Brain Structural and Amyloid Correlates of Recovery From Semantic Interference in Cognitively Normal Individuals With or Without Family History of Late-Onset Alzheimer’s Disease
Abstract
Failure to recover from proactive semantic interference (frPSI) has been shown to be more sensitive than traditional cognitive measures in different populations with preclinical Alzheimer’s disease. The authors sought to characterize the structural and amyloid in vivo correlates of frPSI in cognitively normal offspring of patients with late-onset Alzheimer’s disease (O-LOAD), compared with individuals without a family history of neurodegenerative disorders (CS). The authors evaluated the LASSI-L, a test tapping frPSI and other types of semantic interference and delayed recall on the RAVLT, along with 3-T MRI volumetry and positron emission tomography Pittsburgh compound B, in 27 O-LOAD and 18 CS with equivalent age, sex, years of education, ethnicity, premorbid intelligence, and mood symptoms. Recovery from proactive semantic interference (frPSI) and RAVLT delayed recall were lower in O-LOAD cases. Structural correlates of both cognitive dimensions were different in CS and O-LOAD, involving brain regions concerned with autonomic, motor, and motivational control in the former, and regions traditionally implicated in Alzheimer’s disease in the latter. Better recovery from retroactive semantic interference was associated with less amyloid load in the left temporal lobe in O-LOAD but not CS. In middle-aged cognitively normal individuals with one parent affected with LOAD, frPSI was impaired compared with persons without a family history of LOAD. The neuroimaging correlates of such cognitive measure in those with one parent with LOAD involve Alzheimer’s-relevant brain regions even at a relatively young age.
Available studies suggest that the characteristic neuropathological features of Alzheimer’s disease are present years to decades before cognitive symptom onset in both early-onset Alzheimer’s disease (EOAD) and late-onset Alzheimer’s disease (LOAD) cases. In vivo detection of such features is critical to predict which at-risk individuals will develop the disease and to eventually enact secondary prevention strategies that target pathophysiological processes leading to symptomatic disease. Although it represents less than 1% of all cases of Alzheimer’s disease, EOAD has received most of the attention in attempts to define the natural history of the disease1; since known autosomic dominant alterations that cause EOAD all affect beta amyloid deposition, the prevalent view on how Alzheimer’s disease develops is, understandably, the amyloid cascade hypothesis.2 However, LOAD, which accounts for over 99% of cases and thus represents a much greater public health problem, is an entity for which polygenic inheritance (including genes not involved in amyloid metabolism) and unknown environmental influences both contribute to clinical symptom onset.1,3,4 LOAD research has mostly referred to clinical forms of the disorder (especially mild cognitive impairment [MCI] and Alzheimer’s-type dementia); relatively few studies have addressed early LOAD phenotypes in individuals at risk. Apart from aging, having a first-degree relative with LOAD is the main risk factor for the disorder, offering an opportunity to detect and characterize its early cognitive and neuroimage phenotypes.4,5,6
Semantic interference tests, such as the Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L), are true cognitive “stress test” paradigms that allow detection of subtle cognitive impairment and are sensitive to very early clinical forms of the disorder.7,8,9 Moreover, our group has recently described that certain LASSI-L variables are very sensitive to cognitive difficulties in asymptomatic, fully functioning middle-aged adults who have one parent with LOAD.10 The purpose of the present study was to discern the structural MRI (subcortical volumes and cortical thickness) and positron emission tomography Pittsburgh compound B (PET-PiB) (in vivo fibrillar amyloid deposition) correlates of performance in a variety of LASSI-L items, particularly those that have revealed the highest discriminatory power, such as failure to recover from proactive semantic interference (frPSI).9,10,11 These items were compared with a traditional and widely used verbal memory task (Rey Auditory Verbal Learning Test; RAVLT) in asymptomatic offspring of patients with late-onset Alzheimer’s disease (O-LOAD) and in individuals without a family history of Alzheimer’s disease or other neurodegenerative disorders (CS). Based upon studies in early clinical Alzheimer’s disease, we hypothesized that frPSI10 would be inversely related to gray matter and directly correlated to β-amyloid deposition in Alzheimer’s-relevant areas. Specifically, we predicted that greater B2 cued recall (an indicator of frPSI) would correlate with less PET-PiB signal in the posterior cingulate, precuneus, temporal, or frontal areas, with an opposite pattern for B2 cued intrusions (another measure of frPSI), a sensitive test of cognitive impairment that measures errors committed during recall.
Methods
Design and Sample
A cross-sectional study was performed to compare cognitive measures of functional [11C]-PIB-PET and structural T1-weighted MRI data between a sample of 27 O-LOAD participants and 18 CS participants. Both groups were comparable in age, gender, education level, and depressive symptoms. All participants provided their written informed consent for the study as approved by the local bioethics committee and in accordance with the Declaration of Helsinki.
The inclusion criteria for O-LOAD were as follows1: at least one parent diagnosed with probable LOAD according to the DSM-5 criteria;2 40–60 years old at the time of recruitment;3 7 or more years of formal education;4 Mini-Mental State Examination (MMSE) score >26;5 no evidence of current progressive neurologic disease or medical conditions likely to impair cognitive function;6 no history of substance abuse (alcohol, marijuana, stimulants, benzodiazepines, cocaine, or other illicit drugs); and6 Hachinski score <4 to screen out subjects with vascular-derived cognitive impairment.
All participants were asked to supply names, dates of birth, causes of death, and clinical information for all affected family members. The information was confirmed with other family members and by interview with the examining physician, discussing the parents’ symptomatology and progression of disease. Only individuals whose parents had lived to age ≥65 were included. For individuals whose parents had received no treatment at Fleni Foundation (N=5), the diagnosis of LOAD was clinician certified. In addition to the clinical definition of LOAD for the subjects’ parents, structural MRIs were available to confirm atrophy changes suggestive of Alzheimer’s disease and absence of significant vascular disease for the parents of 15 participants. Of these, three parents had a positive PET-PiB test.
The volunteers in the CS group had the same inclusion and exclusion criteria described above but were required to have no family history of neurodegenerative disease. None of the participants were amyloid+ as per clinical standards in the visual inspection of the scans by an experienced neuroradiologist (SV).
Traditional Cognitive Assessment
The traditional neuropsychological battery used in this study included the MMSE as a screening test of cognitive function,12 and the RAVLT to assess verbal episodic memory.13–15 The Beck Depression Inventory-II 16 was administered to screen for presence and severity of depressive symptoms that could impact cognitive performance. To estimate premorbid intelligence the Word Accentuation Test–Buenos Aires version was administered.17
LASSI-L Cognitive Stress Test
In addition to the standard assessment, a novel cognitive stress test was implemented: the LASSI-L, which has proved to be very effective in discriminating Alzheimer’s disease from MCI and healthy subjects. Studies with the LASSI-L show strong relationship to amyloid load in older adults who presented normal scores on traditional neuropsychological measures.8
This test assesses the effects of proactive and retroactive interference (PSI and RSI, respectively) and frPSI after controlled cued learning and recall of two lists comprised of different stimuli but sharing the same semantic categories. The complete administration procedures are described below.
Subjects are presented with a 15-word list (List A) comprised of common fruits, musical instruments, and clothing items (five words per semantic category) written on individual cards in a random order. They are instructed to read each word out loud and remember them. Subjects are then asked to recall the words immediately after exposure. Following this free recall trial, subjects are required to recall the words pertaining to each semantic category. Then, examinees are presented for a second time with List A and are asked to immediately recall the words for each semantic category. This trial allows further acquisition and storage of the material. Next, participants are presented with a competing List B, which follows the same format as List A and contains the same semantic categories but different stimuli. The same procedure is then followed with List B as with the first list. A free recall of the items immediately after they are presented is followed by a recall organized by the semantic categories (B1 cued recall). Then, a second presentation of the list is introduced, and participants are asked to recall the items by semantic categories (B2 cued recall). After acquisition and recall of List B, subjects are asked to remember the List A stimuli through free and cued recall (A3 free recall and A3 cued recall, respectively). Finally, after a 20-minute period, subjects are asked to freely recall as many words as possible from either list with no need to reference each stimuli’s source (delayed recall).7
For this study we selected LASSI-L cued recall and intrusion error measures that have shown the highest degree of discriminability and relationship to volumetric reductions within the brain in previous studies.9,18 These included List B1 cued recall and intrusions (susceptible to proactive interference), List B2 cued recall and intrusions (susceptible to frPSI), List A3 free recall an intrusions, A3 cued recall and intrusions (susceptible to RSI), as well as delayed recall and intrusions.
The traditional neuropsychological assessment and the LASSI-L were administered in separate 30-minute sessions, so as to avoid interference effects among semantic items.15 All tests were administered and scored by a trained neuropsychologist blind to the participant’s group.
All participants were cognitively asymptomatic, neuropsychological performance was within normal limits, and none of the individuals met criteria for MCI or dementia.
MRI Imaging
MRI images were acquired on a 3 T GE Signa HDxt MRI machine with an eight-channel head coil. A high-resolution T1 3D fast SPGR-IR image was acquired. One hundred and sixty-six sagittal contiguous slices were obtained in an acquisition matrix of 256×256, TR=7.256 ms, TE=2.988 ms, flip angle 8°, FOV=26 cm, and slice thickness=1.2 mm.
Cortical reconstruction and volumetric segmentation were performed with the FreeSurfer image analysis program version Linux-centos6_x86_64-stable-v6-beta−20151015, which is documented and available for download online (http://surfer.nmr.mgh.harvard.edu/). Briefly, the FreeSurfer pipeline performs removal of nonbrain tissue using a hybrid watershed/surface deformation procedure,19 automated Talairach transformation, segmentation of the subcortical white matter (WM) and deep gray matter (GM) volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles),20,21 intensity normalization,22 tessellation of the GM/WM boundary, automated topology correction,23,24 and surface deformation following intensity gradients to optimally place the GM/WM and GM/cerebrospinal-fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class.25,26,27
The morphometric evaluation of each hemisphere was performed independently. Each volume and surface obtained was carefully reviewed by two investigators blind to the participant’s study condition (BDA, SMS) and edited manually by one of these investigators (BDA) as necessary to conform the anatomically determined limits. Major topological inaccuracies were corrected with vertex edits or control points. Finally, surface maps were smoothed by using a Gaussian kernel of 10 mm.
Once the cortical models were complete, we used the program to measure the whole-brain cortical thickness. The latter was calculated as the shortest distance between the GM/WM boundary and pial surface at each vertex across the cortex. The maps were created using spatial intensity gradients across tissue classes and were therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and are thus capable of detecting submillimeter differences between groups. Subcortical volumes were also measured automatically using the FreeSurfer software. In this procedure, each voxel in the normalized brain volume was assigned to one of 40 regions of interest (ROIs; hippocampus, amygdala, thalamus, caudate, putamen, pallidum, among others), using a probabilistic atlas, from which we obtained the volume measurements. Additionally, total intracranial volume was also calculated using FreeSurfer and used to normalize the ROIs’ volumetric data.
Finally, in order to map all of the subjects’ brains to a common space and perform a comparison between groups, we registered all of the cortical thickness maps to a spherical atlas that is based on individual cortical folding patterns to match cortical geometry across subjects28 and create a variety of surface-based data.
Amyloid PET Imaging
11C-PIB synthesis was carried out in a GE TRACER lab FXC PRO module, a compact, automated radiochemistry system that generates C-11 labeled radiochemicals from 11C-CO2. The module includes a high performance liquid chromatography (HPLC) system for purification. The HPLC is a separation method that allows the isolation of the labeled product from radioactive by-products and organic impurities. In-house HPLC was performed with 0.009M sodium citrate ethanol/water (60/40) as a mobile phase with a flow rate of 3 mL/min and a reverse phase high performance liquid chromatographic column. Chromatograms were registered using an ultraviolet detector and a radioactivity detector in series. The product peak containing the [11C]-PIB was cut from the chromatographic system by valve switching from waste to product line. The [11C] PIB fraction (retention time, 11–12 minutes) was transferred into flask and diluted with 30 mL of saline solution to reduce the ethanol percentage. Hence, the final total volume was 36 mL. After this step, the PIB solution was fractionated after sterile filtration. The 10 mCi (adjusted by weight) was administered and image acquisition proceeded 50 minutes post−11CPIB administration. Subsequently, dynamic tomographic images, 3D mode took 20 minutes. PET images were processed along with MRI volumetric T1 images. MRI T1 images were obtained and then analyzed in FreeSurfer as previously described in the T1 image processing section, above. Once the T1 images were processed, an analysis was performed using the PETSurfer scripts, which provides a series of elements for PET image analysis. The procedures used can be found online (https://surfer.nmr.mgh.harvard.edu/PetSurfer) and consist of the following steps: 1) creation of a high-resolution segmentation (gtmseg.mgz) used to run the partial volume correction (PVC) methods to correct limited tissue sampling,29 2) coregistration of the PET and structural T1, 3) application of PVC method from which the PVC uptake of each region relative to pons was obtained together with volumes of corrected voxel-wise values of cortical and subcortical GM, and 4) a surface-based analysis was carried out to sample those volumes onto each of the individual subject’s surfaces.
General Statistical Procedures
Differences between groups were calculated by using a t test for independent samples for continuous variables; chi square tests were used for categorical variables. An FDR correction was applied to multiple intergroup comparisons (https://www.sdmproject.com/utilities/?show=FDR). Correlations between imaging-derived variables and cognitive and clinical data were evaluated using Pearson correlation coefficients. All tests were two-tailed, and the significance level was set at p<0.05. All statistical analyses were performed using the SPSS version 22.0 software (SPSS, Chicago).
Cortical and Subcortical Statistical Analysis
Entire cortex analyses were performed to explore cortical thickness in O-LOAD and CS versus LASSI-L variables. Statistical maps were generated using the command-line group analysis stream in FreeSurfer, which implements the general linear model to estimate the differences in cortical morphometric data produced by the FreeSurfer processing stream for each hemisphere. Multiple comparisons were corrected with a Monte Carlo simulation using a two-tailed p value set at <0.05. The results were visualized by overlaying significant cortical areas onto semi-inflated cortical surfaces. Subcortical volumes were automatically derived from outcomes of FreeSurfer, and SPSS 20.0 was used to analyze the differences between groups. A significance level of p<0.05 (Bonferroni multiple comparisons correction) was used.
PET Statistical Analysis
The same procedure as described for cortical and subcortical statistical analyses was performed, but in this case the observed data for the general linear model were the PIB volumes obtained after the PVC (step 3).
Results
Table 1 depicts the demographic, clinical, and neuropsychological characteristics of the present sample. Both groups were comparable in age, gender, years of education, and premorbid intelligence, and attained values within the normal range in all tests (Table 1). Offspring of LOAD patients (O-LOAD) performed worse in delayed RAVLT, as well as B2 cued recall and B2 cued intrusions on the LASSI-L (sensitive to frPSI) compared with individuals without family history of Alzheimer’s disease. The present study shows additional significant differences on other various LASSI measures—B1 cued intrusions (sensitive to PSI), A3 free recall (sensitive to RSI), A3 intrusions (sensitive to RSI), and delayed recall intrusions. O-LOAD subjects also obtained lower scores in the MMSE. After controlling for false discovery rate,30 only RAVLT delayed recall and LASSI-L B2 cued intrusions remained significantly different between groups (Table 1).
| Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CS | O-LOAD | |||||||||
| Characteristic | Mean | Frequency | SD | % | Mean | Frequency | SD | % | Statistic | p |
| Female | 15 | 83.3 | 19 | 70.4 | 0.983 | 0.482 | ||||
| Maternal inheritance | N/A | N/A | 15 | 55.6 | ||||||
| Age (years) | 51.22 | 8.94 | 55.26 | 7.04 | –1.612 | 0.117 | ||||
| Education level (years) | 17.65 | 3.35 | 16.96 | 2.84 | 0.696 | 0.492 | ||||
| WAT-BA | 43.33 | 5.09 | 41.81 | 5.64 | 0.939 | 0.353 | ||||
| BDI-II | 9.41 | 7.96 | 8.81 | 6.62 | 0.258 | 0.798 | ||||
| MMSE | 29.50 | 0.79 | 28.74 | 1.23 | 2.529 | 0.015 | ||||
| RAVLT Delayed R | 10.83 | 2.26 | 8.41 | 3.07 | 3.054 | 0.004 | ||||
| LASSI-L | ||||||||||
| B1 cued recall | 8.50 | 3.16 | 7.94 | 2.11 | 0.594 | 0.558 | ||||
| B1 cued intrusions | 0.75 | 1.00 | 2.41 | 2.67 | –2.393 | 0.026 | ||||
| B2 cued recall | 12.38 | 1.86 | 11.59 | 1.50 | 1.333 | 0.193 | ||||
| B2 cued intrusions | 0.56 | 0.51 | 1.88 | 1.65 | –3.134 | 0.005 | ||||
| A3 free recall | 8.44 | 2.58 | 6.18 | 3.59 | 2.085 | 0.046 | ||||
| A3 intrusions | 0.38 | 0.62 | 1.47 | 1.94 | –2.212 | 0.039 | ||||
| A3 cued recall | 9.81 | 2.37 | 8.29 | 3.10 | 1.586 | 0.123 | ||||
| A3 cued intrusions | 1.13 | 1.15 | 2.06 | 2.14 | –1.577 | 0.127 | ||||
| Delayed recall | 22.38 | 3.34 | 20.24 | 3.93 | 1.688 | 0.102 | ||||
| Delayed intrusions | 0.19 | 0.40 | 0.65 | 0.70 | –2.323 | 0.028 | ||||
TABLE 1. Clinical and Demographic Characteristics of the Study Participantsa
Table 2 exhibits Pearson correlations between RAVLT delayed recall and the LASSI-L variables for each group (CS and O-LOAD), which show that the LASSI-L and the RAVLT are distinct tests that assess different cognitive processes. Tables 3 and 4 show Pearson correlations between LASSI-L measurements for CS and O-LOAD, respectively.
| CS | O-LOAD | |||
|---|---|---|---|---|
| Measure | r | p | r | p |
| B1 cued recall | 0.408 | 0.116 | 0.135 | 0.606 |
| B1 cued intrusions | –0.521 | 0.039 | –0.370 | 0.144 |
| B2 cued recall | 0.361 | 0.170 | –0.202 | 0.436 |
| B2 cued intrusions | –0.288 | 0.279 | –0.129 | 0.621 |
| A3 free recall | 0.509 | 0.044 | –0.004 | 0.987 |
| A3 Intrusions | 0.140 | 0.605 | 0.108 | 0.681 |
| A3 cued recall | 0.497 | 0.050 | 0.186 | 0.476 |
| A3 cued intrusions | –0.088 | 0.747 | –0.108 | 0.681 |
| Delayed recall | 0.383 | 0.143 | 0.061 | 0.817 |
| Delayed intrusions | –0.237 | 0.377 | 0.007 | 0.979 |
TABLE 2. Correlation Coefficients Between RAVLT Delayed Recall and LASSI-L Measuresa
| Measure | B1 Cued Recall | B1 Cued Intrusions | B2 Cued Recall | B2 Cued Intrusions | A3 Free Recall | A3 Intrusions | A3 Cued Recall | A3 Cued Intrusions | Delayed Recall |
|---|---|---|---|---|---|---|---|---|---|
| B1 cued intrusions | –0.105 | ||||||||
| 0.698 | |||||||||
| B2 cued recall | 0.692 | –0.197 | |||||||
| 0.003 | 0.464 | ||||||||
| B2 cued intrusions | –0.144 | 0.553 | –0.166 | ||||||
| 0.595 | 0.026 | 0.538 | |||||||
| A3 free recall | 0.265 | –0.575 | 0.464 | –0.400 | |||||
| 0.320 | 0.020 | 0.070 | 0.125 | ||||||
| A3 intrusions | –0.375 | 0.162 | –0.130 | –0.079 | 0.141 | ||||
| 0.153 | 0.550 | 0.630 | 0.772 | 0.603 | |||||
| A3 cued recall | 0.449 | –0.358 | 0.365 | –0.566 | 0.809 | 0.142 | |||
| 0.081 | 0.173 | 0.165 | 0.022 | >0.001 | 0.600 | ||||
| A3 cued intrusions | –0.165 | –0.087 | –0.180 | –0.354 | –0.357 | 0.211 | –0.187 | ||
| 0.541 | 0.748 | 0.505 | 0.178 | 0.174 | 0.432 | 0.489 | |||
| Delayed recall | 0.542 | –0.289 | 0.759 | –0.248 | 0.690 | 0.024 | 0.623 | –0.326 | |
| 0.030 | 0.278 | 0.001 | 0.354 | 0.003 | 0.929 | 0.010 | 0.218 | ||
| Delayed intrusions | –0.183 | 0.289 | –0.367 | 0.101 | –0.469 | –0.033 | –0.379 | –0.198 | –0.352 |
| 0.497 | 0.277 | 0.162 | 0.710 | 0.067 | 0.920 | 0.148 | 0.462 | 0.181 |
TABLE 3. Correlation Coefficients Between LASSI-L Measurements for Healthy Individuals Without a Family History of Alzheimer’s Disease or Other Neurodegenerative Disordersa
| Measure | B1 Cued Recall | B1 Cued Intrusions | B2 Cued Recall | B2 Cued Intrusions | A3 Free Recall | A3 Intrusions | A3 Cued Recall | A3 Cued Intrusions | Delayed Recall |
|---|---|---|---|---|---|---|---|---|---|
| B1 cued intrusions | –0.051 | ||||||||
| 0.846 | |||||||||
| B2 cued recall | 0.644 | –0.002 | |||||||
| 0.005 | 0.994 | ||||||||
| B2 cued intrusions | –0.325 | 0.818 | –0.297 | ||||||
| 0.203 | >0.001 | 0.246 | |||||||
| A3 free recall | 0.464 | –0.106 | 0.616 | –0.207 | |||||
| 0.061 | 0.686 | 0.008 | 0.426 | ||||||
| A3 intrusions | 0.130 | 0.708 | 0.156 | 0.603 | –0.031 | ||||
| 0.620 | 0.001 | 0.549 | 0.010 | 0.907 | |||||
| A3 cued recall | 0.635 | –0.220 | 0.807 | –0.481 | 0.680 | 0.038 | |||
| 0.006 | 0.397 | >0.001 | 0.051 | 0.003 | 0.885 | ||||
| A3 cued intrusions | 0.098 | 0.796 | 0.242 | 0.692 | 0.170 | 0.808 | 0.044 | ||
| 0.708 | >0.001 | 0.350 | 0.002 | 0.515 | >0.001 | 0.865 | |||
| Delayed recall | 0.538 | –0.105 | 0.769 | –0.149 | 0.546 | 0.116 | 0.733 | 0.199 | |
| 0.026 | 0.688 | >0.001 | 0.567 | 0.023 | 0.658 | 0.001 | 0.443 | ||
| Delayed intrusions | 0.027 | 0.149 | –0.324 | 0.285 | –0.197 | 0.175 | –0.524 | 0.015 | –0.399 |
| 0.917 | 0.568 | 0.204 | 0.267 | 0.449 | 0.501 | 0.031 | 0.995 | 0.113 |
TABLE 4. Correlation Coefficients Between LASSI-L Measurements for Asymptomatic Offspring of Individuals With Late-Onset Alzheimer’s Diseasea
Figure 1 depicts correlations between B2 cued recall performance and cortical thickness throughout the brain. Figure 1B reveals that for O-LOAD, B2 cued recall (sensitive to frPSI) on LASSI-L was related to greater cortical thickness in the left medial occipital cortex and right hemisphere superior frontal gyrus, precentral gyrus, and postcentral gyrus. In contrast, B2 cued recall in CS was associated with greater cortical thickness in right orbitofrontal cortex and left precentral and middle frontal gyri (Figure 1A). In O-LOAD participants, B2 cued intrusions (indicative of frPSI) were inversely correlated (a larger number of B2 cued intrusions indicates poorer performance) with cortical thickness at the level of the left medial posterior parietal cortex, left temporo-occipital cortex, and right superior frontal gyrus (Figure 2B). Number of B2 cued intrusions was not correlated with cortical thickness in the CS. Greater RAVLT-D in CS correlated with decreased thickness in the right hemisphere occipital cortex adjacent to the calcarine fissure extending onto the posterior parietal cortex (Figure 3A). No correlations were found among O-LOAD participants (Figure 3B). A3 cued recall performance structural correlates in CS (Figure 4A) showed a remarkable resemblance to those of RAVLT-D (Figure 3A): namely, decreased cortical thickness in the right posterior parietal/occipital cortex adjacent to the calcarine fissure and right anterior frontal cortex, in addition to a small area of the right inferior temporal gyrus (Figure 4A). O-LOAD showed an opposite structural-functional relationship regarding LASSI-L A3 cued recall, such that greater performance in this item was related to greater cortical thickness in the right middle and posterior cingulate cortex, right precentral gyrus, left frontal pole, and bilateral occipital cortex (Figure 4B).
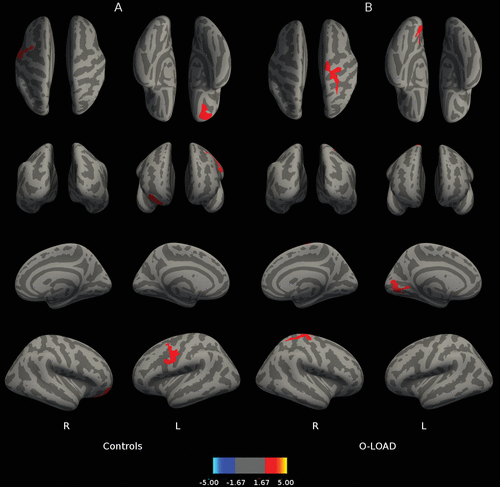
FIGURE 1. Correlations Between LASSI-L B2 Cued Recall and Cortical Thicknessa
a Panel A shows the correlation between Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) B2 cued recall and cortical thickness in healthy individuals without a family history of neurodegenerative disorders. Panel B shows the correlation between LASSI-L B2 cued recall and cortical thickness in asymptomatic offspring of individuals with late-onset Alzheimer’s disease.
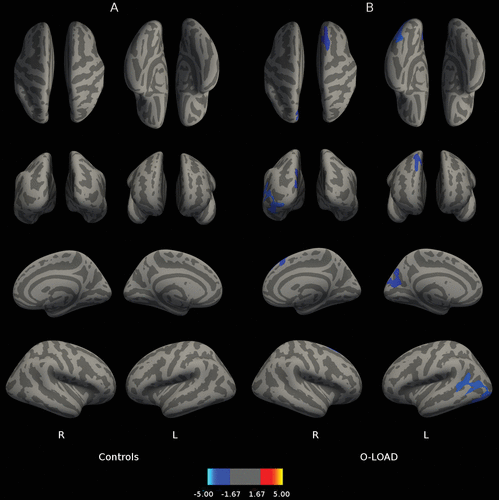
FIGURE 2. Correlation Between LASSI-L B2 Cued Intrusions and Cortical Thicknessa
a Panel A shows the correlation between Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) B2 cued intrusions and cortical thickness in healthy individuals without a family history of neurodegenerative disorders. Panel B shows the correlation between LASSI-L B2 cued intrusions and cortical thickness in asymptomatic offspring of individuals with late-onset Alzheimer’s disease. The displayed anatomical parameters are the same as the parameters in Figure 1.
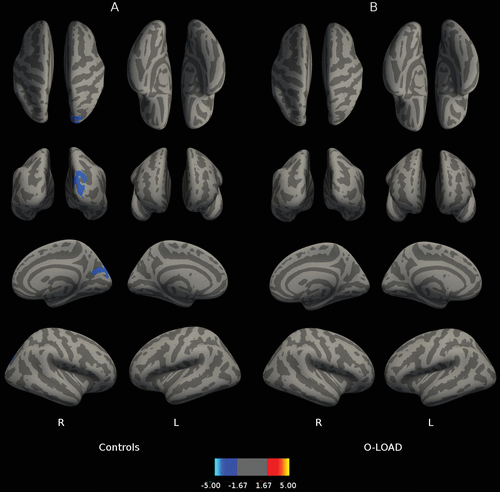
FIGURE 3. Relationship Between Cortical Thickness and Delayed Recall on the Rey Verbal Auditory Learning Test (RAVLT)a
a Panel A shows the relationship between cortical thickness and delayed recall on the RAVLT in healthy individuals without a family history of neurodegenerative disorders. Panel B shows the relationship between cortical thickness and delayed recall on the RAVLT in asymptomatic offspring of individuals with late-onset Alzheimer’s disease. The displayed anatomical parameters are the same as the parameters in Figure 1.
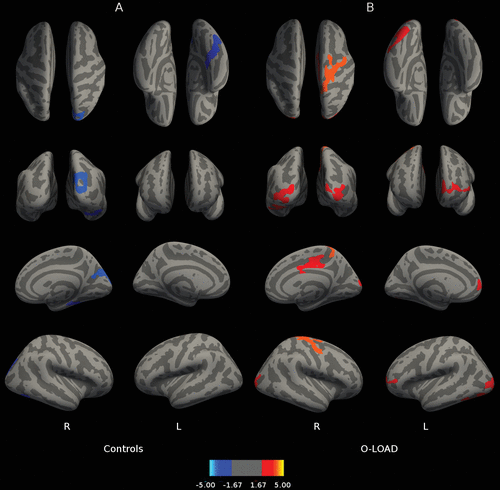
FIGURE 4. Relationship Between LASSI-L A3 Cued Recall and Cortical Thicknessa
a Panel A shows the relationship between Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) A3 cued recall and cortical thickness in healthy individuals without a family history of neurodegenerative disorders. Panel B shows the relationship between LASSI-L A3 cued recall and cortical thickness in asymptomatic offspring of individuals with late-onset Alzheimer’s disease. The displayed anatomical parameters are the same as the parameters in Figure 1.
Figure 5 shows better performance in A3 cued recall was related to decreased amyloid load in the left temporal lobe in O-LOAD. No relationships were observed between PET-PiB and measures of rSI in CS (Figure 5).
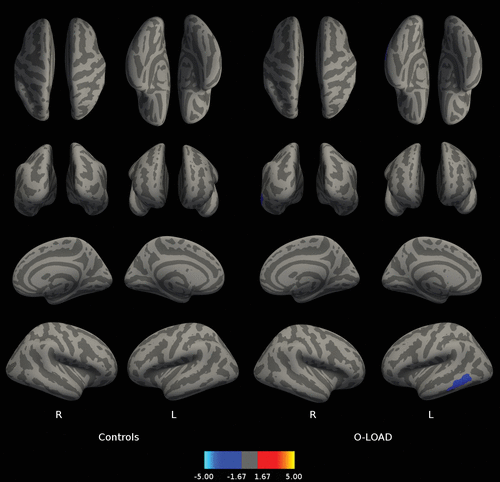
FIGURE 5. Relationship Between the Amount of In Vivo Fibrillar Amyloid Deposition as Assessed by PET-PiB Signal and LASSI-L A3 Cued Recalla
a Panel A shows the relationship between the amount of in vivo fibrillar amyloid deposition as assessed by positron emission tomography Pittsburgh compound B (PET-PiB) and the Signal and Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) A3 cued recall in healthy individuals without a family history of neurodegenerative disorders. Panel B shows the relationship between the amount of in vivo fibrillar amyloid deposition as assessed by PET-PiB and LASSI-L A3 cued recall in asymptomatic offspring of individuals with late-onset Alzheimer’s disease. The displayed anatomical parameters are the same as the parameters in Figure 1.
Discussion
In the present study we have observed that 1) individuals with O-LOAD exhibit a decreased performance compared with CS on B2 cued recall and cued intrusions on the LASSI-L and delayed RAVLT recall, as we reported elsewhere10; 2) LASSI-L B1 cued intrusions, A3 free recall, A3 intrusions, and delayed recall intrusions were also more impaired in O-LOAD relative to CS subjects, even though differences became nonsignificant when using stringent correction for multiple measurements; 3) LASSI-L measures of frPSI, particularly related to B2 intrusions among O-LOAD cases, were associated with increased cortical thickness in Alzheimer’s-prone regions with minimum involvement in CS subjects; 4) retroactive semantic interference, as evidenced by A3 cued recall performance, was related to relative preservation of Alzheimer’s-relevant cortical areas and lower amyloid load in the left temporal cortex in O-LOAD; and 5) A3 cued recall exhibited an inverse relationship with cortical thickness in CS. Remarkably, the structural correlates of retroactive semantic interference in this group were almost identical to RAVLT correlates.
As previously described in clinical9 and preclinical samples of persons at increased risk for LOAD,10 frPSI measures on the LASSI-L discriminate between CS and asymptomatic O-LOAD subjects. In the present study, additional variables indicative of PSI and RSI were found to discriminate between the two groups. These results provide further support for the LASSI-L semantic interference testing paradigm as a sensitive tool for early detection of LOAD-related cognitive decline.10 Interestingly, multiple LASSI-L measures of intrusion-type errors on items related to proactive and retroactive interference as delayed recall show significant differences between O-LOAD and CS, with the former group exhibiting higher scores. Unlike reported similar findings in MCI and Alzheimer’s disease patients,9 the amount of intrusions found in our sample did not approach or exceed the correct responses on the corresponding recall trials. This is most likely explained by the fact that the subjects selected for this study were clinically asymptomatic for cognitive impairment. Nonetheless, these results suggest that LASSI-L items related to the inability to properly access source memory may be more cognitively demanding for middle-aged O-LOAD than CS participants. These findings underscore the relevance of proactive and retroactive interference and intrusions measurement as markers of subtle cognitive decline that might only be detected on cognitive stress tests such as the LASSI-L, which, by magnifying semantic interference effects, enables early detection of subtle decline in cognitive performance.
Both interference effects and intrusion-type errors likely reflect an interplay between memory and executive processes, therefore providing greater emphasis to executive functions in the study of early cognitive decline in asymptomatic at-risk subjects besides the traditional memory measurements. Simons and Spiers,31 as well as Duarte and his team,32 proposed that the interaction between medial temporal and medial orbital frontal regions are involved in discrete and elaborate representations of to-be-remembered targets involved in learning. These areas work in concert to reactivate, monitor, and differentiate semantic associations and representations. In particular, deficits within this system may interfere with source memory that leads to semantic intrusion errors.32,33 Not surprisingly, both O-LOAD and CS display structural correlates involving the prefrontal cortex, including superior frontal gyri, related to working memory operations.34 This matter is being further explored in ongoing research by our team.
Recovery from proactive semantic interference, as measured by B2 cued recall, was associated with increased cortical thickness in areas related to autonomic (medial orbitofrontal cortex) and cognitive control and motivation (left dorsolateral prefrontal cortex) in normal individuals without family history of LOAD. B2 cued recall in O-LOAD instead involved increased cortical thickness in what are traditionally Alzheimer’s-prone cortical regions including posterior parietal and temporo-parietal cortex.9,11 Moreover, the inverse relationship between the left precuneus and temporo-parietal cortex (also Alzheimer’s disease prone regions) with B2 cued intrusions present only in O-LOAD cases, lends further support to the possibility that, in the presence of sensitive cognitive stress for Alzheimer’s disease, there may be some compensatory mechanisms occurring in Alzheimer’s-prone regions before significant atrophy arises. On the other hand, in O-LOAD, better A3 cued recall performance is related to preserved cortical thickness in a different set of regions than those related to RSI involving bilateral occipital areas concerned with high-order visual processing, prefrontal cortical areas involved in motor and cognitive control, and right cingulate cortex regions presumably participating in motivational and sensory-motor control circuits.9
Finally, recovery from retroactive interference (A3 cued recall) is inversely related to PET-PiB in the left temporal lobe in O-LOAD only, which probably underscores the importance of this LASSI-L item in this group of individuals in addition to the well-established value of frPSI in diverse groups of individuals with preclinical LOAD.7,8,9,11 Moreover, it might be worth exploring its value in very early LOAD neuropathology as the only cognitive measure whose performance seems sensitive to amyloid deposition in left temporal cortex, as most available data report a lack of correlation between in vivo amyloid load and cognitive function deficits in at-risk or early clinical Alzheimer’s disease samples.
The present study has some limitations. As it is not a prospective study, we cannot know if LASSI-L abnormalities and their imaging correlates are predictive of the eventual development of clinical LOAD. Our sample was homogeneous in regards to ethnicity, geographical area, culture, and years of education, thus probably limiting generalizability of the results. Also, the relatively small sample size might have obscured significant clinical-neurobiological correlates in persons with or without family history of LOAD. Furthermore, given the size of the sample, the absence of correlations for certain tests may reflect low power of the study. Nonetheless, the current results have significant implications for understanding the earliest pathogenesis of LOAD and provide observations worthy of further research.
In sum, in the present paper we describe a series of correlations between GM integrity and in vivo amyloid deposition and the ability to recover from semantic interference in cognitively normal individuals with or without family history of LOAD. The relationships between Alzheimer’s-related brain regions and semantic interference in persons with family history of LOAD underscore the potential value of recovery from semantic interference, and LASSI-L measures in particular, to detect the impact of very early Alzheimer’s disease-related neuropathology in neuropsychological functioning, including both cortical thickness and amyloid deposition. Moreover, our group is currently studying direct structural and in vivo amyloid deposition differences between the two groups. Early detection of LOAD phenotype changes is critical for preclinical detection of cases and eventually for the application of etiological secondary prevention, warranting the use of LASSI-L for this purpose.
1 : Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016; 12:292–323Crossref, Medline, Google Scholar
2 : Alzheimer’s disease: the amyloid cascade hypothesis. Science 1992; 256:184–185Crossref, Medline, Google Scholar
3 : Structural correlates of early and late onset Alzheimer’s disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry 2005; 76:112–114Crossref, Medline, Google Scholar
4 : Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:280–292Crossref, Medline, Google Scholar
5 : Family history of Alzheimer’s disease and hippocampal structure in healthy people. Am J Psychiatry 2010; 167:1399–1406Crossref, Medline, Google Scholar
6 : Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 2013; 12:207–216Crossref, Medline, Google Scholar
7 : A novel cognitive assessment paradigm to detect pre-mild cognitive impairment (PreMCI) and the relationship to biological markers of Alzheimer’s disease. J Psychiatr Res 2018; 96:33–38Crossref, Medline, Google Scholar
8 : A novel cognitive stress test for the detection of preclinical alzheimer disease: discriminative properties and relation to amyloid load. Am J Geriatr Psychiatry 2016; 24:804–813Crossref, Medline, Google Scholar
9 : Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer’s disease. Assessment 2018; 25:348–359Crossref, Medline, Google Scholar
10 : Failure to recover from proactive semantic interference and abnormal limbic connectivity in asymptomatic, middle-aged offspring of patients with late-onset Alzheimer’s disease. J Alzheimers Dis 2017; 60:1183–1193Crossref, Medline, Google Scholar
11 : Recovery from proactive semantic interference and MRI volume: a replication and extension study. J Alzheimers Dis 2017; 59:131–139Crossref, Medline, Google Scholar
12 : “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
13 : L’examen clinique en psychologie [The Clinical Psychological Examination]. Paris, Presses Universitaires de France, 1964Google Scholar
14 : Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, Western Psychological Services, 1996Google Scholar
15 : Validation of the Spanish version of the LASSI-L for diagnosing mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 2017; 56:733–742Crossref, Medline, Google Scholar
16 : Beck Depression Inventory-II. San Antonio, Tex, The Psychological Corporation, 1996Google Scholar
17 : Estimation of premorbid intelligence: the Word Accentuation Test—Buenos Aires version. J Clin Exp Neuropsychol 2000; 22:677–685Crossref, Medline, Google Scholar
18 : Recovery from proactive semantic interference in mild cognitive impairment and normal aging: relationship to atrophy in brain regions vulnerable to Alzheimer’s disease. J Alzheimers Dis 2017; 56:1119–1126Crossref, Medline, Google Scholar
19 : A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004; 22:1060–1075Crossref, Medline, Google Scholar
20 : Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Crossref, Medline, Google Scholar
21 : Sequence-independent segmentation of magnetic resonance images. Neuroimage 2004; 23(Suppl 1):S69–S84Crossref, Medline, Google Scholar
22 : A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998; 17:87–97Crossref, Medline, Google Scholar
23 : Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging 2001; 20:70–80Crossref, Medline, Google Scholar
24 : Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imaging 2007; 26:518–529Crossref, Medline, Google Scholar
25 : Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999; 9:179–194Crossref, Medline, Google Scholar
26 : Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci 1993; 5:162–176Crossref, Medline, Google Scholar
27 : Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000; 97:11050–11055Crossref, Medline, Google Scholar
28 : High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999; 8:272–284Crossref, Medline, Google Scholar
29 : Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. Neuroimage 2016; 132:334–343Crossref, Medline, Google Scholar
30 : Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995; 57:289–300Google Scholar
31 : Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci 2003; 4:637–648Crossref, Medline, Google Scholar
32 : Orbito-frontal cortex is necessary for temporal context memory. J Cogn Neurosci 2010; 22:1819–1831Crossref, Medline, Google Scholar
33 : Source monitoring and memory distortion. Philos Trans R Soc Lond B Biol Sci 1997; 352:1733–1745Crossref, Medline, Google Scholar
34 : Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 2003; 7:415–423Crossref, Medline, Google Scholar



