The Neural Correlates of Impaired Self-Monitoring Among Individuals With Neurodegenerative Dementias
Abstract
Objective:
Self-monitoring is a crucial component of human empathy and necessary for the formation and repair of social relations. Several studies have brought to light possible neuronal substrates associated with self-monitoring, but the information that they have provided is inconclusive. The authors, therefore, studied a large group of patients with dementia to assess what brain structures are necessary for the self-monitoring function.
Methods: Seventy-seven patients with dementia of various types were screened using voxel-based morphometry to assess possible volume reduction in the brain structures of patients with self-monitoring problems, and the decrease of socioemotional expressiveness and modification of self-presentation was estimated using the Revised Self-Monitoring Scale. Regression analysis was employed to investigate the correlation between gray matter loss and deficient self-monitoring.
Results: The socioemotional expressiveness scores were associated with decreased gray matter volume in the right olfactory cortex, inferior frontal gyrus, superior temporal pole, parahippocampal gyrus, insula, and medial temporal gyrus bilaterally. Self-presentation scores were associated with bilateral gray matter volume reduction in the olfactory cortex, insula, rectus gyrus and inferior frontal gyrus, right superior temporal pole, and parahippocampal gyrus, as well as the left medial temporal gyrus and anterior superior frontal gyrus.
Conclusions: These results suggest that patients with dementia present decreased ability of self-monitoring, probably due to impaired insula and orbitofrontal cortex and their disconnection from structures of the salience network.
Humans are social beings, and much of their progress over the centuries is due to their ability to effectively adapt to their social environment. The cognitive process by which people evaluate their behavior to ensure that it is consistent with how they are expected by others to behave, based on indirect or implicit feedback from others, is called self-monitoring (1, 2). Self-monitoring, defined as the understanding of others’ social cues and modifying one’s behavior accordingly, is a crucial component of human empathy and has significant advantages for the formation and repair of social relations (3).
Self-monitoring is a substantial aspect of theory of mind, as it requires evaluation and adaptation of one’s behavior to what is expected of others in order to explain and predict other peoples’ behavior. Several functional imaging studies of theory of mind have brought to light possible components of the neuronal substrates associated with self-monitoring, but the relevant information is inconclusive and contradictory. In particular, some studies suggest that the anterior paracingulate cortex is a key structure for mentalizing—the ability to read the mental states of other agents (4, 5). However, previous neuroimaging studies have also shown activation in the anterior paracingulate cortex in additional tasks that may include various components of self-monitoring, such as visual self-recognition, (6) autobiographical memory, (4, 7) verbal self-monitoring, (8) and self-generated thoughts (9) concerning one’s own mental state rather than other people’s, but may also subserve other functions.
In addition, neuroimaging studies have shown that the superior temporal sulci are also crucial structures in the initial analysis of social cues (10–12). Moreover, the temporal poles have also been thought of as a store for personal and episodic memories, subserving the mentalizing process (13–16). Previous studies have suggested that the amygdala is also activated for rapid and automatic response to salient social stimuli (12, 17). Baron-Cohen et al (18). have reported increased activity in the orbitofrontal cortex, leading to the suggestion that this brain area is important for the processing of the rewards and punishments that are required for adaptive social behavior (18). Taken together, these studies lead to the conclusion that many brain structures that are already know to subserve other cognitive functions are equally involved in self-monitoring.
Furthermore, evidence from clinical studies has shown the crucial role of specific brain areas for self-monitoring. Patients with the behavioral variant of frontotemporal dementia (bvFTD) are characterized by early, severe lack of awareness of their behavior and personality decline, and sometimes of their cognitive deficits. These deficits suggest an inability for self-monitoring, likely due to early impairment of specific brain circuits in the temporal lobe bilaterally (possibly involving basic emotion reading) and right temporal regions (ability to change one’s behavior) (19). In particular, bvFTD has been associated with early deficits in functional connectivity of the anterior insula (AI) and anterior cingulate cortex (ACC) with the salience network (SN). As a result, socioemotional symptoms, such as loss of empathy and impaired self-awareness and emotion recognition, appear in patients with bvFTD early in the course of the disease (20, 21). In addition, temporal damage in patients with bvFTD has been associated with the inability to identify sarcasm, whereas damage to the dorsomedial frontal cortex has been associated with failure to identify others’ intentions. Moreover, previous studies in patients with bvFTD referred to reduced perspective-taking ability due to ventromedial orbitofrontal cortex impairment (22, 23). In contrast, patients with Alzheimer’s disease are often spared social functioning until later in its course (24). In addition, patients with a semantic variant of primary progressive aphasia presented with loss of knowledge of social interaction scripts except for the simplest and most concrete, likely due to atrophy in the anterior temporal lobes (25, 26). Previous studies have shown that although progressive supranuclear palsy (PSP) is mainly a motor disorder, it is commonly presented with bvFTD behavior symptoms (emotional blunting and disinhibition) and reduced socioemotional sensitivity due to the disruption of the subcortical regions with SN (27–29). In addition, corticobasal syndrome is also characterized by behavior and personality changes similar to those observed in frontotemporal dementia. Previous studies have shown impaired ability to recognize and express facial emotional expressions (28, 30–33). In view of the fact that large tracts of the temporal, frontal, and limbic brain appear to be involved in self-monitoring as well as other functions, the need arises to ascertain what structures are necessary for what aspects of self-monitoring
One model of self-monitoring (2) suggests that it involves two specific aspects: socioemotional expressiveness (i.e., the ability for intuitive sensitivity to others’ subtle social cues) and modification of self-presentation (i.e., the ability to modify one’s behavior when one feels it is not appropriate for the prevailing social circumstances). Thus, illustrating the neural networks that underlie these self-monitoring aspects could help us better understand the self-monitoring process in its totality. We hypothesized that both subscales would be associated with specific brain areas and that the associated brain areas would be common in part for both subscales.
To test the above hypotheses, we retrospectively studied a large group of healthy controls (HCs) and patients with dementia by means of voxel-based morphometry to identify gray matter changes between the dementia and HC groups. Then, for these specific brain structures, a regression analysis model was employed to investigate the correlation between gray matter loss and lack of self-monitoring.
Methods
Participants
A total of 77 patients and 31 HC subjects were recruited in this retrospective study from an outpatient memory clinic and a day-care center for third graders. Among the patients, 39 were diagnosed as meeting core clinical criteria for Alzheimer’s disease according to the McKhann criteria (34, 35). Thirty-eight patients were diagnosed with one of the frontotemporal dementia syndromes, including 13 with bvFTD (Rascovsky criteria) (36); 11 with semantic variant of primary progressive aphasia (Gorno-Tempini criteria); four with nonfluent variant of primary progressive aphasia (Gorno-Tempini criteria) (37); six with a corticobasal syndrome (Armstrong criteria), (38) and four with PSP motor syndrome (Höglinger criteria) (39). Patients’ diagnoses were derived by a multidisciplinary team of neurologists, neuropsychologists, and psychiatrists who performed extensive behavioral, neuropsychological, and neuroimaging assessment. The diagnoses were confirmed through brain MRI. Greek as native language was also an inclusion criterion.
Informed consent to participate in the study was obtained from all participants and their informants or caregivers. The study was conducted in compliance with the regulations of the local ethics committee and in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Self-Monitoring Testing
The Revised Self-Monitoring Scale (RSMS) is a questionnaire designed to assess the degree to which subjects attend to others’ socioemotional signals and allow those signals to influence their behavior. It consists of two subscales designed to measure cognitive elements of empathy: the Expressive Behavior (EX) subscale, which measures the subjects’ sensitivity to the expressive behavior of others, and the Self-Presentation (SP) subscale, which measures the subjects’ tendency to monitor their self-presentation. An informant (a close relative) was asked to rate on a 6-point Likert scale (1=certainly, always false to 6=certainly, always true) how well each of the 13 statements of the questionnaire described the patient’s ability to modulate his or her behavior in various social situations.
MRI Data Acquisition
All participants underwent a routine MRI exam within 1 month from the time of neurocognitive and neuropsychiatric assessment. A standard clinical brain MRI protocol was employed, which included a three-dimensional, high spatial resolution T1-weighted (three-dimensional HR-T1) gradient echo pulse sequence for the acquisition of detailed anatomical images. MRI scans were performed in four different diagnostic imaging centers equipped with 10 different MR scanners. Therefore, acquisition parameters varied depending on the MR scanner used; however, the data were compatible with the minimum requirements of voxel-based morphometry analysis. All imaging data were screened by an experienced neuroradiologist using standard neuroradiological criteria for the detection of anatomical abnormalities or pathologies and the presence of image artifacts (e.g., due to gross motion).
MRI Data Preprocessing and Analysis
Volumetric analysis was conducted using the Computational Anatomy Toolbox (CAT12) and a toolbox of statistical parametric mapping (SPM12; Wellcome Department of Cognitive Neurology, www.fil.ion.ucl.ac.uk/spm/software/spm12) implemented on MATLAB R2016b (MathWorks, Natick, MA). All three-dimensional-HR T1 images were segmented into gray matter, white matter, and cerebrospinal fluid and then normalized using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL), which provides six iterations, using an already existing DARTEL template in the Montreal Neurological Institute space derived from 555 HCs of the IXI Dataset (http://www.brain-development.ord). Thus, the creation of sample-specific DARTEL templates was not necessary. During this registration preprocessing, local gray matter and white matter volumes were conserved by modulating the image intensity of each voxel by the Jacobian determinants of the computed deformation fields. Registered image and preprocessing parameters exported were quantitatively assessed, and data with weighted overall quality measure lower than C+ were excluded from further analysis. The remaining normalized and modulated gray matter images were smoothed with an 8mm full-width-at-half-maximum isotropic Gaussian kernel via a standard module of SPM12.
The preprocessed images were then entered into t test models in SPM12. Between-group whole-brain differences on gray matter density were determined using the two-sample t test with gender, years of education, total intracranial volume, MRI scanner, and Addenbrooke’s Cognitive Examination-Revised (ACE-R) scores as nuisance variables to account for any potentially contributing effect on the pattern of local gray matter changes (40–43). The statistical threshold was set at p<0.05, corrected for multiple comparisons with family-wise error (FWE) correction. The statistically significant clusters were used as masks in the next step of regression analysis.
Self-Monitoring Analysis
Correlation analyses between volume reduction and RSMS subscale scores were performed for the group with dementia to show the relationship between self-monitoring scores and gray matter volume. To this purpose, the multiple regression design function of SPM12 was used. Thus, RSMS scores were treated as a covariate of interest, whereas age, gender, total intracranial volume, MRI scanner, years of education, and ACE-R scores were treated as confounding variables. The statistical maps were thresholded at p<0.05, applying FWE correction for multiple comparisons. Anatomical regions of interest covering the entire volumes of clusters were defined using the WFU_PickAtlas tool of SPM12 (44, 45) and automated anatomical labeling (46). To investigate the unique effect of each subscale, separated design matrices were performed for both subscales. The effect of each self-monitoring function was tested by importing the RSMS scores (subscales EX and SP) into a t-contrast statistical model.
Statistical Analysis
Descriptive metrics were calculated and between-group differences were conducted for all available demographic and clinical data, using t test and chi-square statistics. SPSS (version 22.0) was used for all analyses, and the level of significance was set at p<0.05.
Results
Demographic and Clinical Characteristics
Significant differences were revealed in years of education, gender, and ACE-R (p=0.006; p=0.009; p=0.001, respectively) (Table 1) when patients’ group means were compared with the HC group; thus, they were included as nuisance covariates in the neuroimaging analysis. In contrast, the two groups were age matched.
| Characteristic | Dementia group (N=77) | Healthy control group (N=31) | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years)b | 69.81 | 8.67 | 66.13 | 8.30 |
| Education level (years)c | 10.53 | 4.74 | 13.19 | 3.33 |
| ACE-Rd | 58.91 | 18.51 | 94.61 | 3.00 |
| RSMS EX | 19.72 | 7.56 | ||
| RSMS SP | 23.84 | 8.10 | ||
TABLE 1. Demographic and clinical characteristics of patients with dementia and healthy control subjectsa
Behavioral Results
The RSMS EX subscale mean score was 19.72 (SD=7.56) and the RSMS SP subscale mean score was 23.84 (SD=8.10) in the group with dementia (Table 1).
Neuroimaging Results
Compared with HCs, patients with dementia showed widespread decreased gray matter density at p<0.05, FWE. The main alterations were located in the bilateral temporal, frontal lobes, cingulum, precuneus, putamen, fusiform, olfactory, hippocampus, parahippocampal, and insula (Figure 1) (for further details, see Table S1 in the online supplement).
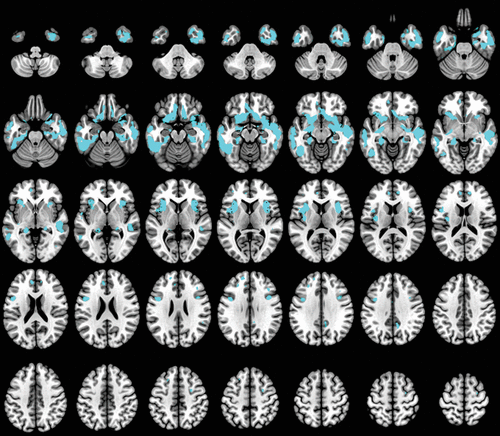
FIGURE 1. Anatomical regions with gray matter volume reductions in patients with dementia compared with healthy control subjectsa
a Significant clusters (depicted in cyan) were found at p value <0.05 (family-wise-error corrected) comparing patients with dementia and healthy control subjects.
Behavior subscale.
Reduced gray matter density in frontal, medial, and temporal anatomical areas, such as right olfactory cortex, inferior frontal gyrus, superior temporal pole, parahippocampal gyrus, insula, and bilateral medial temporal gyrus was strongly correlated with decreased scores on the EX subscale (p<0.05, FEW) (Table 2, Figure 2).
| Subscale score and cluster | Anatomical labeling | Voxel | Montreal Neurological Institute coordinate (x, y, z) (mm) | T |
|---|---|---|---|---|
| Expressive behavior subscale score | ||||
| 1 | 337 | 25.5, 7.5, –15 | 4.51* | |
| Temporal_Pole_Sup_R | ||||
| Olfactory_R | ||||
| ParaHippocampal_R | ||||
| Insula_R | ||||
| Frontal_Inf_Orb_R | ||||
| 2 | 54 | –48, 6, –33 | 4.50* | |
| Temporal_Mid_L | ||||
| 3 | 29 | 57, –1.5, –16.5 | 4.36* | |
| Temporal_Mid_R | ||||
| Self-presentation subscale score | ||||
| 1 | 682 | 27, 15, –27 | 5.99* | |
| Insula_R | ||||
| Temporal_Pole_Sup_R | ||||
| Frontal_Inf_Orb_R | ||||
| ParaHippocampal_R | ||||
| Rectus_R | ||||
| Olfactory_R | ||||
| 2 | 874 | –16.5, 9, –16.5 | 5.72* | |
| Insula_L | ||||
| Olfactory_L | ||||
| Rectus_L | ||||
| Frontal_Inf_Orb_L | ||||
| Frontal_Sup_Orb_L | ||||
| 3 | 123 | –63, –19.5, –12 | 4.47* | |
| Temporal_Mid_L |
TABLE 2. Expressive behavior and self-presentation subscale scores positively correlated with gray matter volume (density)a
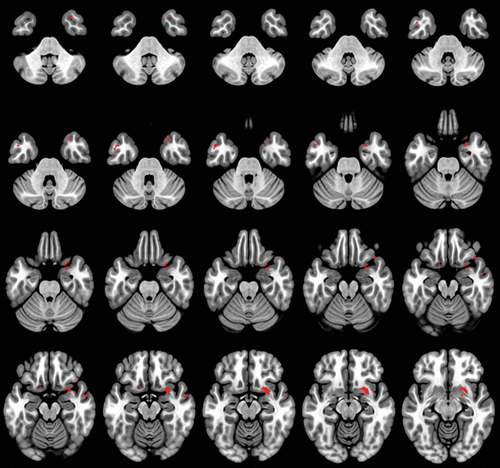
FIGURE 2. Decreased expressive behavior subscale scores associated with decreased gray matter volume in anatomical regions in patients with dementiaa
a Significant clusters from regression (depicted in red) were found at p value <0.05 (family-wise-error corrected), correlating decreased expressive behavior subscale cores with decreased gray matter volume.
Self-Presentation subscale.
The correlation analyses between decreased SP subscale scores and reduced gray matter density showed associations located in frontal structures including bilateral olfactory cortex, rectus gyrus, and inferior frontal gyrus, as well as left anterior superior frontal gyrus; in temporal structures including right superior temporal pole and parahippocampal gyrus, left medial temporal gyrus, and insula bilateral (p<0.05, FWE whole-brain correction) (Table 2, Figure 3).
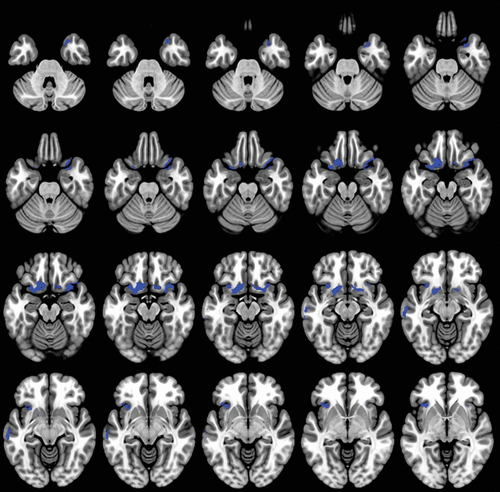
FIGURE 3. Decreased self-presentation subscale scores associated with decreased gray matter volume in anatomical regions in patients with dementiaa
a Significant clusters from regression (depicted in blue) were found at p value <0.05 (family-wise-error corrected), correlating decreased expressive behavior subscale scores with decreased gray matter volume.
Discussion
A caregiver-based measure of self-monitoring was correlated with MRI-derived gray matter density in patients with dementia. Our results confirm the gray matter changes in patients with dementia compared with the HC group (47–50) and also suggest that anatomical regions such as the orbitofrontal, anterior prefrontal, and temporal cortex, as well as specific limbic areas, have a crucial role in the self-monitoring function in patients with dementia. In our study, social self-monitoring loss was correlated with bilateral gray matter loss located in the olfactory cortex, rectus gyrus, inferior frontal gyrus, and insula. Additionally, it was associated with reduced gray matter density in the right temporal pole and hippocampal gyrus, and in the left superior frontal gyrus and medial temporal gyrus.
We observed that the lateral and medial orbitofrontal cortex (OFC) contributed significantly to self-monitoring in patients with dementia. The OFC, as a part of the SN, contributes to the perception of social cues and interpretation of information in the current circumstances (21, 51, 52). In particular, (53) the OFC is an essential brain structure in signaling the expected rewards/ or punishments for an action, given the particular details of a social frame. Furthermore, Kringelbach and Rolls (54) have reported that medial OFC is crucial for the ongoing monitoring of the reward value of amplifiers, whereas the lateral OFC is involved in the evaluation of the punishment value of amplifiers leading to a change in the current behavior. Previous studies also indicated that orbitofrontal damage impairs self-monitoring, precluding generation of social emotions associated with the resolution of social mistakes (55). Damage to the OFC has been associated with the ability to state the rules with a failure to apply these rules to ongoing behavior (56, 57). Additionally, the reduced reward-related attention to social cues has been directly associated with many of the socioemotional deficits observed in bvFTD patients (58, 59).
The insula cortex was also significantly associated with decreased self-monitoring in our study. The insula is involved in experiencing emotions and representing the emotional state of other people (51). Additionally, the insula was considered a crucial brain structure in the evaluation of the incoming stimuli for personal and social salience (60). In particular, the AI integrates highly processed sensory stimuli with homeostatic, affective, motivational, and hedonic information, proving a fundamental basis for emotional awareness in a social setting due to interconnectivity with SN nodes (52, 61–63). According to Berntson et al., (64) damage to the insula causes reduced response to both unpleasant and pleasant visual stimuli, suggesting a role for the insula in emotional processes. In addition, it has been associated with abnormal decision-making under uncertainty and risk (65, 66).
In our study, the anterior temporal pole was correlated with self-monitoring. Previous studies have implicated the temporal lobe in understanding social behavior, deriving social meaning, and maintaining social bonds in a continuously changing social frame (67). In particular, the temporal pole provides supportive information about whether a stimulus is salient concerning emotional and social aspects by linking sensory representation with emotional and social memory (68). Furthermore, it has been suggested that damage to the temporal lobe could lead to a hypoemotionality to visual stimuli and poor understanding in social words versus nonsocial words, (6) as well as a loss of person knowledge (68, 69). In addition, the temporal pole is a crucial brain structure for faultlessly inferring others’ intentions and thoughts by retrieving semantic and autobiographical information (70).
The ventral medial prefrontal cortex (vmPFC) was associated with decreased self-monitoring. The vmPFC plays an important role in representation of and reasoning about others’ emotions. It has been reported that the socioemotional process between the self and the person being evaluated (self-relatedness) may be underlined by vmPFC, which probably mediates the qualities of the withdrawn information for this procedure (71–74). Additionally, previous research has indicated the coactivation of vmPFC, along with the default mode network, during imaging of one’s own feelings or retrieving an autobiographical memory (71, 75–77). In addition, activation in the subgenual ACC (sgACC) may reflect a bottom-up information sensitivity and its potential for self-other evaluation in a positive light (78, 79). Recent studies have implicated the sgACC in detecting subjectively rewarding opportunities (78, 80, 81) and evaluating social threats related to the self (82). Moran and colleagues suggested that sgACC is responsive to the emotional valence of information, but only for traits that were judged to be self-descriptive (80).
Our findings demonstrate that self-monitoring is associated with decreased brain volumes in the left dorsolateral prefrontal cortex (dlPFC) and right ventrolateral prefrontal cortex (vlPFC). According to Sollberger and colleagues, (60) behavioral regulation in a manner appropriate to the social context is mainly mediated by dlPFC and vlPFC. In particular, composition of one’s appropriate behavior response, in accordance with the external social context, is regulated through the suppression of selfish behavior and active memory retrieval (83) by the dlPFC and vlPFC, respectively. Decety and Jackson (3) suggested that dlPFC mediates inferring others’ intentions and imagining others’ knowledge or feelings, thus temporarily inhibiting perspective taking. Previous studies have suggested that the damage to the dlPFC is associated with poor performance in perspective-taking tasks (3, 84, 85). Furthermore, patients with lesions in the dlPFC also showed deficits in using social cues to make interpersonal judgments (86).
Thus, it appears that dementia could impair both sensitivity to the expressive behavior of others and subjects’ tendency to monitor their self-presentation. Once a social cue is presented, a low-level salience detection process is performed to separate stimulus with personal relevance from noise. Lateral OFC, including the olfactory cortex and rectus gyrus, and the insula are key structures for this process (60). It is possible that the impaired OFC in patients with dementia could lead to an inability to evaluate their behavior and compare expected reward or punishment with the delivered reward or punishment, along with a failure to perceive their social mistakes and adapt their behavior to social rules (87). Insula damage may result in an inability to recognize and evaluate risk during decision-making, as well as identify social norm violations (88). Furthermore, temporal lobe loss suggests the important role of this brain region in modulation of high-level social behaviors, which may be due to disruption of connectivity with other limbic regions through the uncinate fasiculus (52, 67, 89, 90) In addition, damage to dlPFC and vlPFC may affect the ability for complex social reasoning and deliberate regulation of social behavior, causing an ineffective top-down control process to emotion-processing regions such as OFC and insula, resulting in the deficits in self-monitoring observed in our patients (91).
In addition, the emotion sharing, emotion understanding, and emotion regulatory mechanisms required for self-other distinction during bottom-up and top-down processes of empathy involve structures mainly in the right fronto-limbic network (92–95). According to previous studies, lower affective and cognitive empathy were associated with smaller volume in right fronto-limbic regions, including the right hippocampus, parahippocampal gyrus, thalamus, fusiform gyrus, inferior temporal gyrus, and dorsomedial and dorsolateral prefrontal cortices in patients with fronto-temporal damage due to neurodegenerative disease (96–100).
Although our study has achieved its aims, there are some limitations. First, the informant reports concerning self-monitoring provided by caregivers or close relatives may be insensitive to aspects of this processing. Second, our study does not include other social and emotional measures or findings from the different dementia groups. Third, the patient group included patients diagnosed with various types of dementia; however, strict threshold p<0.05 FWE was applied to hedge the inhomogeneity of the patients’ group. Fourth, as our study conducted by means of voxel-based morphometry, specific elements of the self-monitoring instead of other social and emotional roles of the above-mentioned regions could not be identified. Future work should focus on the clarification of these aspects of self-monitoring using fMRI or single-photon emission computed tomography neuroimaging techniques.
In summary, our research has revealed that the lateral OFC, insula, temporal pole, dlPFC, and vlPFC are essential brain areas for the self-monitoring process. Damage to these areas could lead to decreased socioemotional expressiveness and modification of self-presentation aspects. Our results suggest that in patients with dementia the decreased ability for both low-level and high-level self-monitoring processes is probably due to impaired insula and OFC and their disconnection from structures in the SN (24). These findings can not only contribute to a more accurate diagnosis but also be used to provide better care to patients with dementia.
1 : Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 2006; 18:871–879. doi:
2 : Revised Self-Monitoring Scale. PsycTESTS Dataset, 1984. doi:
3 : The functional architecture of human empathy. Behav Cogn Neurosci Rev 2004; 3:71–100. doi:
4 : A functional imaging study of cooperation in two-person reciprocal exchange. Proc Natl Acad Sci USA 2001; 98:11832–11835. doi:
5 : Reading the mind in cartoons and stories: an fMRI study of “theory of mind” in verbal and nonverbal tasks. Neuropsychologia 2000; 38:11–21. doi:
6 : Recognizing one’s own face. Cognition 2001; 78:B1–B15. doi:
7 : Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 1999; 9:54–61. doi:
8 : Functional neuroanatomy of verbal self-monitoring. Brain 1996; 119:907–917. doi:
9 : Brain activity during stimulus independent thought. Neuroreport 1996; 7:2095–2099Medline, Google Scholar
10 : Interacting minds—a biological basis. Science 1999; 286:1692–1695. doi:
11 : Imaging the intentional stance in a competitive game. Neuroimage 2002; 16:814–821. doi:
12 : Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci 2002; 5:277–283. doi:
13 : Functional delineation of the human occipito-temporal areas related to face and scene processing: a PET study. Brain 2000; 123:1903–1912. doi:
14 : Neural substrates for recognition of familiar voices: a PET study. Neuropsychologia 2001; 39:1047–1054. doi:
15 : Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage 2000; 11:203–209. doi:
16 : Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci 1996; 16:4275–4282Crossref, Medline, Google Scholar
17 : Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci 1999; 11:1891–1898. doi:
18 : Recognition of mental state terms: clinical findings in children with autism and a functional neuroimaging study of normal adults. Br J Psychiatry 1994; 165:640–649Crossref, Medline, Google Scholar
19 Shdo SM, Adhimoolam B, Miller B, et al: Specific right-temporal contributions to distinct behavioral subcomponents of empathy in neurodegenerative disease. Presented at the proceedings of the 43rd Annual Meeting of the International Neuropsychological Society, Denver, February 2015Google Scholar
20 : Individual differences in socioemotional sensitivity are an index of salience network function. Cortex 2018; 103:211–223. doi:
21 : Evidence for scripts in semantic dementia: Implications for theories of semantic memory. Cogn Neuropsychol 2001; 18:323–341Crossref, Medline, Google Scholar
22 : Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol 2005; 18:28–36. doi:
23 : Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage 2009; 47:2005–2015. doi:
24 : Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol 2006; 60:660–667. doi:
25 : Evidence for scripts in semantic dementia: implications for theories of semantic memory. Cogn Neuropsychol 2001; 18:323–341. doi:
26 : Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol 2001; 49:433–442. doi:
27 : Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome). Neurology 1996; 46:922–930Crossref, Medline, Google Scholar
28 : Neuropsychiatric aspects of progressive supranuclear palsy. Neurology 1996; 47:1184–1189Crossref, Medline, Google Scholar
29 : Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann Neurol 2013; 73:603–616Crossref, Medline, Google Scholar
30 : Clinical profile of PiB-positive corticobasal syndrome. PLoS One 2013; 8:e61025. doi:
31 : The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology 2000; 55:1368–1375. doi:
32 : Neuropsychiatry of corticobasal degeneration and progressive supranuclear palsy. Int Rev Psychiatry 2013; 25:197–209. doi:
33 : Dysfunctional facial emotional expression and comprehension in a patient with corticobasal degeneration. Neurocase 2007; 13:165–168. doi:
34 : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269. doi:
35 : Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34:939–944. doi:
36 : Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134:2456–2477. doi:
37 : Classification of primary progressive aphasia and its variants. Neurology 2011; 76:1006–1014. doi:
38 : Criteria for the diagnosis of corticobasal degeneration. Neurology 2013; 80:496–503. doi:
39 : Clinical diagnosis of progressive supranuclear palsy: the Movement Disorder Society criteria. Mov Disord 2017; 32:853–864. doi:
40 : Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 2010; 53:1244–1255. doi:
41 : Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology 2010; 75:857–863. doi:
42 : Interpreting scan data acquired from multiple scanners: a study with Alzheimer’s disease. Neuroimage 2008; 39:1180–1185. doi:
43 : Relationship between brain age-related reduction in gray matter and educational attainment. PLoS One 2015; 10:e0140945. doi:
44 : Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage 2004; 21:450–455. doi:
45 : An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003; 19:1233–1239. doi:
46 : Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289. doi:
47 : Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry 2017; 88:908–916. doi:
48 : Clinicopathological study of patients with C9ORF72-associated frontotemporal dementia presenting with Delusions. J Geriatr Psychiatry Neurol 2015; 28:99–107. doi:
49 : Behavioral variant frontotemporal dementia with corticobasal degeneration pathology: phenotypic comparison to bvFTD with Pick’s disease. J Mol Neurosci 2011; 45:594–608. doi:
50 : Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 2008; 29:280–289. doi:
51 : The social brain: neural basis of social knowledge. Annu Rev Psychol 2009; 60:693–716 716.doi.org/10.1146/annurev.psych.60.110707.163514Crossref, Medline, Google Scholar
52 : How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 2009; 10:59–70. doi:
53 : Does the orbitofrontal cortex signal value? Ann N Y Acad Sci 2011; 1239:87–99. doi:
54 : The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72:341–372. doi:
55 : Are shame, guilt, and embarrassment distinct emotions? J Pers Soc Psychol 1996; 70:1256–1269. doi:
56 : Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 2000; 10:295–307. doi:
57 : Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 1994; 57:1518–1524. doi:
58 : Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci 2013; 7:467. doi:
59 : Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 2002; 125:2286–2295. doi:
60 : Social cognition. Continuum (Minneap Minn) 2010; 16(4 Behavioral Neurology):69–85. doi:
61 : How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Medline, Google Scholar
62 : Neural systems supporting interoceptive awareness. Nat Neurosci 2004; 7:189–195Crossref, Medline, Google Scholar
63 : Deconstructing empathy: Neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia 2018; 116(Pt A):126–135. doi:
64 : The insula and evaluative processes. Psychol Sci 2011; 22:80–86. doi:
65 : Exploring the neurological substrate of emotional and social intelligence. Brain 2003; 126:1790–1800. doi:
66 : Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 2008; 131:1311–1322. doi:
67 : Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci 2013; 8:123–133. doi:
68 : The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 2007; 130:1718–1731. doi:
69 : The neural basis of human social values: evidence from functional MRI. Cereb Cortex 2009; 19:276–283. doi:
70 : Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia 2007; 45:1591–1607. doi:
71 : Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage 2006; 31:440–457. doi:
72 : Clan mentality: evidence that the medial prefrontal cortex responds to close others. J Neurosci 2010; 30:13906–13915. doi:
73 : Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci Biobehav Rev 2012; 36:1043–1059. doi:
74 :.Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 2012; 16:147–156. doi:
75 : The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 2006; 44:2189–2208Crossref, Medline, Google Scholar
76 : A default mode of brain function. Proc Natl Acad Sci USA 2001; 98:676–682Crossref, Medline, Google Scholar
77 : The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry 2018; 83:638–647. doi:
78 : Three ways in which midline regions contribute to self-evaluation. Front Hum Neurosci 2013; 7:450Crossref, Medline, Google Scholar
79 : Neural substrates of socioemotional self-awareness in neurodegenerative disease. Brain Behav 2014; 4:201–214. doi:
80 : Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 2006; 18:1586–1594Crossref, Medline, Google Scholar
81 : The role of the neural reward circuitry in self-referential optimistic belief updates. Neuroimage 2016; 133:151–162Crossref, Medline, Google Scholar
82 : The negative consequences of threat: an fMRI investigation of the neural mechanisms underlying women’s underperformance in math. Psychol Sci 2008; 19:168–175Crossref, Medline, Google Scholar
83 : Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychol Sci 2011; 22:60–70. doi:
84 : Investigating the functional anatomy of empathy and forgiveness. Neuroreport 2001; 12:2433–2438. doi:
85 : Functional imaging of “theory of mind”. Trends Cogn Sci 2003; 7:77–83. doi:
86 : Impairment of social perception associated with lesions of the prefrontal cortex. Am J Psychiatry 2004; 161:1247–1255. doi:
87 : Cortical regions activated by the subjective sense of perceptual coherence of environmental sounds: a proposal for a neuroscience of intuition. Cogn Affect Behav Neurosci 2008; 8:318–328. doi:
88 : Damage to the insula is associated with abnormal interpersonal trust. Neuropsychologia 2015; 71:165–172. doi:
89 : White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex 2012; 48:216–229. doi:
90 : Reduced prefrontal connectivity in psychopathy. J Neurosci 2011; 31:17348–17357Crossref, Medline, Google Scholar
91 : The Human Frontal Lobes: Functions and Disorders. New York, The Guilford Press, 2007Google Scholar
92 : Right fronto-limbic atrophy is associated with reduced empathy in refractory unilateral mesial temporal lobe epilepsy. Neuropsychologia 2015; 78:80–87. doi:
93 : Dissecting the neural mechanisms mediating empathy. Emot Rev 2011; 3:92–108Crossref, Google Scholar
94 : Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev 2011; 35:903–911Crossref, Medline, Google Scholar
95 : Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 2011; 54:2492–2502Crossref, Medline, Google Scholar
96 : Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase 2001; 7:145–160Crossref, Medline, Google Scholar
97 : Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci 2003; 15:324–337Crossref, Medline, Google Scholar
98 : Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cogn Behav Neurol 2005; 18:55–67Crossref, Medline, Google Scholar
99 : Structural anatomy of empathy in neurodegenerative disease. Brain 2006; 129:2945–2956Crossref, Medline, Google Scholar
100 : Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009; 132:617–627Crossref, Medline, Google Scholar



