Sleep Patterns and Neuropsychiatric Symptoms in Hospitalized Patients With Dementia
Abstract
The authors examined 28 dementia inpatients receiving treatment as usual. Beginning-to-end differences in neuropsychiatric symptoms and actigraphic sleep patterns were measured. Using a mixed-model, the authors regressed neuropsychiatric symptoms on average sleep minutes (between-subjects effect) and each night’s deviation from average (within-subject effect). Sleep did not significantly differ from beginning to end of participation, whereas neuropsychiatric symptoms did. Average sleep minutes predicted average neuropsychiatric symptoms (p=0.002), but each night’s deviation from the average did not predict next day’s symptoms (p=0.90). These findings raise questions about the immediate benefits of treating sleep-wake disturbances on neuropsychiatric symptoms in hospitalized inpatients with dementias.
The circadian cycle changes as people age. Healthy elderly subjects nap more during the daytime1 and have lower total sleep time and efficiency compared with younger healthy adults.2 Five percent of people older than 65 years of age are severely demented; mild to moderate dementia affects approximately 10%−15% additionally.3 Sleep/wake cycle disturbances (SWDs) are prominent in dementia, may cause significant distress to both patients and caregivers, and are a frequent reason for institutionalization.4 Some researchers have linked SWDs to “sundowning” and agitation, representing a potential “state” association between SWDs and short-term changes in neuropsychiatric symptoms.5,6 Melatonin has been used to treat sundowning in AD, with resulting improvement in SWDs,7,8 supporting the “state” association. Other researchers have linked SWDs to the long-term deterioration in cognition and progression of AD, representing a “trait” association between SWDs and dementia.9–14 Perhaps both a “state” association between SWDs and short-term changes in neuropsychiatric symptoms, and a “trait” association between SWDs and durable neuropsychiatric impairment in dementia, exist. The hypothesized “state” association would most likely be observable in day-to-day changes in acute treatment settings (e.g., an inpatient dementia unit admitting patients because of agitation, depression, or psychotic symptoms). However, most studies on SWDs in dementia have been conducted in outpatient or nursing home settings6,10 and therefore are less likely to address the “state” component.
Research on the associations between SWDs and dementia has employed self- and caregiver reports, polysomnography (PSG), and activity monitoring (actigraphy). Although the physiological “gold standard” is PSG, it is time-limited (one or two days), not widely available, and expensive. Actigraphs, as an alternative to PSG, detect movements in three axes and record subjects’ activity levels over days to weeks. Using validated algorithms to infer wakefulness and sleep, actigraphs have been shown to accurately represent SWDs in dementia compared with PSG.15 Actigraphs do not have any known significant adverse effects.
Clinical observations suggest a possible association between improvement (or worsening) in neuropsychiatric symptoms and improvement (or worsening) in SWDs in dementia. The aims of the present study were to a) quantify SWDs in dementia patients hospitalized for acute neuropsychiatric symptoms using actigraphy, and b) measure improvement in SWDs and neuropsychiatric symptoms over the course of hospitalization and examine associations between them. We hypothesized there would be a) substantial SWDs in these patients, and b) observable improvements in both SWDs and neuropsychiatric symptoms over the course of hospitalization. Moreover, c) changes in SWDs would be associated with changes in neuropsychiatric symptoms.
Methods
The study was approved by the university’s institutional review board.
Subjects
We recruited dementia patients acutely hospitalized in a university inpatient medical psychiatry unit for acute behavioral disturbances, usually agitation and aggression. The study was observational; therefore, subjects received inpatient clinical care as usual. Subjects remained in the study until hospital discharge. To be included, study subjects needed to have a DSM-IV diagnosis of dementia, be aged 50 or older, and be able to wear the actigraphs. We also used the unpublished ConnCog Examination, a structured brief cognitive examination developed locally (available on request), to screen for orientation, attention, word generation, memory, prosody, aphasia, praxis, visuospatial performance, and abstraction ability. Subjects who met the inclusion criteria but who were too agitated to be able to wear actigraphs, were actively suicidal or homicidal, or whose participation in the study was considered unsuitable for other reasons by their clinical team were excluded from study participation.
All patients admitted to the Medical Psychiatry unit were screened for eligibility. The main reason for screen failure among patients with dementia was unwillingness or inability to give informed consent. In subjects with impaired medical decision-making capacity, the legal guardian or person with durable power of attorney had to give informed consent after the patient gave verbal assent and/or demonstrated no signs of opposition to participation. Additionally, even in subjects with intact medical decision-making capacity, a family member was required to cosign the informed consent document. Because of the variable time it took to obtain these outside signatures, subjects were enrolled within 1 to 5 days after admission to the medical psychiatry unit. The sample was enrolled between 2004 and 2006.
Assessments
Clinical assessment measures included a) the Neuropsychiatric Inventory (NPI),16 which covers 12 major behavioral areas and has good interrater and test-retest reliability. The majority of the NPIs were scored by the first author, otherwise by the treating clinician. Except in unusual circumstances, the same clinician scored the NPI to the patient throughout the course of the study. Clinical assessment measures also included b) the Rating Scale for Aggressive Behavior in the Elderly (RAGE),17 scored by the nursing staff to quantify the most common aggressive behaviors in patients with dementia; c) the Disruptive Behavior Rating Scale (DBRS),18 which quantifies the severity of physical aggression, verbal aggression, agitation, and wandering, also scored by the nursing staff; d) the Cornell Scale for Depression in Dementia (CSDD),19 scored by the first author based on an interview; and e) the Montgomery-Åsberg Depression Rating Scale (MADRS),20 which is sensitive to change in intensity of depressive symptoms in response to treatment, also scored by the first author based on an interview.
Assessments were conducted at time intervals consistent with the expected rates of change of the measured construct. Aggression (RAGE), disruptive behaviors (DBRS), and neuropsychiatric symptoms (NPI) were assessed daily (except Saturday and Sunday) in order to provide the most accurate data for comparison with sleep actigraphy data taken continuously. Accordingly, we modified the NPI, RAGE, and DBRS scoring for daily administration. Measures of depression (MADRS, CSDD) were assessed weekly. For each of these measures, a higher score denotes more psychopathology.
A standard time in bed (9:00 p.m.) and out of bed (7:30 a.m.), consistent with the unit routine, defined the down time (measurement) interval. During that interval, no visitors were allowed. Lighting on the unit closely resembled ambient conditions. Actigraphs were initialized using the ACT software (Ambulatory Monitoring, Ardsley, N.Y.). They were placed on the subject’s nondominant wrist, remained there at all times, and recorded data continuously throughout participation. Actigraphy data were recorded in one-minute epochs using the zero crossing mode and were analyzed with the action3 software (Ambulatory Monitoring, Ardsley, NY). Actigraphy-measured sleep parameters were obtained based on validated algorithms incorporated in the software (Cole-Kripke scoring algorithm).21–25
Data Analyses
In order to minimize within-subject variability of sleep and neuropsychiatric data, the average of the first 2 nights or days, respectively, was compared with the average of the last 2 nights or days. Difference scores (first two nights/days minus last two nights/days) were calculated for each subject for the primary sleep measures, and for the primary neuropsychiatric measures (viz., NPI, RAGE, DBRS). These difference scores then were divided (weighted) by days of participation (representing daily rate of change) in order to account for the subjects’ differing lengths of stay. Paired t tests were applied. Associations between beginning-to-end weighted changes in sleep and neuropsychiatric symptoms were summarized using Pearson product-moment correlations.
Mixed model linear regression was used to define temporal associations between the sleep and neuropsychiatric variables obtained on a daily basis. We a priori selected NPI score, as well as NPI Agitation and Irritability subscale scores (separately), as our outcome variables since patients were admitted to the medical psychiatry unit for acute behavioral disturbances, usually agitation and/or aggression. We did not enter other symptoms as outcomes in the mixed model analysis because they were likely to be either a) less frequent (e.g., psychosis) or b) less prone to change with treatment (e.g., cognition). Our predictor variables were mean sleep minutes (between-subjects effect) and the deviation of each night’s sleep minutes from that mean (within-subject effect). In order to reduce the risk of Type I error from multiple comparisons, we set our level of significance to p<0.01.
Results
We recruited 28 dementia inpatients (see Table 1) with mean age 82 years (SD=9). Dementia diagnoses included Alzheimer’s disease, vascular dementia, dementia with Lewy bodies, frontotemporal dementia, and dementia not otherwise specified. Five subjects had more than one dementia diagnosis: four had concomitant diagnoses of Alzheimer’s and vascular dementia, and one had Alzheimer’s and frontotemporal dementia. Based upon clinical information, including bedside cognitive examination, history provided by relatives, and functional status, the first author made a qualitative determination of dementia severity (i.e., mild, moderate, or severe; Table 1).
| Characteristic | Frequency (N) | % |
|---|---|---|
| Sex | ||
| Male | 13 | 46.43 |
| Female | 15 | 53.57 |
| Dementia severity | ||
| Mild | 3 | 10.71 |
| Moderate | 10 | 35.71 |
| Severe | 15 | 53.57 |
| Alzheimer's dementia | ||
| Yes | 15 | 53.57 |
| No | 13 | 46.43 |
| Vascular dementia | ||
| Yes | 9 | 32.14 |
| No | 19 | 67.86 |
| Dementia with Lewy bodies | ||
| Yes | 4 | 14.29 |
| No | 24 | 85.71 |
| Dementia not otherwise specified | ||
| Yes | 5 | 17.86 |
| No | 23 | 82.14 |
| Number of dementia diagnoses | ||
| One | 23 | 82.14 |
| More than one | 5 | 17.86 |
| Patient age (years) | ||
| N | Mean | SD |
| 28 | 81.61 | 9.3 |
TABLE 1. Sample Frequencies and Age
The group mean (SD) days of participation was 13.3 (SD=8.6). Group mean average sleep minutes were 381 (SD=165) during the first 2 nights and 387 (SD=178) during the last 2 nights. Mean sleep efficiency was 76.6% (SD=19.9%) and 74.4% (SD=19.0%). Mean NPI total scores were 37 (SD=17) during the first 2 days and 19 (SD=13) during the last 2 days. Mean NPI agitation/aggression subscales scores were 5 (SD=4) and 2 (SD=3); mean DBRS total scores were 7 (SD=2) and 6 (SD=1); and mean RAGE total scores were 9 (SD=9) and 3 (SD=6), respectively. Additional sleep and neuropsychiatric symptom scores appear in Table S1 in the data supplement accompanying the online version of this article.
None of the actigraphy-measured weighted sleep parameters significantly differed between the beginning and end of treatment. In contrast, beginning-to-end differences in weighted NPI total score (p<0.0001), and NPI agitation/aggression (p=0.002), RAGE (p=0.0002), DBRS (p=0.003), and several other NPI subscale (e.g., agitation/aggression, irritability, disinhibition, apathy/indifference) scores all significantly differed in the expected direction (see Table S2 in the online data supplement). NPI depression subscale, MADRS, and CSDD did not significantly differ between the beginning and end of treatment (see Table S2 in the online data supplement).
Because both sleep and neuropsychiatric change scores were calculated as beginning minus end, the expected sign of the correlation between improvement in sleep and improvement in psychiatric symptoms would be negative—that is, a larger improvement in sleep (a more negative value) would be associated with a larger improvement in symptoms (a more positive value). The correlation between weighted change in total sleep minutes and weighted change in total NPI score was negligible (r=−0.08, p=0.71, 95% CI [−0.44, 0.32]) (Table 2). Thus, sleep change (which was trivial) accounted for less than one percent of the variance in total NPI score change. Correlations for each sleep measure with each neuropsychiatric symptom measure are presented in Table S3 in the online data supplement.
| Assessment Measure | Sleep Minutes (N=27) | Sleep Efficiency (N=24) | Wake Minutes (N=27) |
|---|---|---|---|
| NPI total score | r=–0.08 | r=–0.03 | r=0.08 |
| p=0.71 | p=0.9 | p=0.71 | |
| NPI agitation subscale | –0.32 | –0.20 | 0.32 |
| 0.10 | 0.36 | 0.10 | |
| NPI irritability subscale | 0.35 | –0.02 | –0.35 |
| 0.08 | 0.93 | 0.08 | |
| DBRS total score | –0.23 | –0.42 | 0.23 |
| 0.25 | 0.04 | 0.25 | |
| RAGE total score | –0.14 | –0.21 | 0.14 |
| 0.47 | 0.34 | 0.47 |
TABLE 2. Correlations Between Sleep Parameters and Neuropsychiatric Symptomsa
In the mixed model analysis, subjects’ mean sleep minutes (the average amount subject slept over the course of his/her participation) significantly predicted their own average total NPI scores over the same period (estimate=−0.05, SE=0.02, 95% CI [−0.08, −0.02], t(138)=–3.11, p=0.002) (Table 3). For every additional 60 minutes of average sleep, NPI score decreased by 3 points (i.e., −0.05*60 minutes). However, subjects’ deviation from their average sleep on a given night did not predict their total NPI score the next day (estimate=−0.001, SD=0.01, 95% CI [−0.02, 0.02], t(21)=–0.13, p=0.901). For each additional 60 minutes of sleep on a given night, NPI score decreased by only 0.06 points. Moreover, we can be 95% confident that the true NPI decrease was no more than 1.2 points (−0.02*60 minutes). The typical (50th percentile) deviation on a given night, averaged across subjects, was a very substantial 97.9 minutes, suggesting that this failure to find a significant effect was not due to low variation in subjects’ deviations from their average sleep. The pattern is also visually depicted on Figure 1.
| Effect | Estimate | Standard Error | df | t | Pr>|t| | Lower 95% CL | Upper 95% CL |
|---|---|---|---|---|---|---|---|
| Outcome variable: total Neuropsychiatric Inventory (NPI) score | |||||||
| Intercept | 41.1167 | 6.462 | 23 | 6.36 | <0.0001 | 27.7490 | 54.4845 |
| Average sleep minutes | –0.05185 | 0.01669 | 138 | –3.11 | 0.0023 | –0.0849 | –0.0188 |
| Individual night's deviation from average | –0.0012 | 0.009601 | 21 | –0.13 | 0.9014 | –0.0212 | 0.0188 |
| Outcome variable: NPI agitation subscale score | |||||||
| Intercept | 5.6297 | 1.4743 | 23 | 3.82 | 0.0009 | 2.5799 | 8.6795 |
| Average sleep minutes | –0.00938 | 0.003796 | 137 | –2.47 | 0.0147 | –0.0169 | –0.0019 |
| Individual night's deviation from average | 0.000088 | 0.002221 | 21 | 0.04 | 0.9687 | –0.0045 | 0.0047 |
| Outcome variable: NPI irritability subscale score | |||||||
| Intercept | 5.7883 | 1.5968 | 23 | 3.63 | 0.0014 | 2.4852 | 9.0915 |
| Average sleep minutes | –0.00903 | 0.004106 | 137 | –2.2 | 0.0296 | –0.0172 | –0.0009 |
| Individual night's deviation from average | 0.001573 | 0.001763 | 21 | 0.89 | 0.3824 | –0.0021 | 0.00524 |
TABLE 3. Random Effects Mixed-Model Regression Solutions for Fixed Effects (N=28)
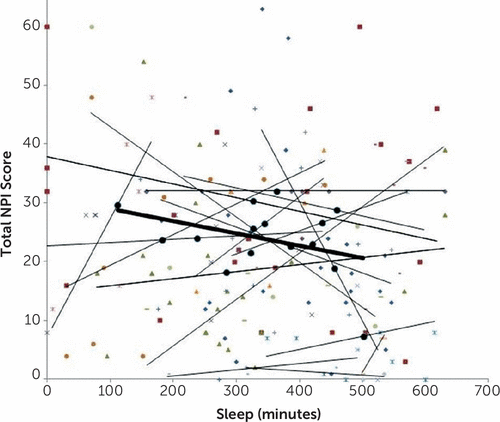
FIGURE 1. Daily Neuropsychiatric Impairment Inventory Total Score as a Function of Minutes of Sleep on the Preceding Nighta
a Small symbols code for individual subjects’ data points. Light lines represent within-subject regression lines. Large black circles represent each subject’s mean. Heavy line represents between-subject regression line for these means. Only subjects for whom at least five paired Neuropsychiatric Inventory (NPI) score sleep data were available (N=15) are shown. See the article text for mixed-model data analysis results on all subjects. It may be seen that no directional pattern emerges for individual subjects’ regression lines. For some subjects, the lines are downward, suggesting that more sleep is followed by less neuropsychiatric impairment the following day. However, for others the lines are upward, and for others, they are flat. However, despite the absence of a consistent pattern of within-subjects, nightly sleep NPI score associations, the between-subject regression line of each subject’s mean is downsloping, suggesting that, over the entire study period, more sleep is associated with less neuropsychiatric impairment, as predicted.
When the agitation or irritability NPI subscale scores replaced total NPI score as the outcome variable, results were similar (Table 3). When apathy, anxiety, and depression NPI subscales were used as outcome variables, they were not significantly predicted by either the individual’s average sleep, or by his/her deviation from that average (not shown).
Discussion
Sleep-wake disturbances (SWDs) in dementia are widely believed to be associated with confusion and agitation (e.g., as in “sundowning”)5,6. Based on this and our previous clinical observations, it was our expectation that patients with dementia would have sleep and neuropsychiatric symptoms that would both improve over the course of hospitalization, and that they would be correlated throughout. Our results only partially support these predictions. In this study, sleep did not significantly improve between the beginning and the end of a course of inpatient treatment. This stands in contrast to the observed substantial improvement in neuropsychiatric symptoms. Moreover, the across-subject correlation between within-subject sleep change (as measured by total minutes asleep) and within-subject change in neuropsychiatric symptoms (as measured by NPI total score) from the beginning to end of participation was negligible—that is, symptom improvement over participation was not accompanied by a corresponding improvement in sleep.
Results of our mixed model regression analyses indicated that, over the course of the observation period, the more subjects slept on average, the less were their psychiatric symptoms. However, how much more or less than their average subjects slept on a particular night did not significantly predict their neuropsychiatric symptoms the following day. This latter negative finding was contrary to expectation. Examination of the confidence intervals (Table 3) militates against this negative finding’s representing a Type II error. Neither is this lack of association explained by symptom intractability, because neuropsychiatric symptoms significantly improved over hospitalization. It was also not explained by a lack of variance in the sleep data, because the SDs were larger in our patients than in elderly subjects with normal cognition collected by others.26,27 Rather, we are inclined to accept this negative finding as valid, and to interpret its implication as meaning that anecdotal impressions of the deleterious effect of a bad night’s sleep on neuropsychiatric symptoms the next day may be exaggerated. Interestingly, other investigators have also reported a lack of correlation between nighttime actigraphic activity and the NPI in inpatients with probable Alzheimer’s disease and agitation.28
It is tempting to interpret the results of the mixed model analysis as supporting sleep disturbance as a “trait” in dementia (a concept supported by much of the literature on dementia and sleep9–14), but not as a “state” influencing daily symptoms. However, we acknowledge several limitations to such an inference. First, we have made the working assumption that sleep quality over the mean 13-day period of subjects’ participation represents a trait, in contrast to nightly sleep, which we assume represents a state. Some might argue that such a 13-day period is too short to accurately determine a sleep “trait,” although we are unaware of any absolute empirical standard for what would constitute such a thing. Here “trait” and “state” are pragmatically defined as relative to each other.
The study has additional limitations. First, the sample size was small. Second, we did not control for potentially confounding factors. Therefore, we cannot evaluate the roles of multiple possible influences on an individual’s sleep during the hospital stay, including medications, medical comorbidities, and the structure of the inpatient setting (with definite rules on bedtime, “lights off,” and time to awake). Additionally, our design also does not allow for causal inferences to be made.
We used actigraphy as our main sleep measure, which, although convenient, practical, and validated against polysomnography,29 also has its limitations. Actigraphs measure movements and not sleep itself. Hence reduced mobility in dementia patients may lead to overestimation of sleep. Actigraphs are best at measuring total sleep time; their accuracy in distinguishing sleep from wake decreases when sleep becomes more fragmented, a trait that increases proportionately with age2 and with dementia.30 Additionally, actigraphy is not as useful as polysomnography at distinguishing between different sleep disorders.31 Nevertheless, actigraphy has been used with success in studies in the elderly32 and those with dementia.11,15,28,33
In summary, the present study found evidence that better “trait” sleep (defined empirically as over the course of hospitalization) was associated with less neuropsychiatric symptomatology. However, the hypothesized association between “state” sleep (on a single night) and next-day neuropsychiatric symptoms was refuted. This latter result raises a question of whether treating SWDs (e.g., pharmacologically) on a given night will confer an immediate benefit in reducing neuropsychiatric symptoms in hospitalized dementia subjects, although a course of such treatment may still confer a benefit over the longer term. We suggest that this question should be explored further in future randomized, controlled treatment studies that target SWDs in dementia.
1 : 24-hour sleep/wake patterns in healthy elderly persons. Appl Nurs Res 1994; 7:75–83Crossref, Medline, Google Scholar
2 : Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol Behav 2002; 76:597–603Crossref, Medline, Google Scholar
3 Cummings JL, Benson DF: Dementia: a clinical approach, 2nd ed. Oxford, United Kingdom, Butterworth-Heinemann, 1992Google Scholar
4 : Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS Drugs 2001; 15:777–796Crossref, Medline, Google Scholar
5 : Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry 2001; 158:704–711Crossref, Medline, Google Scholar
6 : Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int 2000; 17:405–418Crossref, Medline, Google Scholar
7 : The use of chronobiotics in the resynchronization of the sleep/wake cycle: Therapeutical application in the early phases of Alzheimer’s disease. Recent Pat Endocr Metab Immune Drug Discov 2011; 5:80–90Crossref, Medline, Google Scholar
8 : Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Curr Neuropharmacol 2010; 8:218–227Crossref, Medline, Google Scholar
9 : Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function. J Sleep Res 1995; 4:15–20Crossref, Medline, Google Scholar
10 : Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging (Milano) 1998; 10:308–315Medline, Google Scholar
11 : Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep 1997; 20:18–23Medline, Google Scholar
12 : Observed sleep/wakefulness and severity of dementia in an Alzheimer’s disease special care unit. J Gerontol A Biol Sci Med Sci 1995; 50:M303–M306Crossref, Medline, Google Scholar
13 : Sleep in non-institutionalized Alzheimer’s disease patients. Aging (Milano) 1994; 6:451–458Medline, Google Scholar
14 : Sleep/wake cycle disturbance in Alzheimer’s disease: how much is due to an inherent trait? Int Psychogeriatr 2002; 14:73–81Crossref, Medline, Google Scholar
15 : Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 1997; 20:24–27Crossref, Medline, Google Scholar
16 : The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44:2308–2314Crossref, Medline, Google Scholar
17 : A rating scale for aggressive behaviour in the elderly—the RAGE. Psychol Med 1992; 22:211–221Crossref, Medline, Google Scholar
18 : Assessment of disruptive behavior associated with dementia: the Disruptive Behavior Rating Scales. J Geriatr Psychiatry Neurol 1989; 2:196–202Crossref, Medline, Google Scholar
19 : Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23:271–284Crossref, Medline, Google Scholar
20 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
21 : Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 2001; 105:185–191Crossref, Medline, Google Scholar
22 : The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 1997; 85:207–216Crossref, Medline, Google Scholar
23 : The actigraph data analysis software: II. A novel approach to scoring and interpreting sleep-wake activity. Percept Mot Skills 1997; 85:219–226Crossref, Medline, Google Scholar
24 : Evaluation of human activities and sleep-wake identification using wrist actigraphy. Psychiatry Clin Neurosci 1998; 52:157–159Crossref, Medline, Google Scholar
25 : Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep 2003; 26:337–341Crossref, Medline, Google Scholar
26 : A longitudinal study of laboratory- and diary-based sleep measures in healthy “old old” and “young old” volunteers. Sleep 1994; 17:489–496Crossref, Medline, Google Scholar
27 : Percentile reference charts for selected sleep parameters for 20- to 80-year-old healthy subjects from the SIESTA database. Somnologie (Berl) 2005; 9:3–14Crossref, Google Scholar
28 : Actigraphy in agitated patients with dementia. Monitoring treatment outcomes. Z Gerontol Geriatr 2007; 40:178–184Crossref, Medline, Google Scholar
29 : Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav 2001; 72:21–28Crossref, Medline, Google Scholar
30 : A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. Int J Geriatr Psychiatry 1999; 14:520–525Crossref, Medline, Google Scholar
31 : The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003; 26:342–392Crossref, Medline, Google Scholar
32 : Circadian rhythms of activity in healthy young and elderly humans. Neurobiol Aging 1989; 10:259–265Crossref, Medline, Google Scholar
33 : Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: results of a randomized, controlled trial. J Am Geriatr Soc 2011; 59:1393–1402Crossref, Medline, Google Scholar



