged nonlinear connectivity analysis in sLORETA in the current study.22,23 This lagged nonlinear connectivity, namely lagged phase synchronization is calculated from the phase information of EEG, which can show functional connectivity between two brain regions placed as regions of interest (ROI), by using the ROI maker 2 in sLORETA. Functional connectivity between two brain regions is quantified here as the “similarity” between time-varying signals recorded at the two regions.22,23 Lagged phase synchronization measures the “similarity” between signals in the frequency domain based on normalized Fourier transforms, which corresponds to a very particular form of nonlinear similarity.22,23
The sLORETA program package computes lagged phase synchronization, which is defined by the following formula: 
In this formula, “ω” is the discrete frequency considered, “x” and ”y” are the EEG sources, “Re” and ”Im” denote the real and the imaginary parts of a complex element; x (ω) and y (ω) denote the discrete Fourier transforms of the two signals of interest x and y at frequency “ω”. The general lagged phase synchronization is defined as the partial coherence between the normalized complex-valued stochastic variables (xω, yω) with the zero-lag effect removed.22,23
To detect changes in resting EEG functional connectivity between the DLPFC, subgenual cingulate cortex, and amygdala, 5 ROIs in total were determined and selected from among 47 Brodmann’s areas in each hemisphere (Table 1). The stimulation site, left DLPFC (BA 9), was placed as a ROI, while the right DLPFC was placed as a negative control ROI. As we did not make ROIs directly in the amygdala using the ROI maker 2 in sLORETA, the parahippocampal gyrus (BA 34/35/36) was alternatively placed as 2 ROIs in the right and left hemispheres. The subgenual cingulate cortex (BA 25) was placed as solo ROI, not pairs in the right and left hemispheres, as its locations was near the center of both hemispheres. Subsequently, we used paired t tests to compare lagged connectivity between baseline and postrTMS, by using sLORETA program package. We made 5 ROIs in this study, and thus the 5ROIs yield 10 distinct coherences (5*4/2). Correction of significance for multiple testing was computed for the two conditions between baseline and postrTMS for each frequency band. For the correction, we applied the randomization statistical nonparametric mapping (SnPM) procedure available in sLORETA program package. T-value threshold was computed by the statistical software implemented in sLORETA, corresponding to statistically p<0.05.
| Brain Region | Hemisphere | Brodmann’s Area |
|---|---|---|
| Dorsolateral prefrontal cortex | Left | 9 |
| Right | 9 | |
| Subgenual cingulate cortex | 25 | |
| Parahippocampal gyrus | Left | 34/35/36 |
| Right | 34/35/36 |
TABLE 1. Brain Regions of Interest (ROIs)
Results
Fourteen patients (9 males, 5 females) with treatment-resistant depression completed the entire study. The mean age of the patients was 43.9 y/o (SD=11.8), and their mean age at onset was 35.4 y/o (SD=11.6). The mean number of previous depressive episode was 3.1 (SD=0.9) and the current depressive episode was 9.8 months (SD=5.5). For changes in the mean HAM-D score, one-way repeated-measures ANOVA and post hoc t tests with Bonferroni corrections showed a significant main effect of time (F(2, 26)=31.1, p<0.01), and the mean HAM-D score decreased significantly from 24.1 (SD=5.5) at baseline to 18.1 (SD=8.9) at week 2 and to 13.6 (SD=7.9) at week 4, respectively (p<0.01) (Figure 1, Figure 2). Four of 14 patients showed remission and 7 patients were responders at week 4.
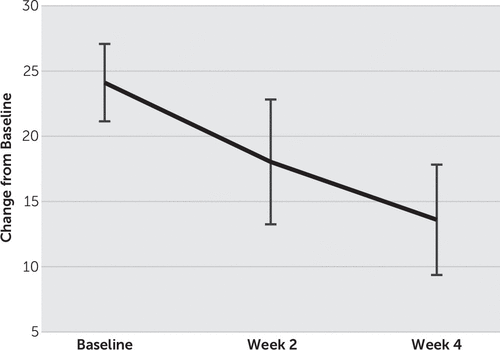
FIGURE 1. Change in the Average Total Score on the Hamilton Depression Rating Scale (HAM-D)a
a The average total HAM-D score decreased significantly from 24.1 (SD=5.5) at baseline to 18.1 (SD=8.9) at week 2 and to 13.6 (SD=7.9) at week 4. Error bars show standard error (2SE).
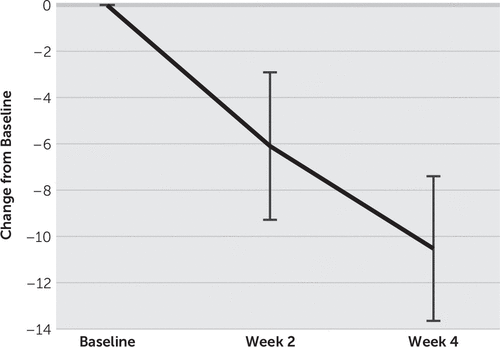
FIGURE 2. Change in the Hamilton Depression Rating Scale (HAM-D) Score from Baselinea
a HAM-D score was decreased from baseline by 6.1 (SD=5.9) at week 2 and by 10.5 (SD=5.8) at week 4. Error bars show standard error (2SE).
Using the lagged nonlinear connectivity analysis in sLORETA, the results showed that there was significant increased functional connectivity in middle beta band (β2, 19-24.5 Hz) between the left DLPFC and parahippocampal gyrus, and between the subgenual cingulate cortex and parahippocampal gyrus (t=2.91, p<0.05, Figure 3), while no significant changes in functional connectivity among the right DLPFC and other regions were observed. The findings indicate that left-prefrontal rTMS modulates more synchronized middle beta band activity between the left DLPFC and limbic regions. There were no significant changes in functional connectivity in other frequency bands. Furthermore, no significant correlations were observed between functional connectivity and HAM-D score changes in any frequency bands.
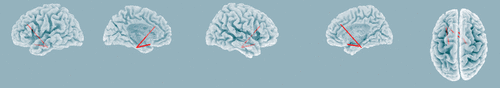
FIGURE 3. Changes in Resting EEG Functional Connectivity in Middle Beta Band Before and After High-Frequency Left Prefrontal Repetitive Transcranial Magnetic Stimulation (rTMS)a
a Significant increased functional connectivity was observed between the left dorsolateral prefrontal cortex (DLPFC) and subgenual cingulate cortex, and parahippocampal gyrus (t=2.91, p<0.05). This indicates that high-frequency rTMS can modulate more synchronized middle betta band activity in the left prefrontal and limbic network.
Discussion
This is the first study to delineate changes in resting EEG functional connectivity in treatment-resistant depression before and after high-frequency left prefrontal rTMS, by using the lagged nonlinear connectivity analysis. We found more synchronized middle beta band activity between the left DLPFC and limbic regions.
According to neuroimaging studies, depressed patients show prefrontal hypoactivity13,16–18 and abnormally increased activity in the limbic regions including the subgenual cingulate cortex and amygdala,1,6,14,15,19–21 compared with healthy control subjects or patients with remission. The DLPFC is functionally connected to the limbic regions, and the dysfunction of the prefrontal and limbic network is involved in regulating emotions and the pathogenesis of depression.13,14 Therefore, we made 5 ROIs in the right and left DLPFC, subgenual cingulate cortex, and bilateral parahippocampal gyrus in order to examine changes in functional connectivity in the prefrontal and limbic network in depressed patients in the current study.
To date, the antidepressant mechanisms of rTMS have still not been elucidated completely, although rTMS has been shown to have multimodal effects on humans.24,25 For instance, high-frequency rTMS over the left DLPFC modulates dopamine release in the subgenual cingulate cortex and orbitofrontal cortex.26 Recent several studies using rTMS and fMRI have shown that rTMS regulates functional connectivity between the left DLPFC and subgenual cingulate cortex, and the baseline functional connectivity with the subgenual cingulate cortex was correlated with clinical improvement.27,28 Therefore, it is difficult to formulate an explanation for the mechanism of rTMS solely in terms of changes in functional connectivity. Our findings of the current study may be explained by one potential contributory mechanism as mentioned below.
A series of studies have demonstrated that down-regulation of GABA-related genes and the dysfunction of GABAergic circuits in the DLPFC,29 subgenual cingulate cortex,30,31 and amygdala32 are implicated in the pathophysiology of depression.33 GABA is the primary inhibitory neurotransmitter and plays a critical role in regulating emotions in the limbic regions.33,34 A recent EEG study disclosed that rTMS produced a significant activation of beta and gamma bands in the frontal areas in healthy control subjects, but compared with them, the frontal EEG responses to rTMS were significantly reduced in the patients with depression.35 These findings suggest, as the authors concluded, that the reduced beta and gamma bands response to rTMS may reflect the dysfunction of GABAergic circuits in depression.35 High-frequency rTMS has been shown to enhance GABA inhibitory circuits.36 An MEG study indicates that GABA concentration is associated with the beta and gamma oscillations.37 Taken together, these findings suggest that synchronized middle beta band activity between the left DLPFC and limbic regions may be involved in modulating GABAergic circuits by high-frequency rTMS.
The current study has some limitations to address. A limitation of this study was to use an open-label design without a sham control. Thus, we could not exclude the possibility that the placebo effect might affect our results. However, we made a negative control in the right DLPFC and revealed that there were no significant changes in functional connectivity among the right DLPFC and other regions. We used “the 5cm rule” to locate the stimulation site using the NeuroStar TMS Therapy System, while there are several issues to address in using of “the 5cm rule” in terms of reproducibility and accuracy to stimulate the left prefrontal cortex because it varies depending on each patient. Next, in the current study, significant changes were observed only in middle beta band activity, and there were no significant changes in other frequency bands. We did not detect any significant correlation between functional connectivity and HAM-D score changes. The exact reason is not clear but it may be due to a relatively small sample size, analysis methods such as making a ROI, stimulation site, and stimulation frequency. Additionally, we used EEG date to assess functional connectivity in the current study, while earlier studies were to use fMRI, showing modulation of anticorrelation between the left DLPFC and medial prefrontal regions of the default mode network.28 These findings appear to be incongruent. As a potential reason, the difference in neuroimaging technique modality should be considered, because fMRI has a low temporal resolution and is not able to measure neural activity directly but instead shows the hemodynamic changes that may occur in response to neural activity. While EEG data directly relate to dynamic post synaptic activity with a high temporal resolution. Lastly, several studies indicate that modulation of GABA function has been proposed as a possible mechanism of action for rTMS.36,38 We assumed that more synchronized middle beta band activity between the left DLPFC and limbic regions might be related to GABAergic circuits modulation. However, we did not investigate directly an association between middle beta band activity and GABA function. To answer these limitations so far, further well-designed studies are needed.
In conclusion, our results of this study reveal that high-frequency rTMS modulates resting EEG functional connectivity between the left DLPFC and limbic regions. These findings provide further insights into the antidepressant mechanisms of rTMS in treating depression.
1 : Recent developments and current controversies in depression. Lancet 2006; 367:153–167Crossref, Medline, Google Scholar
2 : Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382:1575–1586Crossref, Medline, Google Scholar
3 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Crossref, Medline, Google Scholar
4 : Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 2014; 156:1–7Crossref, Medline, Google Scholar
5 : Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA 2014; 111:E4367–E4375Crossref, Medline, Google Scholar
6 : Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007; 10:1116–1124Crossref, Medline, Google Scholar
7 : Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 2007; 8:559–567Crossref, Medline, Google Scholar
8 : Changes in regional cerebral blood flow after repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in treatment-resistant depression. J Neuropsychiatry Clin Neurosci 2008; 20:74–80Link, Google Scholar
9 : The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 2013; 26:13–18Crossref, Medline, Google Scholar
10 : Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62:1208–1216Crossref, Medline, Google Scholar
11 : Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67:507–516Crossref, Medline, Google Scholar
12 : Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125:2150–2206Crossref, Medline, Google Scholar
13 : Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59–72Crossref, Google Scholar
14 : Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156:675–682Medline, Google Scholar
15 : Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004; 61:34–41Crossref, Medline, Google Scholar
16 : Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 1989; 46:243–250Crossref, Medline, Google Scholar
17 : Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry 1990; 147:1313–1317Crossref, Medline, Google Scholar
18 : Depression and the hyperactive right-hemisphere. Neurosci Res 2010; 68:77–87Crossref, Medline, Google Scholar
19 : Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45:651–660Crossref, Medline, Google Scholar
20 : Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 2008; 64:461–467Crossref, Medline, Google Scholar
21 : The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 2015; 20:32–47Crossref, Medline, Google Scholar
22 Pascual-Marqui RD: Instantaneous and lagged measurements of linear and nonlinear dependence between groups of multivariate time series: frequency decomposition. arXiv:0711.1455v1 [stat.ME], http://arxiv.org/abs/0711.1455v1Google Scholar
23 Pascual-Marqui RD, Biscay-Lirio RJ: Interaction patterns of brain activity across space, time and frequency. Part I: methods. arXiv:1103.2852v2 [stat.ME], http://arxiv.org/abs/1103.2852v2Google Scholar
24 : Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry 1999; 56:300–311Crossref, Medline, Google Scholar
25 : Transcranial magnetic stimulation in depression, in Brain Stimulation in Psychiatric Treatment, 1st ed. Edited by Lisanby SH. Washington, DC, American Psychiatric Publishing, 2004, chap 1, 1–16Google Scholar
26 : rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 2009; 4:e6725Crossref, Medline, Google Scholar
27 : Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 72:595–603Crossref, Medline, Google Scholar
28 : Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76:517–526Crossref, Medline, Google Scholar
29 : GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 2011; 14:721–734Crossref, Medline, Google Scholar
30 : Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis 2011; 42:116–124Crossref, Medline, Google Scholar
31 : Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 2012; 169:1194–1202Crossref, Medline, Google Scholar
32 : Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 2012; 17:1130–1142Crossref, Medline, Google Scholar
33 : The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011; 16:383–406Crossref, Medline, Google Scholar
34 : Anxiety disorders and GABA neurotransmission: a disturbance of modulation. Neuropsychiatr Dis Treat 2015; 11:165–175Medline, Google Scholar
35 : Shared reduction of oscillatory natural frequencies in bipolar disorder, major depressive disorder and schizophrenia. J Affect Disord 2015; 184:111–115Crossref, Medline, Google Scholar
36 : The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res 2006; 174:403–412Crossref, Medline, Google Scholar
37 : Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage 2011; 55:616–621Crossref, Medline, Google Scholar
38 : Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin Neurosci 2011; 13:139–145Medline, Google Scholar



