Cognitive Impairments and Dysexecutive Behavioral Disorders in Chronic Kidney Disease
Abstract
The purpose of this study was to characterize cognitive impairments and behavioral disorders in a sample of patients with chronic kidney disease (CKD). A total of 52 patients with CKD were prospectively recruited over a 344-day period. Cognitive functions (memory, action speed, executive function, and language) and behavioral characteristics were assessed with a standardized comprehensive battery. The patients’ performances were interpreted with a validated method on the basis of normative data from 1,003 healthy control subjects. Brain MRI and biological data were collected. Multivariable linear regression models and bootstrap analyses were used to identify risk factors for cognitive impairment. Cognitive impairment was observed in 32.5% (95% confidence interval: 17%–48%) of the 40 included patients with full data sets. Action speed and executive functions were the most frequently impaired domains. Dysexecutive behavioral disorders were observed in 27% of patients, and depression was observed in 32.5%. Cognitive impairment was independently associated with stroke volume, high serum parathyroid hormone and uric acid levels, and low serum glucose levels (adjusted R2=0.54, p<0.001 One-third of patients with CKD had cognitive impairments (action speed and executive functions), behavioral dysexecutive disorders (hypoactivity with apathy, irritability, or anosognosia), or depression.
Chronic kidney disease (CKD) is a substantial public health problem that affects almost 8% of the U.S. population.1 CKD may be associated with an increased risk of cognitive impairment, from the earliest stages of the disease to the latest.2 It may predispose patients to disease progression as a result of worse adherence to CKD risk reduction strategies (i.e., the use of nephroprotective treatments, dietary restriction, and regular laboratory monitoring) and alter their quality of life.
The prevalence of cognitive impairment among persons with CKD ranges from 30% to 60%.3 The estimated prevalence of cognitive impairment in this population varies in accordance with the function of the ages of the CKD samples studied, the cognitive assessments used in various studies as well as the manner in which the results of such assessments are interpreted (i.e., raw scores vs. normative scores), and the criteria used to define cognitive impairment.4 As a result of these differences in study populations and methods, both the prevalence and the character of cognitive impairments in CKD are not fully elucidated. However, the available evidence suggests that executive functions are among the most prominent impairments observed to date.2 Given the apparent predominance of executive dysfunction in CKD, dysexecutive behavioral disorders5—including hypoactivity (e.g., apathy), emotional dyscontrol (e.g., irritability), and unawareness of illness (anosognosia)—might be anticipated but have been neither reported nor formally evaluated in the CKD population. Although the pathoetiology of cognitive impairment in CKD is not fully established, both vascular and nonvascular risk factors appear to be contributory.4 Mechanisms that contribute to the development of cognitive impairment in CKD have not yet been fully characterized, however.
We sought to characterize cognitive impairments and behavioral disorders (prevalence, profile, and risk factors) in a sample of patients with CKD who were prospectively recruited. To this end, we applied a validated methodology on the basis of normative data from a large group of healthy control subjects.
Methods
Design and Setting
We conducted a prospective, single-center study of a population of patients with CKD, consulting at Amiens University Medical Center (Amiens, France) between March 30, 2012, and December 3, 2013. The study was approved by the local investigational review board (CPP Nord Ouest II, Amiens, France; reference ID RCB 2011-AO1448–33). Informed consent was obtained from each participant or, among participants unable to offer independent informed consent, from a legally authorized relative.
Patients
All patients between 18 and 80 years old who were referred for confirmed CKD (i.e., two measurements of the estimated glomerular filtration rate, as calculated with the abbreviated Modification of Diet in Renal Disease equation formula [<80 ml/min/1.73 m, 3 months apart]) were eligible for inclusion in the study.
Exclusion criteria were chronic inflammatory syndrome or a chronic infectious disease (e.g., chronic osteitis); an acute cardiovascular event (myocardial infarction or stroke) in the previous 3 months; abdominal aortic aneurysm; cardiac bypass surgery; primary hyperparathyroidism; acute degradation of renal function in the last 3 months, with a decrease in creatinine clearance >30%; pregnancy or breastfeeding; mental retardation; illiteracy; previously diagnosed cognitive impairment or dementia; psychiatric disorders (current schizophrenia or psychosis or past psychiatric disorders requiring a stay of more than 2 days in a psychiatric unit); legal guardianship or curatorship; cognitive comorbidities (respiratory, liver, or heart failure); and contraindication to MRI. Dialysis was not an exclusion criterion.
Assessments at Baseline
Clinical variables.
The following data were collected: disease stage (the level of kidney function, according to the Kidney Disease Outcomes Quality Initiative CKD classification6), age, sex, education level, current medications, vascular risk factors (hypertension, defined as a previous diagnosis of hypertension [>140/90 mmHg] or the current use of antihypertensive medication; active smoking; dyslipidemia; diabetes; overweight [a body mass index >25 kg/m2]; and alcohol consumption), a history of stroke, vascular rigidity (as assessed by the pulse wave velocity), and disability (on the modified Rankin Scale7).
Neuropsychological assessment.
Cognitive domains were evaluated according to the French adaptation of the Harmonization Standards battery.8 This comprehensive battery assesses the following domains: It measures general cognitive efficiency with the Mini-Mental State Examination (MMSE)9 and the Montreal Cognitive Assessment (MoCA),10 and it assesses language with the shortened Boston Naming Test.11 The battery assesses visuospatial and constructive capacities with the Albert cancellation test12 and the Rey–Osterrieth complex figure test (copying).13 Long-term memory is assessed with the Free and Cued Selective Recall Test (FCSRT),14 the Baddeley door test,15 and the Rey–Osterrieth complex figure test (3-minute recall).13 Executive function and action speed are measured with the GREFEX version of the Trail Making Test,5 Stroop5 category fluency (1-minute animal score) and letter fluency (1-minute letters P, V, and R),16 the Wechsler Adult Intelligence Scale (WAIS) Digit Symbol Substitution subtest,17 and the Behavioral Dysexecutive Syndrome Inventory.5 The battery measures depressive symptoms using the Center for Epidemiologic Studies Depression Scale18 and anxiety using the Goldberg Questionnaire.19 According to previously validated diagnostic criteria for executive dysfunction, any detected disorders must not be more readily explained by perceptuomotor, psychiatric, or other cognitive (language, memory, and visuospatial) disturbances.5
The results were analyzed with normative data from 1,003 healthy volunteers in the GRECogVasc study, included according to previously reported criteria5 and methods.20 Each patient’s performance was analyzed with regard to a validated framework for the interpretation of normative cognitive data, including normative data for MMSE and MoCA scores. In brief, the standardized residuals of cognitive scores (after adjustment on significant demographic factors, e.g., age and educational level) were used to compute summary scores for five cognitive domains: language (score on the Boston Naming Test), visuoconstructive abilities (the complex figure copying score), long-term memory (the FCSRT scores), action speed (completion time in the Trail-Making Test and the WAIS Digit Symbol Substitution subtest), and executive function (letter and category fluencies; errors in the Trail-Making Test, Part B). The global cognitive summary score corresponded to the average of the scores in the five domains. Impairment on the above measures was defined by a score below the 5th percentile, on the basis of the GRECogVasc study normative data.20
MRI.
At baseline, we obtained MRI scans on 3-Tesla systems (SIGNA HDxt, General Electric Medical, Waukesha, WI) by using three-dimensional T1-BRAVO sequences (repetition time [TR]/echo time [TE]=11.4/5.4 ms, slice thickness=1.0 mm, no interslice gap), fluid-attenuated inversion recovery (repetition time/echo time/inversion time [TR/TE/TI]=9,002/153.5/2250 ms, slice thickness=5 mm, no gap), and T2*-weighted gradient echo imaging sequences (TR/TE=460/13 ms, slice thickness=5 mm, no gap).
We visually assessed images for the presence of cerebral infarct and hemorrhage (including microbleeds) and the extension of white matter hyperintensities by using three-dimensional-T1, T2*, and fluid-attenuated inversion recovery sequences. Lesions were segmented on the raw MRI dataset, and the resulting lesion mask was used to compute the stroke volume. The volume of brain tissue (normalized for the subject’s head size) was determined on SIENAX and T1-weighted images.21 Hippocampal atrophy on each side of the brain was evaluated with the Scheltens score22 on three-dimensional-T1 coronal sequences (total score=left+right hippocampal score). The extent of white matter hyperintensities was assessed with the Fazekas score.23 Microbleeds were defined as small (<10 mm in diameter) areas of signal void with associated blooming seen on T2*-weighted MRI and assessed according to the Brain Observer MicroBleed Scale criteria.24
Laboratory tests.
Biological and biochemical measurements included the estimated glomerular filtration rate and serum levels of creatinine, albumin, glucose, sodium, potassium, chloride, carbon dioxide, anion gap, urea, albumin, calcium, phosphorus, hemoglobin, hematocrit, erythrocyte, leukocyte and platelet counts, C-reactive protein, lipid (total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride), parathyroid hormone, calcitriol (1,25[OH] vitamin D3), calcidiol (25[OH] vitamin D3), uric acid, and three uremic toxins (indole-3-acetic acid, p-cresyl sulfate, and indoxyl sulfate).
Data Analyses
The prevalence of cognitive impairment (expressed as a percentage [95% confidence interval]) was determined from the global cognitive summary score. To define the cognitive pattern, we performed an analysis of variance with repeated measures for the cognitive domains (language, visuoconstructive abilities, long-term memory, action speed, and executive function). The sensitivity of cognitive tests was examined with Cohen’s d and its 95% confidence interval (CI); d values >0.8 were considered to reveal good sensitivity. The diagnostic accuracy (sensitivity, specificity, negative predictive value [NPV], and positive predictive value [PPV]) of the two screening tests (the MMSE and the MoCA) was examined. We determined actors associated with the global cognitive summary score (the dependent variable) using a stepwise linear regression to obtain the estimate (E), standard error (SE), and adjusted R2. All clinical, MRI, and biological factors with p<0.1 in the bivariate analysis were fed into the regression analysis. The robustness of the regression model was examined in a bootstrap analysis (N=1,000 permutations). All statistical analyses were performed with R software (http://www.r-project.org/). All tests were two-sided, and the threshold for statistical significance level was set to 5%.
Results
Of the 52 prospectively recruited patients, 12 were excluded because of missing data. The demographic, clinical, biological, and MRI characteristics of the 40 included patients are summarized in Table 1. Seven (17.5%) patients presented with slightly reduced kidney function (stage 2), 19 (47.5%) presented with moderately reduced kidney function (stage 3), 8 (20.0%) presented with severely reduced kidney function (stage 4), and 6 (15.0%) presented with end-stage kidney failure (stage 5; two of the latter were on dialysis, i.e., at stage 5D). Nine (22.5%) patients presented apparent cavitation on three-dimensional T1 MRI sequences (hemorrhage: N=2; infarct: N=6; both features: N=1); eight had experienced a stroke, and one had experienced a silent brain infarct. Other types of vascular abnormality (notably related to white matter hyperintensities) were observed in 28 (70%) patients.
| Characteristic | N | % | Mean | SD |
|---|---|---|---|---|
| Demographic and clinical data | ||||
| Age (years) | — | — | 62.58 | 11.01 |
| Male gender | 22 | 55.0 | — | — |
| Educational level (years) | — | — | 10.7 | 3.2 |
| Right-handedness | 39 | 97.5 | — | — |
| Chronic kidney disease stage: 2, 3, 4, 5a | 7, 19, 8, 6 | 17.5, 47.5, 20.0, 15.0 | — | — |
| Hypertension | 33 | 84.6 | — | — |
| Weight (kg) | — | — | 76.3 | 14.84 |
| Body mass index (kg/m2) | — | — | 27.5 | 5.92 |
| Diabetes | 16 | 41.0 | — | — |
| Hypercholesterolemia | 25 | 64.1 | — | — |
| Smoker | 14 | 43.8 | — | — |
| Pulse-wave velocity (m/s) | — | — | 9.9 | 3.37 |
| MRI features | ||||
| Normalized brain tissue volume (cm3) | — | — | 1,414.2 | 66.5 |
| Stroke volume (mm3) | — | — | 3,678.9 | 2,0265 |
| Total hippocampal atrophy | — | — | 2b | 1–4b |
| White matter hyperintensities burdenc | 12, 19, 4, 5 | 30, 47.5, 10, 12.5 | — | — |
| Presence of microbleeds | 6 | 15 | — | — |
| Infarct and hemorrhage | 9 | 22.5 | — | — |
| Metabolic data | ||||
Estimated glomerular filtration rate (mL/min/1.73 m2)d | — | — | 42.48 | 17.71 |
| Creatinine (µmol/L) | — | — | 219.29 | 168.01 |
| Anion gap (mmol/L) | — | — | 12.34 | 3.1 |
| Prothrombin time | — | — | 93.3 | 16.64 |
| C-reactive protein (mg/L) | — | — | 4.22 | 2.96 |
| Albuminemia (g/L) | — | — | 42.4 | 3.88 |
| Leukocyte count (103/mm) | — | — | 7.13 | 1.9 |
| Hemoglobin (g/dL) | — | — | 12.92 | 1.64 |
| Hematocrit (%) | — | — | 38.98 | 4.5 |
| Erythrocyte count (103/mm) | — | — | 4.28 | 0.61 |
| Cholesterol (mmol/L) | — | — | 4.44 | 1.22 |
| Low-density lipoprotein cholesterol (mmol/L) | — | — | 2.31 | 0.93 |
| Triglycerides (mmol/L) | — | — | 1.72 | 1.43 |
| High-density lipoprotein cholesterol (mmol/L) | — | — | 1.38 | 0.39 |
| Parathyroid hormone (pg/mL) | — | — | 145.18 | 162.6 |
| Calcitriol 1.25(OH)vitamin D3 (pg/mL) | — | — | 36.70 | 21.14 |
| Calcidiol 25(OH)vitamin D3 (pg/mL) | — | — | 26.276 | 10.818 |
| Glycemia (mmol/L) | — | — | 6.065 | 2.306 |
| Calcium (mmol/L) | — | — | 2.34 | 0.156 |
| Uric acid (µmol/L) | — | — | 475.33 | 131.395 |
| Indole–3-acetic acid (µmol/L) | — | — | 1.52 | 0.81 |
| P-cresyl sulfate (µmol/L) | — | — | 81.34 | 84.47 |
| Indoxyl sulfate (µmol/L) | — | — | 25.08 | 32.9 |
TABLE 1. Main Clinical, MRI, and Metabolic Features of the Study Population
Cognitive Impairments and Behavioral Disorders
We analyzed the following results by using normative data from 1,003 healthy volunteers in the GRECogVasc study (gender: 36% male; mean age: 62 years [SD=11.3]; education level: primary school, 27%, junior high, 37.4%, high school, 35.6%; MMSE score: 28.7 [SD=1.4]; MoCA score: 26.5 [SD=2.6]). Among patients with CKD, cognitive impairment was present in 32.5% (95% CI=18–47). The MMSE score was abnormal (i.e., <5th percentile) for 12 patients, eight of whom showed cognitive impairment in formal neuropsychological assessment (sensitivity: 0.62; specificity: 0.85; PPV: 0.67; NPV: 0.82; odds ratio: 9.2, 95% CI=1.97–42.9). The MoCA score was abnormal (i.e., <5th percentile) for eight patients, all of whom showed cognitive impairment in formal neuropsychological assessment (sensitivity: 0.62; specificity: 1; PPV: 1; NPV: 0.84; odds ratio: 5.67, 95% CI=2.72–11.8).
Analysis of Cohen’s d (Table 2) showed that four tests assessing action speed and executive function (including behavioral dysexecutive disorders) had a sensitivity value >0.8. The cognitive scores (Figure 1) differed from one domain to another (F=2.9, df=4, 36, p=0.035). A contrast analysis indicated that the scores for the action speed (−1.045 [SD=1.229]) and executive (−0.888 [SD=1.214]) domains were lower (p<0.05, for both) than those for language (−0.517 [SD=0.930]), visuoconstructive abilities (−0.523 [SD=1.087]), and memory (−0.490 [SD=1.214]). Accordingly, the frequency of impairment was higher for action speed and executive functions (Figure 1).
| Measure | Cohen’s d | 95% CI | p |
|---|---|---|---|
| Mini-Mental State examination | 0.792 | 0.468–1.116 | 0.0001 |
| Montreal Cognitive Assessment | 0.597 | 0.279–0.915 | 0.0100 |
| Boston Naming Test | 0.729 | 0.359–1.099 | 0.0001 |
| Complex figure (copying) | 0.523 | 0.206–0.840 | 0.0012 |
| Free and Cued Selective Recall Test | |||
| Free recall sum | 0.446 | 0.123–0.770 | 0.0068 |
| Total recall sum | 0.188 | –0.139–0.516 | 0.2588 |
| Recognition | 0.033 | –0.287–0.353 | 0.8391 |
| Delayed free recall | 0.169 | –0.153–0.492 | 0.3022 |
| Delayed total recall | –0.098 | –0.420–0.224 | 0.5513 |
| Baddeley Doors test | 0.158 | –0.163–0.479 | 0.3339 |
| Complex figure (recall) | 0.136 | –0.186–0.459 | 0.4071 |
| Trail-Making Test | |||
| Part A completion time | 0.682 | 0.361–1.002 | 0.0001 |
| Part B completion time | 1.147 | 0.820–1.474 | 0.0001 |
| Test errors B–A | 0.786 | 0.453–1.118 | 0.0062 |
| Digit Symbol Substitution Test | 0.939 | 0.620–1.258 | 0.0001 |
| Letter fluency | 0.809 | 0.495–1.123 | 0.0001 |
| Category fluency | 0.631 | 0.320–0.942 | 0.0001 |
| Behavioral Dysexecutive Syndrome Inventory | 0.825 | 0.610–1.041 | 0.0002 |
TABLE 2. Sensitivity of the Cognitive Tests Applied in the Study
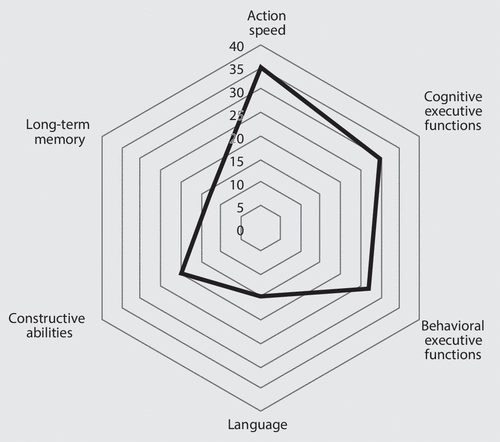
FIGURE 1. The Pattern of Cognitive Impairments in Patients With Chronic Kidney Diseasea
a The scale corresponds to the percentage of patients with impairment.
Anxiety and depressive symptoms were observed in 16 (40%) patients (depression: N=13 [32.5%]; anxiety: N=11 [27.5%]). Impairment in the Behavioral Dysexecutive Syndrome Inventory was observed in 16 (43.2%) patients; six also displayed anxiety or depressive symptoms. Thus, a dysexecutive behavioral syndrome was observed in 10 patients (27%) and was most prominent for global hypoactivity with apathy (N=8), irritability-aggressiveness (N=7), and anosognosia (N=6).
Risk Factors of Cognitive Impairment
In a bivariate analysis (Table 3), eight factors (weight, normalized brain tissue volume, stroke volume, hemoglobin levels, glycemia, albuminemia, and serum parathyroid hormone and uric acid levels) had a p value <0.1 and were therefore fed into the regression analysis. It is noteworthy that the estimated glomerular filtration rate and the serum levels of the three protein-bound uremic toxins were not associated with cognitive impairment. The results of the stepwise linear regression (Table 4) indicated that stroke volume (E=−7.221×10−2, SE=3.074×10−2, p=0.026), high serum parathyroid hormone levels (E=−1.655×10−3, SE=5.673×10−4, p=0.007), high serum uric acid levels (E=−1.815×10−3, SE=7.230×10−4, p=0.018), and low glycemia (E=1.059×10−1, SE=4.780×10−2, p=0.035) were strongly and independently associated with cognitive impairment (adjusted R2=0.54; p<0.001). Furthermore, these four factors were the most frequently selected in a bootstrap analysis.
| Features | Cognitive Impairment Present (N=13) | Cognitive Impairment Absent (N=27) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | Mean | SD | N | % | Mean | SD | ||
| Demographic and clinical data | |||||||||
| Age (years) | — | — | 62.08 | 14.14 | — | — | 62.81 | 9.45 | 0.84 |
| Male gender | 7 | 53.9 | — | — | 15 | 55.6 | — | — | 1 |
| Education level (years) | — | — | 10.54 | 2.2 | — | — | 10.74 | 3.59 | 0.85 |
| Right handedness | 13 | 100 | — | — | 26 | 96.3 | — | — | 1 |
| Hypertension | 12 | 92 | — | — | 21 | 77.8 | — | — | 0.20 |
| Weight (kg) | — | — | 70.46 | 10.97 | — | — | 79.92 | 13.17 | 0.06 |
| Body mass index (kg/m2) | — | — | 25.39 | 3.41 | — | — | 28.46 | 6.61 | 0.16 |
| Diabetes | 4 | 33.3 | — | — | 12 | 44.4 | — | — | 0.77 |
| Hypercholesterolemia | 8 | 66.7 | — | — | 17 | 63.0 | — | — | 1 |
| Smoker | 4 | 40.0 | — | — | 10 | 45.4 | — | — | 1 |
| Pulse wave velocity (m/s) | — | — | 9.23 | 1.31 | 10.24 | 4.01 | 0.42 | ||
| MRI features | |||||||||
| Normalized brain tissue volume (cm3) | — | — | 1,364.4 | 68.2 | — | — | 14,379.4 | 51.6 | 0.0005 |
| Stroke volume (mm3) | — | — | 11,302.7 | 35233.3 | — | — | 8.16 | 24.34 | 0.0048 |
| Total hippocampal atrophy | — | — | 2a | 0–5a | — | — | 2a | 1–3a | 0.83 |
| White matter hyperintensities burdenb | — | — | 1a | 1–1a | — | — | 1a | 0–1a | 0.93 |
| Presence of microbleeds | 2 | 15.4 | — | — | 4 | 14.8 | — | — | 1 |
| Metabolic data | |||||||||
| Estimated glomerular filtration ratec (mL/min/1.73cm2) | — | — | 41.3 | 21.71 | — | — | 43 | 16.21 | 0.81 |
| Creatinine (µmol/L) | — | — | 229.36 | 144.82 | — | — | 215.19 | 179 | 0.82 |
| Anion gap (mmol/L) | — | — | 12.36 | 2.34 | — | — | 12.33 | 3.43 | 0.97 |
| Albuminemia (g/L) | — | — | 44.18 | 3.58 | — | — | 41.64 | 3.79 | 0.07 |
| Prothrombin time | — | — | 88.92 | 22.92 | — | — | 95.4 | 12.66 | 0.27 |
| C-reactive protein (mg/L) | — | — | 3.59 | 1.04 | — | — | 4.48 | 3.44 | 0.41 |
| Parathyroid hormone (pg/mL) | — | — | 222.33 | 249.01 | — | — | 109.58 | 87.59 | 0.04 |
| Calcium (mmol/L) | — | — | 2.25 | 0.22 | — | — | 2.38 | 0.1 | 0.015 |
| Calcitriol 1.25(OH)vitamin D3 (pg/mL) | — | — | 35.18 | 20.23 | — | — | 37.43 | 21.97 | 0.78 |
| Calcidiol 25(OH) vitamin D3 (pg/mL) | — | — | 23.63 | 9.5 | — | — | 27.45 | 11.33 | 0.32 |
| Leukocytes (103/mm) | — | — | 6.44 | 1.26 | — | — | 7.44 | 2.07 | 0.13 |
| Hemoglobin (g/dL) | — | — | 12.64 | 1.73 | — | — | 13.26 | 1.69 | 0.07 |
| Low-density lipoprotein cholesterol (mmol/L) | — | — | 1.93 | 0.58 | — | — | 2.14 | 0.99 | 0.13 |
| Triglycerides (mmol/L) | — | — | 2.0 | 2.3 | — | — | 1.58 | 0.77 | 0.42 |
| High-density lipoprotein cholesterol (mmol/L) | — | — | 1.34 | 0.38 | — | — | 1.4 | 0.4 | 0.68 |
| Glycemia (mmol/L) | — | — | 4.55 | 0.4 | — | — | 6.77 | 2.49 | 0.004 |
| Uric acid (µmol/L) | — | — | 521.36 | 126.83 | — | — | 439.48 | 98.88 | 0.03 |
| Indole–3-acetic acid (µmol/L) | — | — | 1.69 | 1.03 | — | — | 1.44 | 0.69 | 0.38 |
| P-cresyl sulfate (µmol/L) | — | — | 75.43 | 59.19 | — | — | 84.30 | 95.58 | 0.76 |
| Indoxyl sulfate (µmol/L) | — | — | 21.68 | 17.59 | — | — | 26.79 | 38.56 | 0.65 |
TABLE 3. Main Clinical, MRI, and Metabolic Features of the Study Population According to the Presence or Absence of Cognitive Impairment
| Potential Predictor | Regression Coefficient (Estimated) | Standard Error of the Regression Coefficient | p | Bootstrap (%) (N=1,000 Permutations) |
|---|---|---|---|---|
| Weight (kg) | 7.251×10–3 | 7.514×10–3 | 0.34 | 45 |
| Stroke volume (mm3) | –7.221×10–2 | 3.074×10–2 | 0.026 | 87 |
| Brain tissue volume (mm3) | 1.485×10–6 | 1.619×10–6 | 0.367 | 41 |
| Parathyroid hormone (pg/mL) | –1.655×10–3 | 5.673×10–4 | 0.007 | 86 |
| Glycaemia (mmol/L) | 1.059×10–1 | 4.780×10–2 | 0.035 | 90 |
| Uric acid (µmol/L) | –1.815×10–3 | 7.230×10–4 | 0.018 | 88 |
TABLE 4. Factors Associated (or Not) With Overall Cognitive Score According to Regression Analysis
Discussion
This study provided four main findings: Cognitive impairment was observed in at least one-third of patients (after we controlled for the false positive rate when interpreting the cognitive test results); cognitive impairment notably affected action speed and both the behavioral and the cognitive domains of executive function; the most commonly applied screening tests (MMSE and MoCA) showed only moderate sensitivity, thus indicating the need to apply a neuropsychological battery; and behavioral dysexecutive disorders and anxiety and depressive symptoms were frequent.
Cognitive Impairments and Behavioral Disorders
The rate of cognitive impairment observed in the present study (32.5%) corresponds to the lower range of previously reported values in CKD populations (30%−60%).4 This low rate might be due to the lower frequency of false positives associated with our use of the global summary score and the 5th percentile threshold, our inclusion of patients at all stages of CKD diseases (in contrast to the severe CKD population in previous studies), or both. The cognitive pattern was characterized by prominent impairments of action speed and executive functions. In contrast, language, long-term-memory, and visuoconstructive abilities were relatively unaffected. Test sensitivity was consistently found to be good for action speed and executive functions. This suggests that executive disorders and action slowing are present from the very early disease stages onward. Our results reinforce the prominence of dysexecutive dysfunction in cognitive impairments among patients with CKD,25 and action slowing is a key feature of these dysexecutive impairments. Given that action slowing may be due to nonexecutive impairments (e.g., subtle sensorimotor impairments), separate analyses of each domain are mandatory.
Behavioral dysexecutive disorders have not previously been studied in adult patients with CKD, but 27% of our patients presented with this type of behavioral disorder (primarily hypoactivity with apathy, irritability, and anosognosia). This finding indicates that mood and behavioral dysexecutive disorders are frequent and should be assessed in further studies. Anxiety and depressive symptoms were observed in 40% of the patients, and depression was observed in 32.5% of patients, which is a substantially higher proportion than the literature value (23%).26
Risk Factors of Cognitive Impairment
Although it is known that more than one mechanism underlies the development of cognitive impairment in CKD, the nature of these factors is subject to debate.27
Vascular hypothesis of cognitive impairment.
The first hypothesis relates to vascular changes in the brain and is supported by several lines of evidence: Both CKD and stroke share the same risk factors, cerebrovascular disease is highly prevalent in CKD,28 and the two diseases may share a common pathogenic mechanism and substrate.29 This hypothesis is supported by our finding that the cognitive pattern in CKD is similar to that observed in stroke30 and that a high stroke volume is independently associated with cognitive impairment. However, other mechanisms are known to be involved.
Metabolic hypothesis of cognitive impairment: hyperparathyroidism, neuronal toxicity of the uremic state, and hypoglycemia.
In line with recent meta-analysis results,31 we did not observe an association between the estimated glomerular filtration rate level and cognitive impairment. However, the majority of cohort studies studying participants with CKD have shown an association between CKD (either low glomerular filtration rate or albuminuria) and cognitive decline.32–34 We found an association between cognitive impairment and biomarkers of impaired kidney function (serum concentrations of uric acid and parathyroid hormone). The present study is the first to report the association with parathyroid hormone. Phosphate metabolism disorders with hyperparathyroidism promote deposition of calcium in soft tissues, vascular calcification, proliferation of smooth muscle cells, and impaired microcirculation in the brain.35 The uremic state is directly toxic for neurons.36 High uremic toxin concentrations (which are up to 10-fold higher in patients with CKD than in healthy individuals) are found in brain regions related to cognition, such as the thalamus, the mammillary bodies, and the cerebral cortex.37
Our study failed to show an association between cognitive impairment and serum levels of specific protein-bound uremic toxins (i.e., indole acetic acid, indoxyl sulfate, and p-cresyl sulfate), although this might have been due to the study’s moderate statistical power. Watanabe et al.38 reviewed the literature on the interactions between 21 uremic toxins and the brain. They concluded that uric acid, parathyroid hormone, indoxyl sulfate, p-cresyl sulfate, interleukin 1-beta, interleukin 6, and TNF-alpha are likely to have an impact on cognition and the central nervous system under uremic conditions.38 It is surprising that low glycemia was found to be associated with lower cognitive performance—a finding that has not been previously reported in CKD. In diabetes mellitus, the relationship between hypoglycemia and dementia is subject to debate. Although a history of severe hypoglycemic episodes is associated with a higher risk of dementia, it is not known whether minor hypoglycemic episodes or chronic low glycemia increase the risk of dementia. Hypoglycemia may alter neuronal plasticity, increase levels of the neurotoxic compound glutamate, and thus lead to cognitive impairment.39
Our study had several limitations. First, the study population was small, and this might have limited our ability to demonstrate the significance of several factors in a regression model. However, we selected factors that were significant in a bivariate analysis, and we analyzed the robustness of our findings with a bootstrap procedure. Second, the study population was heterogeneous because it included patients at all stages of CKD. Although this is representative of a clinical population, the small numbers of patients at each disease stage prevented us from determining the corresponding frequency and profile of cognitive impairments. Finally, our population presented a large age range and demonstrated that cognitive impairment in an 18-year-old person should be treated (and studied) very differently than cognitive impairment in an 80-year-old person. We considered stratification by age, but analysis was limited by the effective size.
Our study also had a number of strengths. The use of a composite score for the cognitive assessment controlled for the false positive rate. We dichotomized cognitive performance (normal vs. impaired) according to thresholds based on cut-offs from a large control population (with scores adjusted for age and educational level), and we used a validated test battery (the GRECogVasc battery) to assess cognitive function. The present study is the first, to our knowledge, to investigate behavioral dysexecutive disorders in patients with CKD. Last, we performed an exhaustive analysis of potential risk factors of cognitive impairment, including demographic, clinical, imaging, and metabolic factors.
Conclusions
Our results highlight the close relationship between a decline in renal function and cognitive impairment. Cognitive impairment was poorly detected by the most frequently applied screening tests. We found that behavioral dysexecutive disorders and depression were frequent in CKD populations. Cognitive impairment was associated with the stroke volume, high serum uric acid and parathyroid hormone levels, and low glycemia. The assessment of cognitive and behavioral functions might be of value for predicting poor adherence to CKD risk reduction strategies. Several pathways probably underlie this association, and further mechanistic studies are warranted.
1 : US Renal Data System 2010 annual data report. Am J Kidney Dis 2011; 57(Suppl 1):A8, e1–526Crossref, Medline, Google Scholar
2 : Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2016; 67:925–935Crossref, Medline, Google Scholar
3 : Neuropsychiatric complications of chronic kidney disease. Nat Rev Nephrol 2010; 6:471–479Crossref, Medline, Google Scholar
4 : Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013; 24:353–363Crossref, Medline, Google Scholar
5 : Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol 2010; 68:855–864Crossref, Medline, Google Scholar
6
7 : The Rankin Scale With Revised Structured Interview: effect on reliability, grading of disability and detection of dementia. Int J Stroke 2012; 7:183Crossref, Medline, Google Scholar
8 : French adaptation of the vascular cognitive impairment harmonization standards: the GRECOG-VASC study. Int J Stroke 2012; 7:362–363Crossref, Medline, Google Scholar
9 : “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
10 : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699Crossref, Medline, Google Scholar
11 : Adaptation française du test de dénomination de Boston, Versions abrégées. Revue europèenne de psychologie appliquée 1992; 42(1): 67–71Google Scholar
12 : A simple test of visual neglect. Neurology 1973; 23:658–664Crossref, Medline, Google Scholar
13 : Test de copie d’une figure complexe. Paris, Les Editions du Centre de Psychologie Appliquée, 1959Google Scholar
14 : L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16); in L’Evaluation des Troubles de la Mémoire. Présentation de Quatre Tests de Mémoire Épisodique. Edited by Van der Linden M, Adam S, Agniel A, . Marseille, France, 2004Google Scholar
15 : Doors and People: A Test of Visual and Verbal Recall and Recognition. Suffolk, United Kingdom, Thames Valley Test Company, 1994Google Scholar
16 : Formal and semantic lexical evocation in normal subjects: performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 1990; 90:207–217Medline, Google Scholar
17 Wechsler D: Wechsler Adult Intelligence Scale, 3rd ed. San Antonio, Tex, Psychological Corporation, 1997Google Scholar
18 : Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997; 12:277–287Crossref, Medline, Google Scholar
19 : Detecting anxiety and depression in general medical settings. BMJ 1988; 297:897–899Crossref, Medline, Google Scholar
20 : Validation of an integrated method for determining cognitive ability: implications for routine assessments and clinical trials. Cortex 2014; 54:51–62Crossref, Medline, Google Scholar
21 : Measurement of brain atrophy in subcortical vascular disease: a comparison of different approaches and the impact of ischaemic lesions. Neuroimage 2008; 43:312–320Crossref, Medline, Google Scholar
22 : Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992; 55:967–972Crossref, Medline, Google Scholar
23 : Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993; 43:1683–1689Crossref, Medline, Google Scholar
24 : Improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke 2009; 40:94–99Crossref, Medline, Google Scholar
25 : Association between renal function and cognitive ability domains in the Einstein Aging Study: a cross-sectional analysis. J Gerontol A Biol Sci Med Sci 2015; 70:764–770Crossref, Medline, Google Scholar
26 : Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int 2013; 84:179–191Crossref, Medline, Google Scholar
27 : Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant 2016; 31:1606–1614Crossref, Medline, Google Scholar
28 : Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol 2014; 13:823–833Crossref, Medline, Google Scholar
29 : Chronic renal failure alters endothelial function in cerebral circulation in mice. Am J Physiol Heart Circ Physiol 2011; 301:H1143–H1152Crossref, Medline, Google Scholar
30 : Is the Montreal Cognitive Assessment superior to the Mini-Mental State Examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke 2011; 42:1712–1716Crossref, Medline, Google Scholar
31 : Dementia risk in renal dysfunction: a systematic review and meta-analysis of prospective studies. Neurology 2017; 88:198–208Crossref, Medline, Google Scholar
32 : Cognitive function and kidney disease: baseline data from the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis 2017; 70:357–367Crossref, Medline, Google Scholar
33 : Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis 2008; 52:227–234Crossref, Medline, Google Scholar
34 : Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009; 73:920–927Crossref, Medline, Google Scholar
35 : Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16:520–528Crossref, Medline, Google Scholar
36 : Neurological disorders in a murine model of chronic renal failure. Toxins (Basel) 2014; 6:180–193Crossref, Medline, Google Scholar
37 : Uremic encephalopathy and other brain disorders associated with renal failure. Semin Neurol 2011; 31:139–143Crossref, Medline, Google Scholar
38 : Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology 2014; 44:184–193Crossref, Medline, Google Scholar
39 : Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia 2007; 55:1280–1286Crossref, Medline, Google Scholar



