Potential Bedside Utility of the Clock-Drawing Test in Evaluating Rapid Therapeutic Response in the Natural Course of Schizophrenia: A Preliminary Study
Abstract
The Clock-Drawing Test (CDT) is a brief, relatively time-efficient, easy to administer at bedside, and well-proven cognitive screening test that assesses a broad range of cognitive abilities in stroke, delirium, and dementia. However, challenges of comprehensive therapeutic outcome evaluations in schizophrenia can also be potentially overcome using CDT. The authors aimed to measure the therapeutic outcome using CDT in 101 schizophrenia patients, irrespective of their diagnostic subtypes. A repeated measures analysis of variance found that improvements on CDT and the Positive and Negative Syndrome Scale were closely correlated, reflecting critical information about therapeutic response measures in schizophrenia.
The Clock-Drawing Test (CDT) has been widely used in clinical neuropsychological practice over the last three decades. The CDT was used traditionally as a “parietal lobe” test,1 but most empirical work on the CDT has focused on its sensitivity and specificity for detecting and differentiating subtypes of dementia.2–4However, there are isolated studies of CDT being used in diagnosing and grading the severity of hepatic encephalopathy,5 predicting rehabilitation outcomes after traumatic brain injury,6 and evaluating cognitions in late-onset institutionalized schizophrenia patients.7
Neurocognitive functions, such as visuospatial ability, constructional ability, attention and concentration, memory, planning, and executive control, are clearly affected in patients with schizophrenia,8 and the CDT measures most of these neurocognitive functions. While these neurocognitive impairments improve with treatment, it is not clear whether parallel improvement in positive symptoms and general psychopathology also accompanies neurocognitive improvement or whether their improvement is independent, and if so, to what extent. In other words, the CDT could be used to assess the progress of therapeutic response and/or outcome in treatment-seeking schizophrenia patients. However, to the best of our knowledge, the CDT has not been studied as a measure for this assessment. The primary aim of the present study was to assess the therapeutic outcome in patients with schizophrenia using the CDT. The secondary aim was to examine the relationship between CDT performance and the Positive and Negative Syndrome Scale (PANSS),9which comprehensively measures the clinical/therapeutic improvement in symptoms of schizophrenia.
Methodology
Sample
We assessed 150 schizophrenia patients, of either gender, who fulfilled DSM-IV-TR criteria for the diagnosis of schizophrenia, irrespective of subtypes, and who were admitted to our inpatient psychiatric services in our tertiary care general hospital in Central India. Purposive sampling was done for cases of untreated first-episode schizophrenia for a period of 12 months from January 2014, and a minimum of 10 years of education was required for inclusion. Patients with psychosis who had delirium, dementia, or other organic diseases of the brain, substance dependence apart from nicotine use, past history of head injury and epilepsy, learning disability, subnormal intelligence, poor eyesight, or hearing problems were excluded at the entry level of study. We enrolled age-, sex-, and education-matched healthy controls (N=131), who were the accompanying relatives of nonpsychiatric patients from the departments of dermatology and obstetrics and gynecology and in whom we could not elicit a family- or person-specific history of any psychiatric disorder (past or present). The study was approved by the Institutional Ethical Committee of Mahatma Gandhi Institute of Medical Sciences, Sevagram, India, in January 2014. All participants and their caregivers provided written consent for participation.
Assessment
During the study period, all patients with schizophrenia who were screened and selected were treated at par with our treatment protocol(s) followed at the center. On the day of admission, a consultant psychiatrist (P.K. or K.M.) independently assessed the patients for clinical diagnosis and PANSS scores, while demographic details and baseline CDT scores were assessed by the senior resident (R.R.). The subsequent CDT and PANSS were administered on day 3, day 7, and weekly thereafter for 4 weeks. For healthy matched controls, the CDT was administered at similar time intervals. The follow-up tests were administered by an examiner (G.S.) who was not involved in the baseline tests and was blinded to the analysis of those tests. For the present study, the Sunderland Method of CDT evaluation was chosen. The CDT was administered by instructing the patient to draw the face of a clock and place the hand of the clock to indicate 10 minutes past 10 or alike time settings. This was scored using the Sunderland scoring system,10 which is a 10-point rating with a clinical description ranging from 1 (an attempt or uninterpretable effort to draw a clock) to 10 (the clock face and number is intact, with the correct position), in which the first 5 points reflected an intact drawing of the clock face with a circle and numbers and the remaining points were attributed to drawing the hands accurately to denote time. The intrarater reliability was 0.91.
Statistical Analyses
Analyses were conducted to measure the therapeutic outcome and compare the CDT and PANSS scores of schizophrenia patients with the CDT scores of healthy controls. Categorical variables were analyzed using chi-square and Fisher’s exact tests. Student’s t tests were performed to analyze the continuous variables of patients and control subjects. Pearson’s correlation coefficients were calculated to determine the relationship among the continuous variables (PANSS and CDT scores). Repeated-measures of analysis of variance were used to measure the difference in therapeutic responses on subsequent evaluations. A p value <0.05 was considered significant, and a p value <0.005 was considered highly significant. All analyses were performed using SPSS software, version 20.0 (SPSS, Cary, N.C.)
Results
Out of 150 schizophrenia patients, 19 were noncooperative because of the severity of their symptoms, 12 refused to undergo all examinations, and 18 did not complete the study because of irregular follow-up/discharge on request and abrupt withdrawal of consent during follow-up, Therefore, we could evaluate the CDT only in 101 patients (65 men [64.4%] and 36 women [35.6%]). The performance of patients was significantly poor (p<0.001) at baseline, compared with healthy controls (3.77±2.51 versus 8.71±1.24). We found negative correlation of the CDT scores with age (r=−0.17, p<0.05) and education (r=−0.25, p<0.05). Furthermore, the CDT scores correlated negatively and significantly with the total (r=−0.43, p<0.001), negative (r=−0.44, p<0.001), and positive (r=−0.21, p<0.05) PANSS scores. This suggests that the greater the symptom severity of schizophrenia, the poorer the score on the CDT, and positive symptoms affect CDT outcome more than negative symptoms.
Using the one-way repeated measure analysis of variance, we found that CDT scores gradually and steadily improved with the clinical improvement in the PANSS scores (Figure 1). A significant difference (p<0.005) was noted between the CDT and PANSS scores (total, general psychopathology, positive and negative symptom scores over 4 weeks from the baseline). The final scores among the patients of schizophrenia at the end of 4 weeks on CDT performance were significantly improved from baseline 3.77±2.51 to 8.05±1.59 but still differed from controls (p<0.001). According to the PANSS scores remission criteria, the effect rate was 89.10%, which is quite significant.
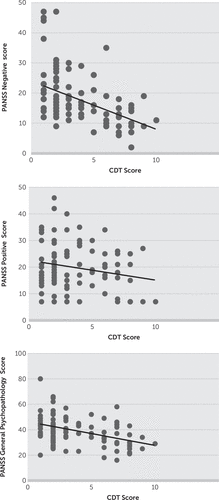
FIGURE 1. Correlation Between Clock-Drawing Test (CDT) Scores and Clinical Variablesa
a The graphs show A) correlation of CDT scores and Positive and Negative Syndrome Scale (PANSS) negative scores; B) correlation of CDT scores and PANSS positive scores; and C) correlation of CDT scores and PANSS general psychopathology scores.
Discussion
To date, the CDT has been used to assess therapeutic outcomes of dementia and delirium, with reliable sensitivity and specificity.11,12 Although one study attempted to measure the cognitive-cum-therapeutic outcome(s) of memantine among patients with schizophrenia using the CDT,13 researchers have not used the CDT in routine clinical practice after intervening with antipsychotics. In the present study, we examined whether the CDT can be used to gauge therapeutic outcomes in patients with schizophrenia, which to our knowledge is the first report in the literature. We found a strong positive correlation of the CDT with PANSS and a negative correlation of the CDT with age and education. This reflects that the CDT can be used to assess the progress of therapeutic outcome in treatment-seeking patients with schizophrenia as we hypothesized. Our findings suggest that the positive, negative, and general psychopathology symptoms as evident from the PANSS scores clearly affect the CDT performance among patients with schizophrenia, as they improved substantially over 4 weeks of treatment with second-generation antipsychotics (Figure 2). This is in contradiction to previously conducted studies, some of which showed poor performance14 on the CDT, with high PANSS positive domain scores, while another study15 showed poor CDT performance with high negative scores. Cognitive domain is independent, and several studies conducted by Addington et al.16 and Keefe et al.17 demonstrated that neurocognitive ability is not strongly correlated with severity of psychotic symptoms in patients with schizophrenia, and our study supports this conformity.
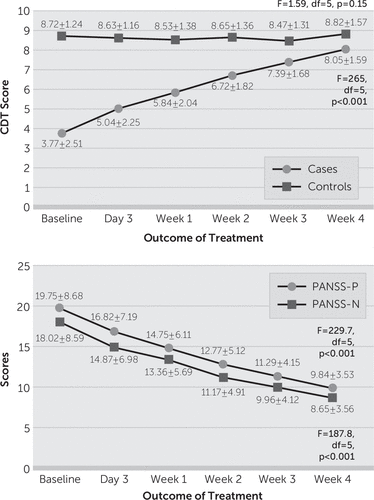
FIGURE 2. Comparison of Clock-Drawing Test (CDT) and Positive and Negative Syndrome Scale (PANSS) Scores (mean±SD) Before and After Treatmenta
a The graphs show A) improvement of CDT scores over 4 weeks; and B) comparison of PANSS positive scores and PANSS negative scores over 4 weeks.
Since most studies18–20 have suggested that CDT performance declines after 60 years of age, and cross-sectional relationships between neurocognition and positive symptoms, particularly hallucinations and delusions, are quite weak,20 previous investigators have mostly focused on elderly patients for CDT analysis, and the present study has, in contrast, assessed the natural course of young, untreated first-episode schizophrenia patients. This is a unique way of using the CDT as a bedside rapid-screening test for therapeutic outcome in contrast to previously conducted studies. However, we acknowledge that a risk of ascertainment bias could remain a major limitation of study
To our surprise, an improved CDT performance was potentially observed within a week of antipsychotic use, which challenges the notion of delayed onset of antipsychotic action21 and thus might be more useful than lengthy and complex neuropsychological tools usually advocated for evaluating domain-specific cognitive components. However, it is too early to consider the CDT a measure as accurate or as comprehensive as batteries such as the Measurement and Treatment Research to Improve Cognition in Schizophrenia22 or the Brief Assessment of Cognition in Schizophrenia,23 which extensively evaluate the important and specific domains of cognitive deficits in schizophrenia. The comparison of the CDT with any such neuropsychological tests is outside the scope of our study; nevertheless, researchers have explored the possible and potential, although limited, clinical utility of the CDT and have called on neuropsychiatrists and neuropsychologists to undertake futuristic studies for qualitative therapeutic response assessment concerning schizophrenia. As the CDT is completed in about 2 minutes, and requires very little training to administer, it can be used even by caregivers, particularly in remote settings, where complicated assessment tools like PANSS may not be easily accessible. If it turns out to be an evidence-based practice, this would pave the way for general physicians and the public to apply simple bedside evaluative approaches for recovery.
1 : The process approach to neuropsychological assessment. Aphasiology 1988; 23-4:309–311Crossref, Google Scholar
2 : Clock-drawing: An assessment technique in dementia. J Clin Exp Gerontol. 1991; 131-2:69–85Google Scholar
3 : Early identification of dementia: predictive validity of the clock test. Arch Clin Neuropsychol 1997; 12:257–267Crossref, Medline, Google Scholar
4 : Quantitative and qualitative analyses of clock drawing in frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc 2006; 12:159–165Crossref, Medline, Google Scholar
5 : Relationship between clock and star drawing and the degree of hepatic encephalopathy. Postgrad Med J 2011; 87:605–611Crossref, Medline, Google Scholar
6 : Executive clock drawing correlates with performance-based functional status in people with combat-related mild traumatic brain injury and comorbid posttraumatic stress disorder. J Rehabil Res Dev 2010; 47:841–850Crossref, Medline, Google Scholar
7 : Relationship between clock-drawing and neuropsychological and functional status in elderly institutionalized patients with schizophrenia. Am J Geriatr Psychiatry 2003; 11:621–628Crossref, Medline, Google Scholar
8 : Cognitive impairment in schizophrenia. Adv Psychiatr Treat 2000; 63:161Crossref, Google Scholar
9 : The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
10 : Clock drawing in Alzheimer’s disease: A novel measure of dementia severity. J Am Geriatr Soc 1989; 37:725–729Crossref, Medline, Google Scholar
11 : Test accuracy of short screening tests for diagnosis of delirium or cognitive impairment in an acute stroke unit setting. Stroke 2013; 44:3078–3083Crossref, Medline, Google Scholar
12 : Further analyses of clock drawings among demented and nondemented older subjects. Arch Clin Neuropsychol 1996; 11:193–205Crossref, Medline, Google Scholar
13 : Addition of memantine to antipsychotic treatment in schizophrenia inpatients with residual symptoms: A preliminary study. Eur Neuropsychopharmacol 2008; 18:117–121Crossref, Medline, Google Scholar
14 : Clock Drawing Test in patients with schizophrenia. Psychiatry Res 2004; 121:229–238Crossref, Medline, Google Scholar
15 : Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res 2003; 59:137–146Crossref, Medline, Google Scholar
16 : Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res 1991; 5:123–134Crossref, Medline, Google Scholar
17 : Implementation considerations for multisite clinical trials with cognitive neuroscience tasks. Schizophr Bull 2008; 34:656–663Crossref, Medline, Google Scholar
18 : Do age and education contribute to performance on the clock drawing test? Normative data for the Greek population. J Clin Exp Neuropsychol 2008; 30:199–203Crossref, Medline, Google Scholar
19 : Clock Drawing Test in institutionalized patients with schizophrenia compared with Alzheimer’s disease patients. Schizophr Res 2003; 59:173–179Crossref, Medline, Google Scholar
20 .: Comparing the influences of age and disease on distortion in the clock drawing test in Japanese patients with schizophrenia. Am J Geriatr Psychiatry 2010; 18:908–916Crossref, Medline, Google Scholar
21 : Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry 2003; 60:1228–1235Crossref, Medline, Google Scholar
22 : Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004; 56:301–307Crossref, Medline, Google Scholar
23 : The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. J Clin Exp Neuropsychol 2006; 28:260–269Crossref, Medline, Google Scholar



