Clinical Application of Findings From Longitudinal Studies of Younger-Onset Dementia: Rapid Review and Recommendations
Abstract
Younger-onset dementia (YOD) presents with heterogeneous symptoms, has a variety of etiologies, and can be difficult to diagnose. The authors conducted a rapid review of longitudinal YOD cohorts and their related substudies to evaluate current literature that may inform the clinical information provided to patients about the progression and duration of illness and to highlight areas for future research. Searches were conducted using MEDLINE, CINAHL, PubMed, PsycINFO, and Web of Science for articles published between January 1966 and June 2018. Four longitudinal YOD cohort studies and their related substudies were identified. Alzheimer’s disease (AD) was reported as the most frequently occurring YOD. The age at onset reported for two cohorts ranged from 53.8 to 60.2 years, depending on the dementia type. Three cohorts yielded substudies that focused on other aspects of YOD, including caregiver outcomes, neuropsychiatric symptoms, and psychotropic drug use. There were conflicting data regarding whether AD or frontotemporal dementia had the greatest rate of cognitive decline. The authors identified a restricted amount of clinical information that may be useful for patients and their families. Limitations included relatively short follow-up periods and types of dementia included. There was also a lack of information on longitudinal changes in neuropsychiatric symptoms and their relationship to biomarkers. These aspects are important considerations for future research, because they may yield information relevant to early diagnosis and disease progression, with improved clinical care for patients with YOD and their families. Streamlining data collection may also improve the ability to generalize results.
Younger-onset dementia (YOD) refers to dementia that is diagnosed when a person is <65 years old (1). The prevalence of YOD has been estimated to be between 51.4 and 67.4 per 100,000 persons (2, 3). YOD has a range of etiologies, including Alzheimer’s disease (AD), frontotemporal dementia (FTD), vascular dementia (VaD), multiple sclerosis, and Huntington’s disease (HD).
YOD presents with a range of symptoms, and diagnostic delay is common, if not the norm (4, 5). Reasons for this delay may include the non-specificity of early symptoms, a predilection for neuropsychiatric presentations, the expectation that dementia only affects older adults, and a lack of knowledge about appropriate services and difficulty accessing them (6, 7). This period of uncertainty can lead to much distress for patients and their caregivers (8). Contributing to this stress may be the age of the patient. The onset of symptoms may occur when patients are in their 40s. Patients at this age are often juggling work, family, and financial responsibilities (3, 9), with potential significant lost productivity costs for them and their caregivers. Cross-sectional studies have reported that patients with YOD have more rapid cognitive deterioration and more frequent behavioral disturbances compared with older patients (10). The literature has also reported that the available services for dementia (usually for late-onset dementia [LOD]) may not always be appropriate for patients with YOD and their caregivers (11).
Once a diagnosis of YOD is finally made, the common follow-up questions are similar to those for dementia in older individuals and pertain to prognosis, duration, and progression of illness, including neuropsychiatric symptoms, and what services may be needed (12).
Longitudinal studies of YOD can potentially be useful in answering these questions, because they provide real-life data, such as characterizing symptoms of illness, investigating changes of these symptoms over time, and correlating them with biomarkers, imaging, and genetics (9). This information may be useful in the diagnostic process and in predicting prognosis. In addition, following up other aspects of YOD, such as caregivers’ perspectives, can provide important information regarding the types of services needed and at what stages of illness, as well as what symptoms are most difficult to manage.
We conducted a rapid review (13) of the available literature on longitudinal YOD studies. Here, we report on demographic data (such as gender, age at onset, and risk factors), symptoms, biomarkers, and caregiver outcomes to inform how clinicians could respond to questions after giving a diagnosis of YOD. We focused on studies that included patient or caregiver clinical information, such as cognitive changes or neuropsychiatric symptoms, in order to optimize clinical utility. We specifically sought to include cohorts that focused on more than one type of YOD to reflect the heterogeneity of clinical populations, as well as to enable comparisons between different types of YOD.
Methods
Search Strategy
Electronic searches were performed using MEDLINE, CINAHL, PubMed, Google Scholar, PsychInfo, Web of Science, and Scopus. Search terms included “younger-onset dementia” OR “early onset” AND dementia. Methodology-related terms were “follow-up” and “cohort” AND “longitudinal.” Searches were limited to studies conducted in English, with human participants, and with persons aged <65 years. We searched studies that were published from January 1, 1966, to June 1, 2018. Gray literature, such as government reports and web-based information, was also included. These strategies were supplemented by searches of reference lists, citation and author searches, and hand searching of key journals. The flow chart of the search strategy is shown in Figure 1.
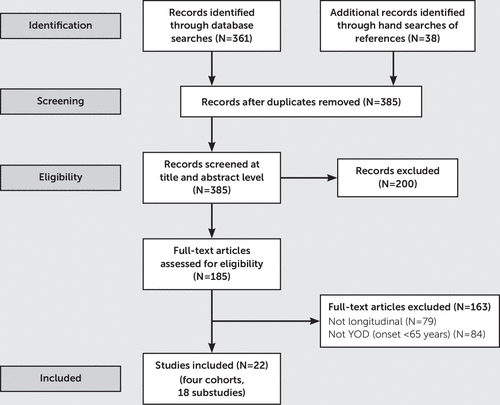
FIGURE 1. Flow diagram of longitudinal studies of patients with younger-onset dementia (YOD)a
a Flow diagram reproduced with permission from Moher et al: the PRISMA Group: Preferred reporting items for systematic reviews and meta-analysis: the PRISMA Statement. PLoS Med 2009; 6(7).
Inclusion and Exclusion Criteria
Criteria for study inclusion were longitudinal qualitative and quantitative research study designs; patients with YOD with a mean or median age at onset of symptoms <65 years old; community, home-dwelling adult, or residential care setting; aims regarding the principle cohort involving longitudinal assessment of patients with YOD; and English-speaking studies only involving human participants. Studies that were based on a principal longitudinal study (referred to as the “substudy”) were assessed if the substudy mentioned the original study in the methods section. The substudy could be either cross-sectional or longitudinal.
Exclusion criteria were principal cohorts that included only one type of YOD; literature without original data, such as editorials and commentaries; studies that did not report follow-up or longitudinal data; and studies that only reported longitudinal changes of imaging or biomarkers without correlating these changes with patient or caregiver clinical information, such as cognitive changes or neuropsychiatric symptoms.
For the principal longitudinal study (referred to as the “cohort”), the aim of the cohort, the main demographic data (e.g., age at onset, gender, type of dementia), and outcome measures are reported, including any follow-up. The main findings for each substudy are presented in Table 1.
| Study and assessment | Major result |
|---|---|
| 1A. Woodburn and Johnstone (18) Lothian cohort | |
| CAMDEX | Patients with alcohol-related dementia demonstrated improved functioning. |
| Clifton Assessment Procedures for the Elderly Behavior Rating Scale | |
| Manchester and Oxford Universities Scale for the Psychopathological Assessment of Dementia | |
| CSSD | |
| National Adult Reading Test | |
| Cognitive Section of the CAMDEX | |
| Brief Neurological Examination | |
| 1B. Woodburn and Johnstone (18) | |
| 1C. Woodburn and Johnstone (18) | |
| 2A. Panegyres and Frencham (15) | |
| Neuropsychology battery, including MMSE | Fifty-five patients had psychiatric diagnoses (depression, bipolar disorder, and anxiety disorders), with stable cognitive function and normal imaging. |
| Imaging (CT, MRI) | |
| Genetics | |
| 2B. Atkins et al. (14) Artemis cohort | |
| MMSE | FTD patients were more likely to have a family history of dementia, greater decline in MMSE and TFC scores, and a history of depression. |
| Global Deterioration Scale | |
| TFC | |
| Cardiovascular risk factors | |
| 2C. Atkins et al. (14) | |
| Cardiovascular risk factors: body mass index, abdominal girth, hypertension, hypercholesterolemia, peripheral vascular disease, statin and hormone replacement therapy use | AD patients were three times more likely to be receiving hormone therapy (3%); APOE ε4 allele: one copy. FTD patients were more likely to be current smokers and had a higher body mass index. |
| 3A. van Vliet et al. (17) NeedYD-study protocol | |
| NPI | Severity: moderate (N=76); moderately severe (N=53); and severe (N=29). |
| CANE | |
| Global Deterioration Scale | |
| Interview for Deterioration of Dementia Scale | |
| MMSE, Frontal Assessment Battery | |
| GRAD | |
| CSSD | |
| QoL-AD | |
| 3B. Bakker et al. (23) | |
| Qualitative case study of one caregiver: Results revealed prolonged time to diagnosis, lack of fit between needs and services, caregiver strain, and services that needed to change to suit the YOD caregiver. | |
| 3C. van Vliet et al. (27) | |
| Semistructured interviews; constant comparative analysis and grounded theory | Patients: Mean duration from symptom onset to diagnosis was 4.6 years. |
| 3D. van Vliet et al. (28) | |
| NPI | NeedYD-study: YOD patients comprised more males, higher education, longer disease duration, and more advanced dementia severity. |
| Global Deterioration Scale | NeedYD-study: NPS was less common overall in YOD, and apathy and aberrant motor behaviors were most common at study entry. |
| MMSE | LOD: Depression and apathy were most common. |
| 3E. Bakker et al. (23) | |
| Caregivers: Year of onset and type of symptoms | NeedYD-study: YOD mean time from symptom onset to admission to an institutional facility was 9 years compared with 4 years for LOD. Apathy was a significant predictor; patients received care at home for longer durations; more severe dementia was present; 33% of patients were in an institutional facility (compared with 44% of LOD patients). |
| NPI | Caregivers’ sense of competence was a significant predictor for both YOD and LOD patients. |
| Competence | Distress-related to NPS did not predict institutionalization. |
| Global Deterioration Scale | Spousal caregivers were less likely to place the patient in an institutional facility; male caregivers were more likely to place the patient in an institutional facility earlier than female caregivers. |
| 3F. van Vliet et al. (28) | |
| Semistructured interviews | Significant predictors for a longer time to diagnosis: Having a YOD; having FTD; |
| Global Deterioration Scale | Higher education and female gender; earlier recognition and help-seeking behavior. |
| MMSE | |
| 3G. Bakker et al. (25) | |
| RUD-Lite | Formal care was provided in 79.9% of cases, with a mean of 79.6 hours per month. |
| Formal services: respite, district nurse, meals on wheels, etc. | Multiple services used: day care (66%), home care (29.7%), and district nurse (7.2%). |
| Global Deterioration Scale | Informal care: 259.6 hours/month |
| Interview for Deterioration in Daily Living Activities in Dementia | Disease severity and increased behavioral problems were significantly associated with the number of formal care hours. |
| NPI | |
| Short Sense of Competence Questionnaire | |
| 3H. Bakker et al. (25) | |
| Patient: QoL-AD, CANE, NPI, Global Deterioration Scale, CSSD | Patients’ quality of life was associated with depression. |
| Caregiver: RAND–36, RUD-Lite, Short Sense of Competence Questionnaire | Caregivers rated their own quality of life lower than the general population; higher levels of pain and physical problems were present. |
| 3I. van Vliet et al. (8) | |
| GRAD | NeedYD-study: YOD patients comprised more males, higher education, and higher dementia severity. Higher level of awareness was present and associated with affective symptoms. |
| NPI | |
| Global Deterioration Scale | |
| 3J. Bakker et al. (24) | |
| Patient and caregiver: NPI, CANE | Areas of need did not change significantly after >2 years of follow-up. (Patients and caregivers reported 18% and 24% of unmet needs at baseline, respectively; 2 years later, this decreased by 8% and 10%, respectively.) |
| FTD patients had higher levels of NPS over time compared with AD patients. | |
| More severe dementia was present, and higher education was associated with increased levels of NPS. | |
| 3K. Millenaar et al. (43) | |
| Global Deterioration Scale | Three major variables were examined: impact of dementia on daily life, coping with the disease, and need for care and support. |
| 3L. Koopmans et al. (32) | |
| Global Deterioration Scale | Fifty-two percent of patients were prescribed at least one drug; 36.2% of patients were taking one drug. |
| Psychotropic drug use | Antidepressants (36.2%) and antipsychotics (17.3%) were most frequently prescribed; the most frequent combination was an antidepressant plus an antipsychotic. |
| NPI | Age and moderate to severe dementia were associated with the total use of drugs. |
| CSSD | No association between rates of NPS and drug use was observed. |
| GRAD | |
| CANE | |
| 3M. Gerritsen et al. (31) | |
| NPI | Progression of dementia was the same across three dementia types. |
| MMSE | AD patients demonstrated the steepest cognitive decline. |
| Global Deterioration Scale | Younger patients had more rapid decline in severity. NPI: Higher psychosis was associated with more cognitive decline; higher affective scores were associated with a slower course. |
| 4A. Hvidsten et al. (16) Nordic protocol | |
| Patients: EuroQoL-5D, QoL-AD | |
| CANE | |
| CSSD, MADRS | |
| Instrumental Activities of Daily Living | |
| Physical Self-Maintenance Scale (awareness) | |
| Reed scale for anosognosia | |
| Cognition: MMSE, clock drawing, Consortium to Establish a Registry for AD, Trail-Making Test, Boston Naming Test, Clinical Dementia Rating | |
| Caregivers: RUD-Lite, CANE, QoL-AD, MADRS, Geriatric Depression Scale, Relative Stress Scale, Locus of Control of Behavior | |
| Informant Questionnaire on Cognitive Decline in the Elderly | |
| 4B. Millenaar et al. (19) | |
| QoL-AD | FTD: Higher MMSE scores, less severe dementia, and more behavior symptoms were present. |
| GRAD: NeedYD | Lower quality of life: More severe depressive symptoms, lower disease awareness, and a higher level of needs were present (no differences were related to dementia severity, behavior symptoms, or activities of daily living). |
| Reed scale for anosognosia: Nordic | |
| 4C. Hvidsten et al. (21) | |
| EuroQoL-5D | Quality of life was higher in patients with YOD |
| QoL-AD | No differences in quality of life between AD patients and FTD patients were observed. |
| CSSD | |
| MADRS |
TABLE 1. Results of longitudinal studies of patients with younger-onset dementia
Screening and Data Extraction
Articles were downloaded using EndNote bibliographic software. Duplicates were removed, and researchers (S.L. and A.G.) double screened the remaining articles for inclusion eligibility. If there was disagreement, abstracts were checked by a third researcher (D.V.), and consensus regarding article retrieval was reached.
Results
Database and hand searches produced a total of 395 nonduplicated articles. Twenty-two studies that were related to four principal longitudinal YOD cohorts were identified (Table 1); of which, two research protocols were used. One of the four principal longitudinal cohorts was an Australian cohort, the Artemis cohort (14, 15); one was a Nordic cohort (16), which was the most recent cohort, with results published in 2014; one was the Needs in Young Onset Dementia (NeedYD-study) cohort, from the Netherlands (17); and one was a cohort from Lothian, Scotland (18), which was the earliest cohort, with results published in 1999. The Artemis cohort, examined in a study by Atkins et al. (14), included patients with a confirmed diagnosis of YOD (N=155), as well as an additional subgroup of patients from a larger group of patients who were referred for suspicion of a YOD (15). Three of the principal YOD cohorts had substudies, with the NeedYD-study cohort (17) yielding the most (N=12). One substudy (19) was derived from both the NeedYD-study and Nordic cohorts.
Sample sizes ranged from 126 study subjects (20) to 226 study subjects (15). Two studies had a 2-year follow-up (16, 17), and the longest duration of follow-up was 7 years (14). The Nordic cohort comprised only AD and FTD dementia types, as did the Atkins et al. substudy (14), whereas the remainder of the cohorts had a more inclusive etiology of YOD. Investigators for the more recent cohorts reported outcome measures at specified regular time points and included caregiver measures (16, 17).
Demographic Characteristics
Approximate mean or median age at onset of symptoms was reported for two cohorts (14, 15, 28), and the mean or median age at enrollment in the study was reported for the remaining cohorts. The percentage of males included in the cohorts ranged from 42.1% (18) to 55% (21). For the different types of dementias, there was a range of gender proportions. Risk factors for YOD, such as family history of dementia or psychiatric conditions, and cardiovascular risk factors were reported for one cohort only (22). Illness variables and demographic characteristics of the study subjects are presented in Table 2.
| Characteristic | Lothian study (18) | Panegyres et al. (15) | NeedYD-study (17) | Nordic (21) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age at symptom onset (years) | ||||||||
| AD | 59.3 | 5.2 | 53.8 | 6.0 | ||||
| FTD | 58.7 | 8.1 | ||||||
| VaD | 60.2 | 4.3 | ||||||
| ARD | 53.4 | 7.1 | ||||||
| MCI | 56.0 | 7.9 | ||||||
| Age at study entry (years) | ||||||||
| Male | 61.8 | 5.0 | ||||||
| Female | 60.2 | 5.9 | ||||||
| AD | 64.0 | |||||||
| FTD | 64.0 | |||||||
| N | % | N | % | N | % | N | % | |
| Male | 53 | 42.1 | 115 | 50.8 | 114 | 54.5 | 48 | 55 |
| AD | 25 | 42 | 15 | 51.7 | 57 | 57.2 | 25 | 50 |
| ARD | 8 | 57 | 5 | 83.3 | ||||
| VaD | 2 | 15 | 3 | 42.8 | ||||
| Mixed | 12 | 48 | ||||||
| FTD | 21 | 48.8 | 23 | 61 | ||||
| MCI | 5 | 45.4 | ||||||
| Frequency of AD | 60 | 52.6 | 29 | 12.8 | 119 | 56.9 | 50 | 56.8 |
| Frequency of bv-FTD | 36 | 15.9 | 28 | 13.4 | 38 | 43.1 | ||
| Frequency of lang-FTD | 7 | 3.1 | 13 | 6.2 | ||||
| Frequency of VaD | 13 | 13 | 7 | 3.1 | 24 | 11.5 | ||
| Frequency of ARD | 14 | 12.3 | 6 | 2.7 | ||||
| Frequency of DLB | 1 | 0.4 | 7 | 3.1 | ||||
| Frequency of other disorders | 27 | 23.7b | 16 | 7.1c | 25 | 11.9 | ||
TABLE 2. Demographic characteristics and etiology of dementia in the study participantsa
AD was the most frequent YOD, with a frequency of approximately 54% reported for three cohorts (17, 18, 21). The frequency of VaD was similar across two cohorts, at approximately 11.5% (17, 18). The frequency of bv-FTD ranged from around 14% (15, 17) to 43.1% (21).
Referral and Recruitment Source
Consecutive patients attending hospital clinics were frequently recruited (15, 17). For the Lothian and Artemis cohorts, the investigators sought to include all patients diagnosed with YOD in a specified time period in a specific area. The Lothian cohort was the only cohort for which patients with YOD living in residential care were included.
Caregiver Outcomes
Caregiver outcome measures were included for the Lothian, Nordic, and NeedYD-study cohorts; however, these measures have only been reported for the NeedYD-study cohort thus far. A variety of formal and informal care services were used. Greater disease severity and increased behavioral problems were associated with increased formal and informal (disease severity only) care hours, and better patient initiative was associated with fewer formal care hours (23). Caregivers supporting patients with a high number of unmet needs reported worse general health, higher levels of pain, and more physical problems (24). Over the course of 2 years, according to the caregivers, the areas of need from the patients did not change significantly (p=0.378).
Pathways to Diagnosis and Care
With regard to pathways to diagnosis and care, the NeedYD-study reported quantitative (25) and qualitative aspects (8, 26, 27). Having more years of education and being female were significant predictors for shorter time to final diagnosis, whereas depressive symptoms and mild cognitive impairment were associated with more than double the amount of time to first dementia diagnosis (25). A dementia diagnosis other than AD was also associated with a longer time to final diagnosis (8).
The major barriers to service provision for younger patients with YOD included lack of knowledge about YOD among primary care doctors and other specialists, which led to difficulty in obtaining a timely diagnosis (23, 27). There was a need for specialized and flexible services unique to patients with YOD and their family members (8, 26).
Regarding residential care, when comparing patients with LOD from all cohorts with the NeedYD-study cohort, apathy was a significant predictor for institutionalization in the NeedYD-study group. In both groups, caregiver report of a lower sense of competence in caring for the person with dementia was a significant predictor for institutionalization (8), whereas caregiver distress related to neuropsychiatric symptoms was not.
Neuropsychiatric Symptoms and Psychotropic Drug Use
In the NeedYD-study, the investigators reported on neuropsychiatric symptoms (28) and insight (29) longitudinally and compared these between the younger-onset and late-onset groups. The younger-onset group had lower prevalence, incidence, and persistence of these symptoms over the 2-year period. Apathy was the most frequently occurring neuropsychiatric symptom in both the younger-onset and late-onset groups (28). Over 1 year, younger-onset patients had better awareness, and this was associated with more depressive symptoms (29). Patients with behavioral variant FTD (bv-FTD) had overall worse neuropsychiatric symptoms over 2 years compared with patients with younger-onset AD (30). Cognitive function, measured with the Global Deterioration Scale, was associated with neuropsychiatric symptoms and type of YOD. Gerritsen et al. (31) reported that higher scores for psychosis and hyperactivity on the Neuropsychiatric Inventory (NPI) were related to a steeper deterioration in cognition, whereas higher affective NPI scores were associated with slower deterioration.
With regard to psychotropic usage reported by caregivers, antidepressants and antipsychotics were most frequently prescribed (36.2% and 17.3%, respectively). Increasing age and depressive symptoms were associated with increased total use of psychotropics (32).
Quality of Life
Quality of life for patients with YOD was reported in two substudies (19, 21). There were no significant differences in quality of life between patients with AD and bv-FTD. Factors associated with worse quality of life included depressive symptoms and met and unmet needs. Hvidsten et al. (16) compared quality of life for patients with YOD and patients with LOD and found that younger patients had a better quality of life. This substudy also suggested that for patients with AD, quality of life was associated with low awareness of disease, and for patients with bv-FTD, quality of life was associated with high awareness.
Progression of Dementia
The progression of dementia over the follow-up period was reported for three cohorts (14, 18, 31). For the Lothian cohort, the investigators found that patients with alcohol-related dementia (ARD) improved their cognition after 12 months (20). There were two contrasting findings. Greater cognitive decline (using the Mini-Mental State Examination [MMSE]), poorer functioning, and poorer survival rates in the bv-FTD group were reported for the Artemis cohort compared with findings from one of the NeedYD substudies (31), which revealed that patients with AD had the greatest cognitive decline when compared with bv-FTD and VaD patients (using the MMSE and Global Deterioration Scale; AD: 2.3 mean point decline, p<0.046). The NeedYD substudy also highlighted that younger age and lower education levels contributed to steeper cognitive decline.
Genetics, Imaging, and Other Biomarkers
Imaging was reported only for substudies related to the Panegyres et al. longitudinal study (15). For the Artemis cohort, Atkins et al. (14) reported that among the 92 patients with AD in their study, white matter hyperintensities were identified in 10, and only one had small or large vessel ischemia. In addition, none of the 63 patients with bv-FTD had white matter hyperintensities or small- or large-vessel ischemia. Serial imaging was not reported. The Artemis cohort is the only cohort for which genetic contributions to YOD, such as the apolipoprotein E gene, were reported.
Discussion
In this rapid review, we reported on four longitudinal YOD cohorts and their associated substudies. Cohorts that included a range of different types of YOD were included to enable comparisons between the different types in terms of neuropsychiatric symptoms, cognitive decline, changes in imaging and biomarkers, and caregiver mental health. Although these cohort studies provide important and much-needed evidence in the field of YOD, there were several inconsistencies with regard to demographic variables and progression of dementia that limit comparisons, pooling of data, and generalizability.
First, there were differences with reporting of age. Age at onset of symptoms was reported for only two cohorts (14, 15, 28), and age at study inclusion was reported for the remaining cohorts. Age at onset arguably is a more accurate and consistent method of reporting, because there can be delay in presentation to services and diagnosis (4, 5). In the epidemiological studies, age at onset was reported for all types of YOD combined. Ikejima et al. (33) reported a mean age at onset of 53.4 years (SD=7.9), and Withall et al. (3) reported a mean age at onset of 55.0 years (SD=9.5). The NeedYD-study reported a mean age at onset comparatively similar to that of these epidemiological studies (mean=54.8 [SD=5.9]). In contrast, Panegyres et al. (15) reported age at onset for the different types of dementia rather than for all types of YOD combined, and thus it was difficult to make comparisons between this cohort and the epidemiological studies.
The proportion of males reported in the included cohorts varied depending on the type of YOD. Overall, the NeedYD-study and Nordic cohorts had similar proportions of males with YOD (55%). Previous epidemiological studies reported sex differences in YOD. In the Harvey et al. (2) study, 58% of patients with YOD were male, and a similar percentage (59.2%) was reported in the study by Ikejima et al. (33). Garre-Olmo et al. (34) and Withall et al. (3) found no significant difference between males and females, which was also reported in the Panegyres et al. study (15). It remains unclear whether biological sex is a risk factor for YOD, which is a similarly unanswered question regarding LOD (35).
Second, it is difficult to ascertain the true frequency of dementia subtypes across a mixed, unselected clinical cohort. The Lothian cohort included most types of YOD (AD, VaD, ARD, and mixed), with AD being the most common type (52.6%). It is interesting that while not specifically excluded, there were no cases of Pick’s disease/FTD. Similarly, Panegyres et al. (15) asserted that they included all people referred for suspicion of a YOD in Western Australia but found a much lower frequency of AD (12.8%) than what was found in the Lothian cohort. The NeedYD-study included the main types of YOD (AD, FTD, and VaD) and identified AD as being the most common type (56.9%). In the three epidemiological studies, different types of YOD were reported as most prevalent: AD (34%) (2), VaD (42.5%) (in the Japanese study) (33), and ARD (22%) (3). Based on the data from these cohorts, the community frequency of various types of YOD remains unknown, which suggests that there are differences in geography, complexity of cases, and possible referral biases. This reiterates the challenge of diagnosing a YOD despite contemporaneous consensus criteria.
Third, there were inconsistencies pertaining to the progression of and survival rates with YOD, with both AD and bv-FTD reported to result in the greatest cognitive decline over time in different cohorts. The reasons for this discrepancy remain unclear. Previous studies have indicated that lower baseline cognition and lower education are potential factors for steeper decline and progression (31). In the Gerritsen et al. (31) substudy, patients with AD had the lowest MMSE scores (mean=17.6 [SD=7.2]) as well as the least years of education compared with patients with VaD and FTD. However, in the Atkins et al. substudy, there was no significant difference in baseline MMSE scores (mean=21 [SD=7]) and years of education (11 years) between the two groups. In this Atkins et al. substudy, patients were followed for up to 7 years, and thus the investigators were able to assess MMSE scores, functioning, and stage of dementia for a much longer duration than in the Gerritsen et al. study (31). Thus, using these two substudies, bv-FTD appears to progress more rapidly compared with AD. More studies are required to corroborate or refute this finding.
One of the more consistent findings from all of the cohorts reviewed in the present article is related to patient quality of life and caregiver outcomes. Quality of life in patients with YOD was explored in two cohorts, comparing younger patients with AD and FTD and patients with LOD. The finding that higher quality of life in AD is associated with low awareness has been reported previously in studies of people with late-onset AD (36). However, the seemingly counterintuitive finding that higher quality of life in FTD is associated with high awareness may be explained by patients’ earlier loss of insight into their illness correlating with fewer depressive symptoms (and hence higher quality of life) and that people with FTD tend to self-rate their own quality of life higher than people with other types of dementia do (37). Having more depressive symptoms was associated with worse quality of life, regardless of age and diagnosis.
The NeedYD substudies provided the most information about caregivers of patients with YOD. The combined literature described most consistently that caregivers were providing a lot of support to their loved ones with dementia, which required a combination of informal and formal care services (27). Service experience issues were reported as a lack of clear diagnostic and assessment pathways, a lack of psychoeducation, and a lack of specific services for younger people (8, 27). Neuropsychiatric symptoms were commonly seen in YOD (but were less frequent compared with LOD), with apathy being a risk factor for institutionalization (8, 28). Although these substudies originated from the original NeedYD-study cohort, these results are similar to those from other studies of caregivers of patients with YOD (38). From these four cohorts, including the NeedYD substudies, there was no information regarding caregiver outcomes in relationship with neuropsychiatric symptoms or cognition in YOD.
Methodological Overview and Limitations
The cohorts varied in terms of the variables described below.
Follow-up: The study by Panegyres et al. (15) and related substudies were characterized by longer periods of follow-up but arguably with less robust frequency of assessments compared with the latter two cohorts (NeedYD and Hvidsten et al. cohorts) and there were no caregiver measures. For the NeedYD (17) and Hvidsten et al. (16) study cohorts, the investigators used a higher number of reliable and valid outcome measures administered at 6-month intervals and included information about caregivers and families, both qualitatively and quantitatively. However, data on imaging and biomarkers were not included for these cohorts, and follow up of cohort members was conducted for a relatively shorter time compared with the earlier studies. This limits some findings, such as results for cognitive progression and survival rates. This is important information to provide to patients with YOD and their families.
YOD etiology: There was variation in the types of dementia that were included in the cohort studies and how diagnoses were made. For the NeedYD cohort, the following dementia types were excluded: dementia caused by traumatic brain injury, dementia related to HIV-AIDS and HD, dementia related to alcohol and substance misuse, FTD and motor neuron disease, and intellectual disability. The inclusion criteria for the Nordic cohort were more restrictive, with inclusion of patients only with dementia caused by bv-FTD and AD. Thus, the generalizability of these results may be restricted. Standard diagnostic criteria that were current for the different types of dementia were used for all cohorts. However, for the Lothian cohort, neurology and psychiatry records were used to identify cases of YOD during 1988 and 1993. During this time, the ICD-9 code for Pick’s disease (ICD code 333.1) was used, and the first consensus criteria for FTD (Lund Manchester criteria in 1994) had not yet been established. It is noteworthy that patients with Pick’s disease/FTD or patients with dementia caused by HD or other medical conditions were not recorded as being included in this cohort. In addition to consensus diagnostic criteria, additional evidence, such as [18F]fluorodeoxyglucose-positron emission tomography (PET), amyloid-PET, or CSF results, should be used to diagnose dementia type if possible. Panegyres et al. (15) stated that in addition to genetic analysis, they used structural and functional imaging as it became available to support their diagnoses of dementia. They were also able to confirm the etiology of dementia via autopsy for patients who died during the study period. Due to the longer follow-up period, this is an advantage in confirming a diagnosis compared with using consensus criteria only.
Recruitment: Almost all of the cohorts comprised only community-dwelling patients with YOD; only the Lothian cohort comprised patients with YOD living in residential care. For the Lothian cohort and in the Panegyres et al. (15) study, all patients with YOD were recruited during the study period, which is more representative and generalizable. Apart from the Lothian cohort, the majority of patients were recruited through convenience sampling (self-selected, consecutive patients attending clinic), which means that participants were potentially likely to be higher functioning, willing to participate in the extra burden of research, and not necessarily representative of the general YOD population. These studies were all conducted in developed countries, suggesting a cultural bias and lack of generalizability of results. We know little about YOD in lower-income and developing countries.
Measures/assessment tools: For the three cohorts for which information on cognitive decline was provided (15–17), the investigators used the MMSE, a brief screening tool that is limited regarding how executive functioning is assessed (39). A more valid and reliable cognitive screening tool, such as the Addenbrooke’s Cognitive Examination–Revised (40) or the Neuropsychiatry Unit Cognitive Assessment Tool (41), with the addition of formal neuropsychological assessment, would provide more comprehensive measures of cognitive decline. Many of the assessments used in these cohorts and substudies have been widely used for patients with LOD and their caregivers. It is possible that these measures are not appropriate for patients with YOD and their caregivers. Thus, the development of specific measures for YOD could be the focus of future research.
What Can We Tell Our Patients With YOD and Their Families?
Despite these differences, the data that these cohorts provide have yielded useful insights on the real-life scenario in YOD. We can inform our patients and their families that bv-FTD (compared with AD) has possibly a faster progression in terms of cognitive decline, functioning, and worse neuropsychiatric symptoms, including apathy. Ensuring that depressive symptoms are managed appropriately may help patients to maintain a relatively reasonable quality of life. Certain neuropsychiatric symptoms (psychosis and hyperactivity) may also be associated with faster deterioration of cognition. Knowledge of challenges regarding pathways to care and services means that we should be clear and specific about to whom patients can be referred for ongoing care and that facilitating this process is important.
Although these cohorts and related substudies have yielded some important information pertaining to cognitive changes in YOD, changes in neuropsychiatric symptoms, and how caregivers cope, correlating these together, as well as linking neuropsychiatric symptoms or cognitive changes with imaging and biomarkers, has yet to be examined. These data could help with early diagnosis and predict progression of the disease. More timely diagnosis of YOD with a specific etiology was shown to be highly important by Sansoni et al. (11), particularly because some YODs are reversible and preventable (e.g., HIV-related dementia). Having more information about the trajectory of the different types of YODs, in terms of prognosis, can enable planning with regard to residential care preparations and strategies to manage neuropsychiatric symptoms. In addition, if it is known that a patient with YOD has imaging changes that correlate with particular neuropsychiatric symptoms, the caregiver can be provided with interventions to manage these symptoms.
Recommendations
We acknowledge the limitations of a rapid review compared with a systematic review. For example, our search may be arguably less comprehensive, but from a clinical perspective, a strength of reviewing cohorts that had a range of YOD etiology reflects the real-life scenario. Conversely, not reviewing studies of cohorts that focused solely on one YOD means that other valuable information (such as symptom and cognitive progression) regarding the individual types of YOD was not evaluated in this review. The NeedYD and Nordic cohorts specifically excluded certain types of YOD, such as HD and dementia caused by multiple sclerosis. We do not know the reasons for this exclusion. It may be because the institutions that conducted these studies do not frequently see patients with the excluded YODs or because these YODs have a comparatively lower prevalence. In addition, although HD is rare, there are already well-established HD cohorts (e.g., PREDICT-HD and Enroll-HD), which is possibly another explanation of why HD was excluded.
Future research in YOD should focus on longitudinal follow-up, with the type of dementia based on consensus diagnostic criteria and additional evidence of neuroimaging and biomarkers (including CSF and blood). Severity of dementia, measure of neuropsychiatric symptoms, and detailed assessment of cognition and functioning should also form part of the assessment. From a research perspective, it has been recommended that biomarkers and other measures should be repeated at least twice, and earlier and more frequent measures might facilitate conclusions about the validity and reliability of particular biomarkers (42). For caregivers and family members, their mental health and burden, as well as pathways to care and diagnosis, could also be included. Streamlining assessment and reporting measures across YOD cohort studies would help with generalizability and drawing conclusions about results. Our recommended measures are outlined in Table 3.
| Measure | Patients with YOD | Caregivers |
|---|---|---|
| Diagnosis | Consensus criteria plus neuroimaging and biomarkers | |
| Neuropsychiatric symptom | Neuropsychiatric Inventory, Cambridge Behavior Inventory | Completed by caregiver |
| Dementia severity | Global Deterioration Scale; Clinical Dementia Rating | |
| Cognition | Neuropsychiatry Unit Cognitive Assessment; Addenbrooke’s Cognitive Examination-Revised | |
| Neuropsychological testing standard batteryb | ||
| Neuroimaging | Structural: MRI brain; functional: fluorodeoxyglucose-PET, amyloid PET | |
| Other biomarker | Cerebrospinal fluid measures of aβ, total-tau and phosphorylated-tau (others: neurofilament) | |
| Mental health | DASS21 | DASS21; Geriatric Depression Scale; Montgomery-Åsberg Depression Rating Scale |
| CSSD | ||
| Caregiver burden | Zarit Burden Inventory; Caregiver Burden Inventory | |
| Quality of life | Quality of Life Alzheimer’s Disease scale; WHOQOL-BREF, EuroQoL-5 dimension | WHOQOL-BREF, EuroQoL-5D |
| Function/dependence | RAND; Dependence scale; Independent Activities of Daily Living | |
| Service usage | Resource Utilization-Lite | |
| Other | Autopsy |
TABLE 3. Recommended measures for patients with younger-onset dementia (YOD) and their caregiversa
More information is required to allow for focused planning and implementation of dedicated services for patients with YOD. Studies that combine the strengths of both early and recent cohorts are needed, with a longer follow-up time, regular valid assessments of patients and their caregivers, and across a number of broad etiologies of YOD, including for patients in residential care. We also recommend including caregiver health information and pathways to care and diagnosis, as well as genetics, longitudinal imaging, and biomarkers, including CSF biomarkers, cognitive profile, and the use of validated and reliable outcome measures. These will assist in providing more information about early diagnosis, progression of the disease, symptoms, and the needs of patients and caregivers.
1 : The diagnosis of young-onset dementia. Lancet Neurol 2010; 9:793–806Crossref, Medline, Google Scholar
2 : The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry 2003; 74:1206–1209Crossref, Medline, Google Scholar
3 : The prevalence and causes of younger onset dementia in Eastern Sydney, Australia. Int Psychogeriatr 2014; 26:1955–1965Crossref, Medline, Google Scholar
4 : Time to diagnosis in young-onset dementia and its determinants: the INSPIRED study. Int J Geriatr Psychiatry 2016; 31:1217–1224Crossref, Medline, Google Scholar
5 : Natural history of frontotemporal dementia: comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord 2004; 17:253–257Crossref, Medline, Google Scholar
6 : Younger people with dementia: diagnostic issues, effects on carers and use of services. Int J Geriatr Psychiatry 1998; 13:323–330Crossref, Medline, Google Scholar
7 : The impact of young onset dementia on the family: a literature review. Int Psychogeriatr 2011; 23:356–371Crossref, Medline, Google Scholar
8 : Time to diagnosis in young-onset dementia as compared with late-onset dementia. Psychol Med 2013; 43:423–432Crossref, Medline, Google Scholar
9 : Younger onset dementia: developing a longitudinal model as the basis for a research agenda and as a guide to interventions with sufferers and carers. J Adv Nurs 1994; 19:659–669Crossref, Medline, Google Scholar
10 : Early-onset dementia is associated with higher mortality. Dement Geriatr Cogn Disord 2008; 26:147–152Crossref, Medline, Google Scholar
11 : Younger onset dementia. Am J Alzheimers Dis Other Demen 2016; 31:693–705Crossref, Medline, Google Scholar
12 : The clinical assessment of the patient with early dementia. J Neurol Neurosurg Psychiatry 2005; 76(Suppl 5):v15–v24Crossref, Medline, Google Scholar
13 : A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009; 26:91–108Crossref, Medline, Google Scholar
14 : The natural history of early-onset dementia: the Artemis Project. BMJ Open 2012; 2:e001764Crossref, Medline, Google Scholar
15 : Course and causes of suspected dementia in young adults: a longitudinal study. Am J Alzheimers Dis Other Demen 2007; 22:48–56Crossref, Medline, Google Scholar
16 : Young onset dementia: a prospective cohort of quality of life and specific needs in persons with young onset dementia and their families. J Clin Trials 2014; 5:1–6Google Scholar
17 : Research protocol of the NeedYD-study (Needs in Young onset Dementia): a prospective cohort study on the needs and course of early onset dementia. BMC Geriatr 2010; 10:13Crossref, Medline, Google Scholar
18 : Measuring the decline of a population of people with early-onset dementia in Lothian, Scotland. Int J Geriatr Psychiatry 1999; 14:355–361Crossref, Medline, Google Scholar
19 : Determinants of quality of life in young onset dementia: results from a European multicenter assessment. Aging Ment Health 2017; 21:24–30Crossref, Medline, Google Scholar
20 : Early-onset dementia in Lothian, Scotland: an analysis of clinical features and patterns of decline. Health Bull (Edinb) 1999; 57:384–392Medline, Google Scholar
21 : Quality of life in people with young-onset Alzheimer’s dementia and frontotemporal dementia. Dement Geriatr Cogn Disord 2018; 45:91–104Crossref, Medline, Google Scholar
22 : Cerebrovascular risk factors in early-onset dementia. J Neurol Neurosurg Psychiatry 2012; 83:666–667Crossref, Medline, Google Scholar
23 : The use of formal and informal care in early onset dementia: results from the NeedYD study. Am J Geriatr Psychiatry 2013; 21:37–45Crossref, Medline, Google Scholar
24 : Unmet needs and health-related quality of life in young-onset dementia. Am J Geriatr Psychiatry 2014; 22:1121–1130Crossref, Medline, Google Scholar
25 : Predictors of the time to institutionalization in young- versus late-onset dementia: results from the Needs in Young Onset Dementia (NeedYD) study. J Am Med Dir Assoc 2013; 14:248–253Crossref, Medline, Google Scholar
26 : Needs in early onset dementia: a qualitative case from the NeedYD study. Am J Alzheimers Dis Other Demen 2010; 25:634–640Crossref, Medline, Google Scholar
27 : Caregivers’ perspectives on the pre-diagnostic period in early onset dementia: a long and winding road. Int Psychogeriatr 2011; 23:1393–1404Crossref, Medline, Google Scholar
28 : Prevalence of neuropsychiatric symptoms in young-onset compared to late-onset Alzheimer’s disease, part 1: findings of the two-year longitudinal NeedYD-study. Dement Geriatr Cogn Disord 2012; 34:319–327Crossref, Medline, Google Scholar
29 : Awareness and its association with affective symptoms in young-onset and late-onset Alzheimer disease: a prospective study. Alzheimer Dis Assoc Disord 2013; 27:265–271Crossref, Medline, Google Scholar
30 : The relationship between unmet care needs in young-onset dementia and the course of neuropsychiatric symptoms: a two-year follow-up study. Int Psychogeriatr 2014; 26:1991–2000Crossref, Medline, Google Scholar
31 : The progression of dementia and cognitive decline in a Dutch 2-year cohort study of people with young-onset dementia. J Alzheimers Dis 2018; 63:343–351Crossref, Medline, Google Scholar
32 : Prevalence and correlates of psychotropic drug use in community-dwelling people with young-onset dementia: the NeedYD-study. Int Psychogeriatr 2014; 26:1983–1989Crossref, Medline, Google Scholar
33 : Prevalence and causes of early-onset dementia in Japan: a population-based study. Stroke 2009; 40:2709–2714Crossref, Medline, Google Scholar
34 : Incidence and subtypes of early-onset dementia in a geographically defined general population. Neurology 2010; 75:1249–1255Crossref, Medline, Google Scholar
35 : Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 2016; 18:437–446Crossref, Medline, Google Scholar
36 : Insight, cognition and quality of life in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2010; 81:331–336Crossref, Medline, Google Scholar
37 : The determinants of quality of life of nursing home residents with young-onset dementia and the differences between dementia subtypes. Dement Geriatr Cogn Disord 2017; 43:320–329Crossref, Medline, Google Scholar
38 : Why aren’t people with young onset dementia and their supporters using formal services? results from the INSPIRED study. PLoS One 2017; 12:
39 : The Mini-Mental State Examination: pitfalls and limitations. Pract Neurol 2017; 17:79–80Crossref, Medline, Google Scholar
40 : The Addenbrooke’s Cognitive Examination (ACE) in the differential diagnosis of early dementias versus affective disorder. Am J Geriatr Psychiatry 2005; 13:218–226Crossref, Medline, Google Scholar
41 : The NUCOG: validity and reliability of a brief cognitive screening tool in neuropsychiatric patients. Aust N Z J Psychiatry 2006; 40:995–1002Crossref, Medline, Google Scholar
42 : A systematic review of longitudinal studies which measure Alzheimer’s disease biomarkers. J Alzheimers Dis 2017; 59:1359–1379Crossref, Medline, Google Scholar
43 : The experiences and needs of children living with a parent with young onset dementia: results from the NeedYD study. Int Psychogeriatr 2014; 26:2001–2010Crossref, Medline, Google Scholar



