Persistence of Mania After Cessation of Stimulation Following Subthalamic Deep Brain Stimulation
The syndrome of mania or hypomania (mood elevation with psychomotor agitation, distractibility, pressure of speech, inflated self-esteem, and impulsivity) may arise following deep brain stimulation (DBS) of the subthalamic nucleus (STN) for Parkinson’s disease (PD).1,2 Previous reports have attributed the onset of mood changes to microlesional effects during neurosurgery,3 the amplitude (voltage) of stimulation,4 or the position of the active electrode contact within the STN.5 Remediation of mania has been described through reducing the amplitude of stimulation,4 moving the active contact,5,6 delivering a more focused stimulation field by using the electrode as the anode,6 or the addition of mood-stabilizing medication.7 Here we report a case of mania with psychotic symptoms in a patient without a personal or family history of bipolar disorder, with persistence after prolonged cessation of stimulation (6 weeks). To our knowledge this is the first such case reported in the literature. This case also describes the phenomenon of a sustained mood change that was positively evaluated by the patient and his family, with emergence of clinically significant symptoms only after activation of an additional electrode contact 9 months after device implantation.
Prior to the commencement of data collection, the full protocol was approved by the Human Research Ethics Committees of the Royal Brisbane and Women’s Hospital, the University of Queensland, the Queensland Institute of Medical Research Berghofer Medical Research Institute, and UnitingCare Health. The patient and his spouse provided written consent to participate in the study.
Case Report
The patient was a 64-year-old married man working as a factory supervisor. He had a 5-year history of PD, manifest with rigidity and tremor of the left upper limb, associated with generalized bradykinesis. His motor symptoms were levodopa responsive, but treatment was limited by intolerable nausea on a range of agents including levodopa, a monoamine oxidase inhibitor, and amantadine. He was referred to a movement-disorders center for consideration of DBS due to significant functional impairment.
In a presurgical assessment the patient was evaluated by a movement-disorders neurologist, a neurosurgeon, a psychiatrist with experience in DBS for PD, and a rehabilitation physician. The diagnosis of PD was confirmed according to the United Kingdom Queens Square Brain Bank criteria,8 with a Hoehn and Yahr scale score of 2.9 His Unified Parkinson’s Disease Rating Scale (UPDRS) Part III motor examination score was 47 (off medication). He had no other significant medical history. He was a nonsmoker and imbibed alcohol infrequently. He gave a psychiatric history of a mild-moderate recurrent depressive disorder, without melancholic features and without any history of suicidality. His most recent episode of depression occurred in the setting of his movement disorder and was treated with escitalopram 20 mg under the care of his general practitioner. He denied a family history of psychiatric or neurological illness. His premorbid personality was described as gregarious, but with social withdrawal since the diagnosis of PD. His Montreal Cognitive Assessment (MOCA) score was 22/30.
Subsequently the patient underwent bilateral stereotactic implantation of quadripolar Medtronic 3389 DBS electrodes. The STN was identified as a midbrain structure on Fluid Attenuation Inversion Recovery (FLAIR) imaging. Intraoperative microelectrode recordings were employed to establish localization within the STN, and the patient was roused from anesthesia to perform test stimulation. A computed tomography (CT) scan confirmed satisfactory postoperative lead placement. Stimulation was commenced at contact 9 in the right STN and contact 1 in the left STN, but subsequent electrode testing yielded the best motor outcome at contacts 11 and 1 (contacts are numbered 8–11 and 0–3 in the ventral-dorsal direction on the right and left DBS electrodes, respectively). The postoperative UPDRS Part III score was 12 (on stimulation), and MOCA (alternate version) score was 28/30. Chronic monopolar stimulation parameters were 2.4 volts (right STN) and 1 volt (left STN) with a pulse-width of 60 µs and frequency of 130 Hz.
The patient reported an immediate nonmotor outcome from stimulation, manifest from the onset of active stimulation. He and his wife undertook a semistructured interview 6 months after his procedure:
The minute I opened my eyes my depression and anxiety have been lifted and I’ve never felt depressed and never felt anxious since that day, would you believe it? I wake up in the morning, I look forward to the day. I enjoy mixing with people now, I always used to shy away from people because I just couldn’t get my thoughts and my words together.
The patient was reviewed by a psychiatrist at each scheduled visit to the movement-disorders center. He displayed a consistent mild elevation of mood with talkativeness. He denied any new impulsive or addictive behaviors. He admitted to episodic verbal irritability:
It’s probably made me a little bit more aggressive. . . . I think it was always in me but I think it comes out a lot more now. . . . I find it hard to control my words. Not to say that I’ve ever got into a fight . . . but I’m very vocal if I believe people are doing the wrong thing by me.
Further adjustments to the DBS device were strongly resisted by the patient and his wife:
I’m deadly worried about going back to being depressed and anxious. . . . I don't know whether I'd be able to pull myself out.
I was worried when he was at home all day… he’d be just sitting in front of the TV or lying in bed but now he’s out all day either with friends or fishing. So that’s a positive, isn’t it?
In order to address residual left-sided motor symptoms, contacts 10 and 11 in the right STN were activated concurrently during an inpatient stay 9 months after the initial surgery. There was no immediate change in mental status, but the patient’s family contacted the center 1 week after discharge to report a new preoccupation with gambling and pervasive irritability. The patient refused to return for device adjustment but was apprehended by the police for dangerous driving. He was held for an emergency psychiatric assessment where he was judged to be floridly manic. He disclosed delusional beliefs that DBS clinicians were conspiring with senior police officials to damage his reputation. He incorporated his wife into these persecutory beliefs and threatened to kill her.
The DBS device was turned off with immediate return of motor symptoms but sustained persistence of mania with psychosis. He was admitted to a psychiatric ward and commenced on lithium, titrated to a serum level of 0.8 mmol/l. Quetiapine was initiated and titrated to a dose of 100 mg nocte. No oral dopaminergic therapies were introduced. Despite these changes, he required an extended admission as an involuntary patient due to persisting symptoms and poor insight. After 6 weeks he was discharged on maintenance therapy with lithium. The patient and his family requested that the DBS device be reactivated, and therapy was gradually retitrated to his previous maintenance level with successful control of his motor symptoms. The patient regained his subjectively positive mood state without a return of mania or psychosis but did not develop insight into the connection between his stimulation and his manic episode, nor did he believe that he had been psychiatrically unwell. He was able to return home with his wife and remains under the care of the movement disorders center as well as a general psychiatrist.
Anatomical Analysis
Preoperative T1-weighted and FLAIR images were coregistered with a postoperative CT scan repeated at 9 months, using an affine transformation within FSL (FLIRT version 6.0).10 These acquisitions were spatially normalized into ICBM_2009b nonlinear asymmetric space using a fast diffeomorphic image registration algorithm (DARTEL) as implemented in SPM12.11 Electrodes were localized using the Lead-DBS toolbox12 and projected onto the BigBrain histological atlas.13 The positions of the electrode contacts were evaluated with reference to a tractrographic parcellation of the STN into limbic, associative, and motor subregions.14 A simplified volume of activated tissue was calculated based upon the patient’s stimulation parameters.15
Visualization (Figure 1) suggested that the left DBS electrode was positioned optimally in the motor aspect of the STN. However, the trajectory of the right DBS electrode appeared to pass through the associative STN, with contact 10 located on the border of the associative and limbic STN. The distances from contacts 9–11 to each subregion of the STN were compared with the proportion of each STN subregion occupied by the volume of activated tissue (Table 1). Although each selected electrode contact was close to all STN subregions, the volume of activated tissue appeared to preferentially include the limbic and associative zones. Given a previous report of mood changes induced by stimulation in the substantia nigra,16 we also extended our VAT analysis to this nucleus, using an atlas developed at high field-strength.17 Our findings suggest that the substantia nigra was not significantly implicated in this patient’s mood changes.
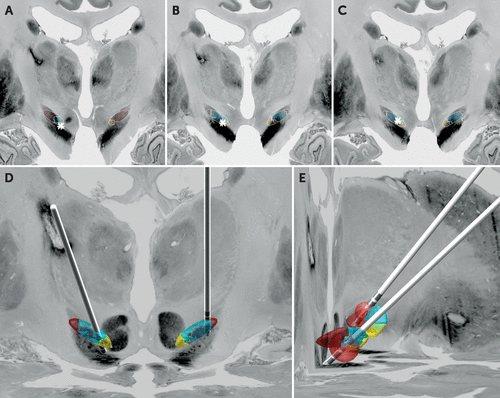
FIGURE 1. Localization of Electrode Contacts Using the Lead-DBS Toolboxa
a Probabilistic parcellation of the subthalamic nucleus (STN) is superimposed upon the BigBrain histological atlas in the midbrain. Within the STN atlas: yellow=limbic territory, blue=associative territory and maroon=motor territory. Coronal views of right electrode contacts 9 (panel A), 10 (panel B), and 11 (panel C); asterisk indicates the contact location. D) Coronal view of the reconstructed electrode trajectories and position. E) Sagittal view with illustrative volume of activated tissue at contact 10 on the right electrode (2.4V) (foreground) and contact 1 (1V) on the left electrode (background).
| Contact on Right Deep Brain Stimulation Electrode | Distance From Limbic STN (mm) | Distance From Associative STN (mm) | Distance From Motor STN (mm) | Percentage of the Limbic STN filled by VAT | Percentage of the Associative STN filled by VAT | Percentage of the Motor STN filled by VAT | Percentage of Substantia Nigra filled by VAT |
|---|---|---|---|---|---|---|---|
| 9 | 1.92 | 1.56 | 1.11 | 14.48 | 7.66 | 8.23 | 4.96 |
| 10 | 0.32 | 0.14 | 0.06 | 52.41 | 37.20 | 16.71 | 4.59 |
| 11 | 0.76 | 0 | 1.48 | 84.83 | 70.68 | 12.59 | 3.94 |
TABLE 1. Distances From Contacts 9–11 to Each Subregion of the Subthalamic Nucleus (STN) Compared With the Proportion of Each STN Subregion Occupied by the Volume of Activated Tissue (VAT)
Discussion
We describe the emergence of mood elevation subsequent to subthalamic DBS in an individual with PD. This phenomenon was positively evaluated by the patient and his spouse. Mania developed 9 months subsequent to initial DBS device implantation, secondary to the activation of an additional contact in the right STN. We employ a novel methodology for localizing and quantifying the field of stimulation in the STN, supporting the role of ventromedial (limbic and associative) subthalamic stimulation in the pathogenesis of postoperative mania. Importantly, we also describe the persistence of mania for 6 weeks after cessation of stimulation, implying that once established, this syndrome may not be responsive to stimulation manipulation.
Existing evidence supports the significance of contact location within the STN. A tripartite functional organization of the STN into limbic, associative, and motor subregions is suggested by primate and human studies.18,19 Moving stimulation between the dorsal and ventral aspects of the nucleus can impair response inhibition20 and modulate behavioral symptoms.21 Stimulation diffusion may also activate the medial forebrain bundle,22 a dopaminergic tract that passes close to the STN as it ascends from the ventral tegmental area.
The persistence of mania after prolonged cessation of stimulation is a novel finding and suggests a degree of plasticity in nonmotor circuits modulated by stimulation. STN DBS modifies the metabolism of cortical regions implicated in response inhibition23–25 and decouples the STN from neural correlates of cognitive conflict.26 A pathological decoupling of this inhibitory network could underlie persistent mood elevation, with subsyndromal changes present in our case for 9 months before mania developed.
In our case, stimulation in the right STN appeared to underlie the patient’s psychiatric symptoms, given the onset of his manic episode after activation of an additional contact in the right DBS electrode, plus the relatively low stimulation intensity in the left electrode. Simulation of a volume of activated tissue gave us additional data about the volume of each STN subregion influenced by stimulation and may help to explain the nonmotor changes observed after DBS. Although anatomical localization may have a role in the practice of DBS, preliminary findings such as this await replication in an appropriately sized sample. We also note that we have not evaluated the modulation of subcortical-prefrontal fibre tracts such as the medial forebrain bundle or hyperdirect pathway. Accurate modelling of connectivity necessitates the preoperative acquisition of high-angular resolution diffusion imaging, which was not available for this patient. The relative contribution of electrode localization within the STN versus the connectivity profile of the stimulation seed is thus an additional research question to be addressed in future work.
1 : Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 2006; 21(Suppl 14):S305–S327Crossref, Medline, Google Scholar
2 : Psychiatric and neuropsychiatric adverse events associated with deep brain stimulation: A meta-analysis of ten years’ experience. Mov Disord 2007; 22:1722–1728Crossref, Medline, Google Scholar
3 : Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson’s disease. Mov Disord 2002; 17:1371–1374Crossref, Medline, Google Scholar
4 : Voltage-dependent mania after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a case report. Biol Psychiatry 2011; 70:e5–e7Crossref, Medline, Google Scholar
5 : Manic episode with psychotic symptoms in a patient with Parkinson’s disease treated by subthalamic nucleus stimulation: improvement on switching the target. J Neurol Sci 2008; 273:116–117Crossref, Medline, Google Scholar
6 : Hypomania as an adverse effect of subthalamic nucleus stimulation: report of two cases. Acta Neurochir (Wien) 2006; 148:895–897, discussion 898Crossref, Medline, Google Scholar
7 : Manic episode with psychotic symptoms induced by subthalamic nucleus stimulation in a patient with Parkinson’s disease. Mov Disord 2003; 18:1382–1384Crossref, Medline, Google Scholar
8 : Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55:181–184Crossref, Medline, Google Scholar
9 : Parkinsonism: onset, progression and mortality. Neurology 1967; 17:427–442Crossref, Medline, Google Scholar
10 : Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23(Suppl 1):S208–S219Crossref, Medline, Google Scholar
11 : A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38:95–113Crossref, Medline, Google Scholar
12 : Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage 2015; 107:127–135Crossref, Medline, Google Scholar
13 : BigBrain: an ultrahigh-resolution 3D human brain model. Science 2013; 340:1472–1475Crossref, Medline, Google Scholar
14 : Brain tissue properties differentiate between motor and limbic basal ganglia circuits. Hum Brain Mapp 2014; 35:5083–5092Crossref, Medline, Google Scholar
15 : Explaining clinical effects of deep brain stimulation through simplified target-specific modeling of the volume of activated tissue. AJNR Am J Neuroradiol 2012; 33:1072–1080Crossref, Medline, Google Scholar
16 : Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 1999; 340(19):1476–1480Crossref, Medline, Google Scholar
17 : Generation and evaluation of an ultra-high-field atlas with applications in DBS planning. SPIE Med Imaging 2016; doi: 10.1117/12.2217126Google Scholar
18 : The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. J Neurosci 2013; 33:4804–4814Crossref, Medline, Google Scholar
19 : Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. Neuroimage 2012; 60:83–94Crossref, Medline, Google Scholar
20 : Mapping go-no-go performance within the subthalamic nucleus region. Brain 2010; 133:3625–3634Crossref, Medline, Google Scholar
21 : Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proc Natl Acad Sci USA 2007; 104:10661–10666Crossref, Medline, Google Scholar
22 : Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery 2009; 64:1106–1114, discussion 1114–1115Crossref, Medline, Google Scholar
23 : Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. Eur J Nucl Med Mol Imaging 2010; 37:1512–1520Crossref, Medline, Google Scholar
24 : STN stimulation alters pallidal-frontal coupling during response selection under competition. J Cereb Blood Flow Metab 2007; 27:1173–1184Crossref, Medline, Google Scholar
25 : Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol 2009; 66:817–824Crossref, Medline, Google Scholar
26 : Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci 2011; 14:1462–1467Crossref, Medline, Google Scholar



