Developing Precision Invasive Neuromodulation for Psychiatry
Abstract
Psychiatric conditions are common and often disabling. Although great strides have been made in alleviating symptoms with pharmacotherapy and psychotherapeutic approaches, many patients continue to have severe disease burden despite the best therapies available. One of the pervasive challenges to improving treatment is that present diagnostic and therapeutic strategies lag behind our modern conceptualization of the pathophysiology of these disorders. Psychiatric symptoms manifest through activity in specific neural circuits; thus, therapies capable of modulating these circuits are attractive. The investigators reviewed recent advances that facilitate treating medically refractory psychiatric disorders with intracranial neuromodulation in a way that intervenes more directly with the underlying pathophysiology. Specifically, they reviewed the prospects for using intracranial multielectrode arrays to record brain activity with high spatiotemporal resolution and identify circuit-level electrophysiological correlates of symptoms. A causal relationship of circuit electrophysiology to symptoms could then be established by modulating the circuits to disrupt the symptoms. Personalized therapeutic neuromodulation strategies can then proceed in a rational manner with stimulation protocols informed by the underlying circuit-based pathophysiology of the most bothersome symptoms. This strategy would enhance current methods in neurotherapeutics by identifying individualized anatomical targets with symptom-specific precision, circumventing many of the limitations inherent in modern psychiatric nosology and treatment.
Many brain disorders are conceptualized within the framework of dysfunctional neural circuits giving rise to pathology, either in the form of behavioral or experiential symptoms. It is well established that a detailed understanding of neuronal circuits that underlie pathophysiology can be leveraged for highly specific and effective therapies. For example, a circuit-based understanding of the motor system has informed the development of highly effective therapies such as deep brain stimulation (DBS) for Parkinson’s disease, dystonia, and essential tremor. Detailed mapping of circuits that underlie psychiatric disorders has been more challenging. In the present article, we discuss some of the reasons for this, along with some technological developments that may facilitate improvements in mapping the causal circuitry of psychiatric disorders, which can lead to more effective targets for neuromodulation. In addition, we focus on depression as a common and representative psychiatric disorder; ultimately, however, we feel the methods discussed here could be applied more broadly.
As a field, psychiatry has lagged in the development of objective markers of disease that relate directly to the underlying pathophysiology. Depression is a representative example: although numerous biological correlates and contributing factors to depression symptomatology have been identified, a unifying pathophysiology is lacking (1, 2). Depression can have numerous routes of origin and varying levels of contribution from genetics, the environment, and the interaction between the two. Ultimately, however, the final pathway that mediates the expression of symptoms is through neural circuits (3). A neural circuit includes multiple discrete processing nodes that may be distributed across different levels of the central nervous system and connected by white matter pathways spanning the brainstem, diencephalon, striatum, cerebellum, and cerebral cortex (4). A circuit-based understanding of brain function is informed by tremendous advances in neuroscience that have occurred over the last several decades and include techniques like optogenetics (5), voltage sensitive dyes (6), and simultaneous recording from multiple brain areas (7, 8). A detailed understanding of neuronal circuits offers the potential for highly specific interventions. Progress to date has been primarily restricted to laboratory animals, although promising human work is gaining momentum (9). Nonetheless, our scientific understanding has yet to translate into clinical tools approaching the spatiotemporal scale relevant for measuring circuit-based pathophysiology or manipulating the function of circuits in a rational way to address underlying symptoms on an individual patient level.
Current Diagnostic Strategy
At present, there are no objective markers of pathophysiology in common clinical use that can aid in a circuit-based diagnosis of disorders such as depression. Available methods for the noninvasive measurement of brain circuit physiology, such as structural MRI, functional MRI (fMRI), or high-density EEG are currently unable to capture the dynamic function of brain circuits at the millimeter and millisecond level of resolution. Without this resolution, measurements capable of elucidating causal circuit-based mechanisms of neuropsychiatric symptoms are likely to remain elusive. Until we develop diagnostic tools that directly relate to the pathophysiology of the disorders, we will remain constrained to diagnosing illness via checklists of primarily subjective symptoms. Depression is one common diagnosis that illustrates how diverse presentations may exist within the same diagnostic category. DSM-5 requires a patient to endorse at least five of nine possible symptoms, leading to 126 unique combinations (10). As a result, illness may vary substantially between two patients—at times to opposite extremes, such as insomnia versus hypersomnia, increased versus decreased appetite, or increased versus decreased psychomotor activity.
Current Treatment
Despite the heterogeneity of symptoms that may contribute to depression and other psychiatric disorders, treatments are typically delivered empirically based on the diagnosis. Treatment options often start with medications and psychotherapy. About one-half to two-thirds of patients do not achieve remission following first-line treatment (11), and one-third remain resistant after multiple medication trials (12). ECT uses electric current to induce a brief generalized seizure. It was developed almost 100 years ago but continues to be regarded as the most efficacious treatment available for severe depression (13), though it is used in <1% of patients because of continued stigma and significant cognitive side effects, such as autobiographical memory loss (14, 15). While the specific therapeutic mechanism is not understood, ECT by nature causes global changes across numerous circuits in the brain (16). Transcranial magnetic stimulation (TMS) is a newer therapy that involves daily application of a milder, magnetically induced electric current to the left prefrontal cortex; it is helpful for about half of patients who do not respond to medication (17). A third method of brain stimulation for depression involves DBS, where electrodes are surgically implanted in the brain. DBS has been highly effective for a subset of patients (18), but industry-sponsored clinical trials (Reclaim and BROADEN) attempting to use the same stimulation site for every individual have been negative and stopped early after futility analyses (19, 20). DBS is largely static in nature, delivered continuously at high frequency to a small volume of tissue surrounding one or two implanted wires that each contain four electrode contacts. Challenges in optimizing invasive neuromodulation are not unique to depression. Studies of DBS for obsessive-compulsive disorder (OCD) have varied on probe placement and treatment response rates (21–24). Furthermore, methods for predicting individual likelihood of response are limited. While DBS is an intervention capable of modulating circuitry with precision (25), the use of a one-location-for-all, constant-stimulation approach does not benefit from individualized electrophysiological correlates of symptoms that could be leveraged to guide personalized stimulation protocols (Figure 1). Especially given the heterogeneity and episodic nature of depression and other psychiatric disorders, a more flexible, patient-centered approach to personalize the stimulation protocol is likely to improve efficacy.
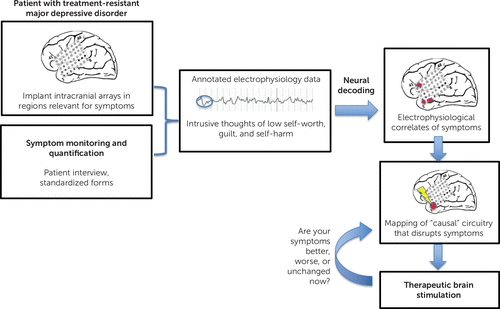
FIGURE 1. Invasive neuromodulation treatment outlinea
a With major depressive disorder as an example, patients with a treatment-resistant course would have electrode arrays implanted intracranially in predetermined regions relevant for current symptoms. They would undergo symptom monitoring and quantification while data from electrodes were continuously recorded. Data would be annotated by the patient when specific intrusive thoughts or worsening symptoms occurred. Neural decoding would occur via machine-learning algorithms to correlate electrophysiological data with ongoing symptoms. Stimulation would then be delivered; if symptoms improved, it would be inferred that a casual neural circuit was identified. Therapeutic brain stimulation could then be delivered in a specific circuit-driven way. The closed-loop nature of this would allow for cycling back to mapping of causal circuitry, thereby improving the stimulation delivered as symptoms change. For a color version of the figure, see the online edition of this article.
While current therapies are helpful for many, they are not informed by the patient’s unique circuit-level pathophysiology. After failing these options, patients are considered refractory to treatment and are often resigned to long-term hardship and disability or increasingly futile medication trials with high side-effect burden. We believe that many developments in the fields of neurosurgery, intracranial electrophysiology, and neuromodulation will provide a new strategy for treating psychiatric disorders refractory to the aforementioned treatments. We describe what these developments are and begin to outline a pathway toward applying these approaches in psychiatry.
Responsive Neurostimulation for Epilepsy as a Model
Currently, the closest model for delivering therapeutic neuromodulation that targets circuit pathology causally related to one’s individualized symptoms is responsive neurostimulation for focal-onset seizures. Carefully chosen patients with focal-onset seizures that have not responded to medications or other conventional therapies can be treated with NeuroPace, an implantable neuro-stimulator (26). First, noninvasive methods are used to localize the seizure onset. This informs the target location for the surgical implantation of intracranial electrodes. These intracranial surface or depth electrodes are used to record neural activity continuously. The data can be transferred wirelessly to an external computer or cloud-based network. Any seizure that occurs can be annotated in a time-locked manner to the recording. After several seizures have been captured, the intracranial recording data can be analyzed to detect electrophysiological features that precede the onset of the seizure. This process, known as neural decoding, often uses machine-learning algorithms to detect electrophysiological features that predict the onset of a seizure. Once enough data have been acquired to accurately predict an upcoming seizure, the device can be programmed to make use of this information to deliver stimulation to disrupt the development of the seizure. The stimulation is delivered via the same electrodes that identify the electrophysiological features preceding the seizure, supporting a causal relationship between the recording and the seizure onset. This system is individualized and adaptive to one’s unique symptoms and simultaneously records and stimulates at a scale congruent with the circuit-based pathophysiology that underlies epilepsy.
Responsive neurostimulation was first approved by the U.S. Food and Drug Administration in 2013; much of the postapproval longitudinal studies evaluating treatment efficacy have been done by the companies themselves. They show that median seizure reduction is 65.7% at 5.4 years and 75% at 9 years (N=191 and N=168, respectively) (27, 28).
Applications in Psychiatry
This general approach to treating epilepsy offers a model from which to develop similar applications for psychiatric indications. Rather than mapping the origin of a seizure, the focus would be on mapping a circuit causally related to a patient’s most disabling symptoms. These circuit-based symptoms may include positive and negative valence, affect, anxiety, obsession, fear, or pain. Once mapped, intervention would occur at the circuit level in a highly personalized way. While technological advances are needed, there is already promising work in this area.
Intracranial Recording
One of the early challenges to overcome in developing precision intracranial neuromodulation is gaining broader spatial coverage than would be allowed with the eight electrodes currently used for NeuroPace in epilepsy. We have a general sense of the areas that population-based studies have implicated in the pathophysiology of specific psychiatric disorders, such as the cingulate cortex, nucleus accumbens, and medial prefrontal and orbitofrontal cortex. These are regions with anatomical heterogeneity at the single-subject level (29). Neural activity in several of these regions could be recorded simultaneously to maximize the probability of identifying the causal circuitry associated with specific symptoms. The use of noninvasive imaging modalities such as fMRI or high-density EEG may further inform the coverage area in an individualized way.
There is no inherent limitation to the number of electrodes from which one could record. When patients with medication-refractory epilepsy are admitted for intracranial monitoring to identify a seizure focus, it is common to record 100–250 contacts simultaneously. However, this intracranial monitoring is limited to 1 or 2 weeks in most cases. The safety and feasibility of long-term electrode implantation requires additional investigation. Developments in intracranial recording equipment may change the landscape in the coming years. Mesh electronics are syringe-implantable probes with a macroporous structure and stiffness comparable to the brain (30). They evoke minimal immune response and have shown long-term stability of neural recording in animal models (31). The size of the probes is comparable to a neuron cell body, and they have recently been implanted in a mouse brain through a 100 µm inner diameter needle (32). Intracranial recordings have the potential for millimeter spatial precision and millisecond temporal resolution and may have improved signal to noise over conventional EEG. Such approaches provide the ability to directly record brain activity while causing minimal disruption to the surrounding tissue and may provide a feasible mechanism for long-term intracranial monitoring.
Linking Symptoms to Neurophysiology
The goal of recording from intracranial electrodes is to identify the electrophysiological features that correspond to the patient’s most disabling psychiatric symptoms. Patients need a way to annotate the intracranial recording with a label for what they are subjectively experiencing. These experiences could range from panic attacks, obsessions, pain, insomnia, and suicidal thoughts to negative rumination. Each example would have its own time course, and this could be incorporated into the method of querying symptoms. Panic attacks, for example, are highly discrete; the method of annotating the electrophysiological recording would mirror that used for seizures with NeuroPace. Other symptoms that occur on a more continuous, fluctuating scale would likely require a combination of approaches. These may include spontaneous reporting of distressing symptoms integrated with prompted periodic monitoring of subjective symptom severity on a continuous scale (e.g., rate mood on scale from 1 to 10) (33).
A patient’s ability to easily record when symptoms occur relative to the ongoing recording is critical for accurately decoding electrophysiological correlates to the symptoms. Accurate temporal relation could be accomplished through mechanisms such as a voice-activated monitor the patient wears or a discrete wearable device the patient could activate by tapping. Through use of a keyword or phrase, the intracranial recording would be seamlessly annotated for later decoding. This information could be corroborated by ancillary data when relevant, such as autonomic measures that may change from baseline in the setting of a panic attack or facial features and vocal patterns that may change in the setting of fluctuations in emotion. The duration of recording would vary depending on the frequency of symptoms. If a patient had hundreds of intrusive thoughts daily on the same subject, enough data could likely be gathered within the same hospitalization as the electrode implantation to decode the electrophysiological correlates. This contrasts with infrequent panic attacks, which may require longer term outpatient intracranial recording.
Decoding the Electrophysiological Correlates of Symptoms
Once an adequate sampling of the symptoms of interest has been acquired with annotated electrophysiological data, the next step will involve neural decoding to extract meaning from the data. Neural decoding typically involves the application of machine-learning algorithms to identify consistent features in the electrophysiological data that correspond to a behavior or outcome of interest. A common approach is to generate an adequate amount of data to train a model and then test the accuracy of the model on data that were withheld from the training. Current intracortical electrodes are already able to record with submillisecond precision, and machine-learning algorithmic approaches can also extract electrophysiological correlates that occur at much longer time scales (34). Much of the methods development of these decoding approaches has occurred with easily measurable phenomena, such as movement (35–37); however, advances have been seen in areas of memory (38), speech (39, 40), and prosthesis control (41, 42).
Recent studies have also demonstrated utility with subjective symptoms directly relevant to psychiatry. Sani et al. (43) used intracranial EEG to decode mood that was predictive at both the patient and group level. While the sample size was small at seven patients, this study was unique in its ability to handle sparse emotional data, with mood assessments every 13 hours on average (43). Limbic networks were largely sufficient for prediction, and the same decoder was significant regardless of when the mood time point was assessed (i.e., hours or days apart). Prediction did not rely on one specific feature, and the most accurate predictions were made when derived from multiple frequency bands being included in the same model. Other groups have similar findings, with beta-frequency coherence between the amygdala and hippocampus significantly related to worsening mood (44) and beta power providing a significant predictive signal of depressive symptoms based on responses to a standardized questionnaire (45). These early findings are promising steps forward in searching for biomarkers of psychiatric illness. They will require replication, and the methods used to produce them need continued development and standardization.
Developing Personalized Stimulation Protocols from Neural Decoding
Identifying the electrophysiological correlates of personalized symptoms will provide a solid starting point for developing more effective neuromodulation interventions. Through the decoding approaches listed above, several electrophysiological correlates of symptoms are likely to be identified. Some may be nonspecific and related to, for example, the process of reporting mood as opposed to the mood state itself. It is likely that new analytical approaches to refine the electrophysiological correlates will be needed. An additional step may be to systematically evaluate whether a causal relationship exists between the decoded electrophysiological correlates and the experience of symptoms. The application of brain stimulation to disrupt the electrophysiological correlates associated with the symptom while noting the effect on the symptom of interest is a method that would provide a cause-and-effect framework that would lead to more insights than are possible through passively recorded data alone. Numerous reports from the literature showcase the immediacy of intracranial stimulation effects in causing subjective changes relevant for psychiatric symptoms. Stimulation of the cingulum led to positive affect and improvements in mood across three patients with epilepsy (46). One patient’s anxiety and pain were able to be so well controlled with stimulation that she was taken off her short-acting sedative and analgesic while undergoing neurosurgery (46). In addition, stimulation of the dorsal anterior insula has been associated with inducing ecstasy (47) and the midcingulate with the will to persevere (48), respectively. Additionally there are several examples of acute improvement in patients with severe depression stimulated at the subgenual anterior cingulate cortex (49, 50). Similarly, significant reductions in both anxiety and depressive symptoms occurred within 30 minutes of reactivating DBS for OCD after a week with the stimulator off in a nonblinded group of 16 patents (51). While these examples of immediate effects of stimulation are encouraging with regard to establishing causal inferences, it is also well established that many neuromodulation therapies take weeks or months before a therapeutic benefit is noted (21, 52, 53). Thus, a lack of immediate effects on symptoms would not rule out the possible therapeutic benefits of individual contacts.
Any causal relationships inferred by electrical stimulation could narrow the therapeutic stimulation protocol to those intracranial contacts demonstrating a causal role in mediating symptoms. The stimulation parameters could be further personalized by the frequency and nature of the patient’s symptoms. Many different strategies are likely to emerge for different symptoms. For example, with sudden-onset events like panic attacks the stimulation protocol could involve an intermittent stimulus delivery that disrupts the process early in its course. For diseases that are largely continuous in nature, there may be a baseline modulation of continuous stimulation that is coupled with other strategies for abrupt episodic worsening or transient symptoms. This would be analogous to current DBS strategies in Parkinson’s disease, where brain stimulation can be adaptively adjusted via a closed-loop system based on oscillations of the beta-rhythm to improve motor symptom fluctuation (54, 55). Strategies for psychiatry may focus on adapting brain stimulation to pathophysiological oscillations or to negative symptoms, while others may activate a competing circuit, such as augmenting positive emotional states as opposed to disrupting circuits that maintain a negative mood (56). This may be conceptualized as the use of healthy circuits to compensate for psychiatric symptoms, such as augmenting the intrinsic circuit-based mechanisms involved in the therapeutic effects of evidenced-based psychotherapy practices.
A combination of approaches may provide ultimate flexibility, which could variably involve continuous stimulation, intermittent stimulation with closed-loop dynamics, or patient-initiated stimulation for episodic relief of debilitating symptoms. Thus, a single approach would not be generalizable to all patients, but each effective strategy could contribute to a collective knowledge base for continued improvements over time.
Anticipated Challenges
The approaches outlined above present several challenges for the scientific community to overcome, three of which we highlight here. First, accurate decoding of the electrophysiological correlates of psychiatric symptoms will require patients to accurately report their symptoms. This requires insight into one’s illness, which not all patients have. If a patient’s depression is driving an increase in anxiety symptoms, having limited insight into the distinction between anxiety and depression may limit the ability of methods to decode symptoms from recorded data, as models are inherently limited by the quality of the data used to train them. Use of differing methods to query mood between research groups will also hinder early development of methods with cross-site replication. Although symptom rating scales provide a common language to researchers for evaluating symptom categories and interpreting results, these broadly used scales may also present challenges for optimally capturing highly personalized symptoms. Connecting electrophysiological signals directly to a subjective experience of psychiatric distress across large groups of patients may lead to the discovery of common electrophysiological correlates of certain classes of symptoms. These features could then inform Bayesian approaches to patient-specific decoding algorithms that may reduce the need for insight into symptoms for select patients that are impaired in this regard.
Second, stimulation of the brain can be adjusted via numerous parameters, such as stimulation location, current intensity, frequency, pulse waveform, current direction, time course (i.e., continuous versus intermittent), and open versus closed loop, among several others. Here, we propose increasing the electrode number several-fold relative to current DBS protocols that use a small number of contacts arranged linearly on a single depth electrode. This will compound an existing problem with DBS, which is difficulty optimizing stimulation patterns with a trial-and-error approach. However, the prior identification of electrophysiological correlates and principled algorithms could greatly speed up this process by limiting the parameter space to a more manageable range. One research team with extensive experience implanting DBS for mood disorders recommends no stimulation adjustment within the first month after placement and only limited manipulations in the subsequent 6 months (57). We hope that decoding the electrophysiological correlates of specific symptoms will limit the number of contacts from which to stimulate and having high quality electrophysiological correlates of the symptoms will provide immediate objective feedback to guide stimulation parameters without waiting several months. Addressing these challenges is likely to rely on the same technology and principles proposed here, including invocation of closed-loop stimulation systems (58), computer-guided or remote internet-based programming (59), and machine-learning techniques, but the feasibility of optimizing stimulation patterns while working with multielectrode arrays remains to be tested.
Finally, the brain is remarkably plastic. This feature of the human brain both facilitates treatment and recovery and could also lead to a relapse of symptoms through alternate circuitry. Psychiatric symptoms are multifactorial and influenced to a large degree by environmental stressors. It is possible that successful modulation of the final common pathway for psychiatric symptoms will not be durable without simultaneously addressing the factors that have contributed to those symptoms to begin with. It will be important to evaluate whether neuromodulation leads to successful treatment for a limited period followed by reversion to the previous disordered steady state, which may explain why patients receiving time-limited neuromodulation therapies have such high relapse rates (60, 61). It remains to be explored whether adaptive stimulation protocols may offer a solution for this symptom recrudescence or whether this aspect of relapse could be effectively addressed by coupling invasive neuromodulation with intensive psychotherapy in which patients learn new coping mechanisms during a therapeutic window where disabling symptoms are transiently improved. Some DBS research in OCD patients suggests promise for such an approach (62).
Safety, Long-Term Efficacy, and Ethics of Intracranial Neuromodulation
The risks associated with intracranial neuromodulation include surgical complications that can occur at the time of implantation or at any time after placement of intracranial hardware. The main intraoperative risk is hemorrhage and the main perioperative and postoperative complication is infection (63). A significant risk factor for infections are surgeries for battery replacement, which may be required as frequently as every 2–3 years (64). Increasingly, rechargeable batteries are used that prolong the lifespan of the battery and minimize the need for repeated surgical intervention.
Local injury to the brain in response to probe placement has been a concern for both safety and long-term efficacy of intracranial electrodes, though experience with DBS and NeuroPace has generally been reassuring in this regard. Nevertheless, this should be carefully monitored when considering broader intracranial coverage with more recording electrodes for psychiatric conditions than are used conventionally with DBS. Inflammation and the proliferation of glial cells, or gliosis, is a common local response to a foreign object implanted in the central nervous system. Gliosis has been studied extensively in relation to implanted brain stimulation devices along with monitoring long-term impedance. Impedance is a proxy of long-term efficacy of stimulation that relates to the effective resistance in a circuit. Data to date show fluctuations in impedance primarily during the first 6 months after implantation, followed by stability or even improvement in impedance with long-term implantation (65–67). Developments in the materials used, such as previously mentioned mesh electronics, may further minimize gliosis (68).
By targeting the underlying pathology believed to be responsible for psychiatric symptoms, we hope to significantly improve the quality of life of patients with disabling psychiatric disease. This strategy raises important ethical implications. Unlike most therapies that target a disease, this intervention would modulate the patient’s subjective experience and behavior in a very direct way. Moreover, the line between pathological behavior and normal, appropriate human experience is not always clear, and neuromodulation may affect both. For example, a patient with severe and disabling melancholic depression may find tremendous relief from intracranial neuromodulation that allows him or her to re-engage in normal activities. Yet the experience of sadness is also a part of everyday life; it could be challenging to treat the depression-related pathology without modifying the adaptive emotional states that everyone experiences. Similarly, there are examples of intracranial stimulation causing its own set of problems, such as patients becoming addicted to self-administration (69) or developing behavioral disorders secondary to stimulation (70). A retrospective cohort of patients with Parkinson’s disease after subthalamic nucleus DBS showed higher than anticipated attempted and completed suicide rates, specifically in the first 3 years (71). Finally, as with any therapy there exists the possibility that it may evolve from a treatment toward a method of enhancement used in the absence of severe disabling pathology. The ethical concerns raised here are not unique to psychiatric neuromodulation; current DBS treatments for movement disorders and psychiatric pharmacotherapeutics present the same challenges and questions, with which ethicists have been grappling for years. Nonetheless, these ethical considerations must be again raised and thoughtfully considered, as for any new psychoactive treatment.
Imagining the Future Implementation
Based on the developments highlighted in this article, it is possible to envision a future in psychiatry that includes personalized invasive neuromodulation. Proof of concept for these approaches should continue to be tested and developed in patient volunteers with epilepsy undergoing intracranial monitoring to detect a seizure focus, as is already happening with mood decoding and stimulation. This experimental paradigm provides invaluable data directly relevant to these goals with negligible risk to subjects, who require the invasive monitoring for other clinical indications.
Leveraging this information, personalized invasive neuromodulation therapy could then be considered first for patients that have exhausted all conventional therapies or who have demonstrated response to noninvasive stimulation and may be presumed to have a “neuromodulation-responsive” form of illness. They may be referred to an interdisciplinary neuromodulation team that includes a psychiatrist with expertise in psychiatric diagnosis and management, a functional neurosurgeon, and a neuromodulation specialist with expertise in intracranial neuromodulation. Together they would evaluate candidacy for an intracranial intervention, akin to the comprehensive evaluations undertaken by current epilepsy and DBS academic centers (21). A plan then could be developed for the implantation of intracranial electrodes spanning several brain regions, informed by leading ideas about the localization of circuits mediating the patient’s most bothersome symptoms derived from population studies (72). Implantation and therapeutic strategies can be further calibrated using noninvasive tools like high-density EEG, TMS, or fMRI.
After the implantation of electrodes, a period of symptom monitoring would begin that could be completed as an inpatient or outpatient, dependent on symptom frequency. Methods to annotate symptoms with continuous electrophysiological recording data would be used. Machine-learning algorithms would be trained on these data to learn predictive features in specific brain areas, with the goal of delivering stimulation through one or more of those same recording contacts.
Ideally, one could infer causality of the electrophysiological correlates of a symptom by administering electrical stimulation that disrupts the electrophysiological features associated with the symptom along with disrupting the symptom itself. Stimulation parameters could then be explored in a systematic way, with the goal of interrupting or preventing the most debilitating symptoms. If long-term intracranial monitoring is ultimately found to be safe and beneficial for psychiatric illnesses, new symptoms could be evaluated as they emerge using the previously implanted contacts. Data would be continuously recorded throughout the course of treatment and uploaded to a cloud-based network where ongoing data analysis could further improve the stimulation protocols. These data could be compared with databases worldwide so that patients with particularly refractory symptoms could have their electrophysiologic signatures compared with patients with similar clinical presentations. Insights gained from success stories would contribute collectively to the improvement of the method and could revolutionize the diagnostic approach to and management of psychiatric illness. Moreover, invasive intracranial methods described here would improve our understanding of the pathophysiology of psychiatric disorders as well as the mechanisms of action for effective therapeutic circuit modulation. It may be possible to leverage these insights to inform the next generation of less invasive, less expensive treatment strategies that are mechanistically informed.
Conclusions
Developing personalized interventions in psychiatry that are intimately related to the pathophysiology of patients’ most disabling symptoms has been a long-standing goal of researchers and clinicians. We have fallen short of this largely because the diagnostic tools available in clinical neuroscience do not match the spatiotemporal resolution necessary to measure and intervene upon specific neural circuits that directly mediate symptoms. We believe the steps outlined above provide a compelling strategy worthy of further investigation. These investigations will provide valuable information about psychiatric neurophysiology critical for enhancing the efficacy of current neuromodulation treatments and may reveal new treatment strategies for managing the most challenging and disabling brain-based disorders.
1 : The molecular neurobiology of depression. Nature 2008; 455:894–902Crossref, Medline, Google Scholar
2 : The neurobiology of depression: an integrated view. Asian J Psychiatr 2017; 27:101–111Crossref, Medline, Google Scholar
3 : Circuit dynamics of adaptive and maladaptive behaviour. Nature 2014; 505:309–317Crossref, Medline, Google Scholar
4 : Illuminating neural circuits: from molecules to MRI. J Neurosci 2017; 37:10817–10825Crossref, Medline, Google Scholar
5 : Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8:1263–1268Crossref, Medline, Google Scholar
6 : Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol 2012; 108:2323–2337Crossref, Medline, Google Scholar
7 : Recent advances in neural recording microsystems. Sensors (Basel) 2011; 11:4572–4597Crossref, Medline, Google Scholar
8 : Chronic in vivo multi-circuit neurophysiological recordings in mice. J Neurosci Methods 2011; 195:36–46Crossref, Medline, Google Scholar
9 : Neural circuit and clinical insights from intraoperative recordings during deep brain stimulation surgery. Brain Sci 2019; 9:173Crossref, Google Scholar
10 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed, Washington, DC,
11 : Systematic review of clinical practice guidelines for failed antidepressant treatment response in major depressive disorder, dysthymia, and subthreshold depression in adults. Can J Psychiatry 2017; 62:11–23Crossref, Medline, Google Scholar
12 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Crossref, Medline, Google Scholar
13 Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003; 361:799–808Crossref, Medline, Google Scholar
14 : The prevalence of electroconvulsive therapy use since 1973: a meta-analysis. J ECT 2016; 32:236–242Crossref, Medline, Google Scholar
15 : Adverse effects of electroconvulsive therapy. Psychiatr Clin North Am 2016; 39:513–530Crossref, Medline, Google Scholar
16 : How electroconvulsive therapy works? Understanding the neurobiological mechanisms. Clin Psychopharmacol Neurosci 2017; 15:210–221Crossref, Medline, Google Scholar
17 : Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci 2018; 30:173–179Link, Google Scholar
18 : A systematic review and meta-analysis of deep brain stimulation in treatment-resistant depression. Prog Neuropsychopharmacol Biol Psychiatry 2018; 82:224–232Crossref, Medline, Google Scholar
19 : A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry 2015; 78:240–248Crossref, Medline, Google Scholar
20 : Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017; 4:839–849Crossref, Medline, Google Scholar
21 : Deep brain stimulation for obsessive-compulsive disorder: a long term naturalistic follow up study in a single institution. Front Psychiatry 2020; 11:55 Crossref, Medline, Google Scholar
22 : Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 2008; 359:2121–2134Crossref, Medline, Google Scholar
23 : Deep brain stimulation for treatment-refractory obsessive compulsive disorder: a systematic review. BMC Psychiatry 2014; 14:214 Crossref, Medline, Google Scholar
24 : Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg 2013; 80:31.e1–31.e8Crossref, Google Scholar
25 : Basal ganglia circuits as targets for neuromodulation in Parkinson disease. JAMA Neurol 2015; 72:1354–1360Crossref, Medline, Google Scholar
26 : Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 2011; 77:1295–1304Crossref, Medline, Google Scholar
27 : Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 2015; 84:810–817Crossref, Medline, Google Scholar
28 , et al: Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology 2020; 95:e1244–e1256Crossref, Medline, Google Scholar
29 : Precision functional mapping of individual human brains. Neuron 2017; 95:791–807.e7Crossref, Medline, Google Scholar
30 : Highly scalable multichannel mesh electronics for stable chronic brain electrophysiology. Proc Natl Acad Sci USA 2017; 114:E10046–E10055Crossref, Medline, Google Scholar
31 : Syringe-injectable mesh electronics for stable chronic rodent electrophysiology. J Vis Exp 2018; 137:58003Google Scholar
32 : Advanced one- and two-dimensional mesh designs for injectable electronics. Nano Lett 2019; 19:4180–4187Crossref, Medline, Google Scholar
33 : Mobile apps for mood tracking: an analysis of features and user reviews, presented at the Proceedings of the American Medical Informatics Association, Washington, DC, 2017Google Scholar
34 : Review: human intracortical recording and neural decoding for brain-computer interfaces. IEEE Trans Neural Syst Rehabil Eng 2017; 25:1687–1696Crossref, Medline, Google Scholar
35 : Characterization and decoding the spatial patterns of hand extension/flexion using high-density ECoG. IEEE Trans Neural Syst Rehabil Eng 2017; 25:370–379Crossref, Medline, Google Scholar
36 : Neural decoding of expressive human movement from scalp electroencephalography (EEG). Front Hum Neurosci 2014; 8:188 Crossref, Medline, Google Scholar
37 : Movement decoding using neural synchronization and inter-hemispheric connectivity from deep brain local field potentials. J Neural Eng 2015; 12:056011 Crossref, Medline, Google Scholar
38 : Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun 2018; 9:365 Crossref, Medline, Google Scholar
39 : Real-time decoding of question-and-answer speech dialogue using human cortical activity. Nat Commun 2019; 10:3096 Crossref, Google Scholar
40 : Speech synthesis from neural decoding of spoken sentences. Nature 2019; 568:493–498Crossref, Medline, Google Scholar
41 : Individual finger control of a modular prosthetic limb using high-density electrocorticography in a human subject. J Neural Eng 2016; 13:026017 Crossref, Medline, Google Scholar
42 : Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006; 442:164–171Crossref, Medline, Google Scholar
43 : Mood variations decoded from multi-site intracranial human brain activity. Nat Biotechnol 2018; 36:954–961Crossref, Medline, Google Scholar
44 : An amygdala-hippocampus subnetwork that encodes variation in human mood. Cell 2018; 175:1688–1700.e14Crossref, Medline, Google Scholar
45 : Pilot study of an intracranial electroencephalography biomarker of depressive symptoms in epilepsy. J Neuropsychiatry Clin Neurosci 2020; 32:185–190Link, Google Scholar
46 : Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. J Clin Invest 2019; 129:1152–1166Crossref, Medline, Google Scholar
47 : The role of the dorsal anterior insula in ecstatic sensation revealed by direct electrical brain stimulation. Brain Stimul 2019; 12:1121–1126Crossref, Medline, Google Scholar
48 : The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron 2013; 80:1359–1367Crossref, Medline, Google Scholar
49 : Deep brain stimulation for treatment-resistant depression. Neuron 2005; 45:651–660Crossref, Medline, Google Scholar
50 : Direct electrical stimulation of lateral orbitofrontal cortex acutely improves mood in individuals with symptoms of depression. Curr Biol 2018; 28:3893–3902.e4Crossref, Medline, Google Scholar
51 : Rapid effects of deep brain stimulation reactivation on symptoms and neuroendocrine parameters in obsessive-compulsive disorder. Transl Psychiatry 2016; 6:e722 Crossref, Medline, Google Scholar
52 : A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry 2017; 174:640–648Crossref, Medline, Google Scholar
53 : The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016; 9:336–346Crossref, Medline, Google Scholar
54 : Pallidal deep-brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in Parkinson’s disease. J Neurosci 2018; 38:4556–4568Crossref, Medline, Google Scholar
55 : The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord 2014; 20(Suppl 1):S44–S48Crossref, Medline, Google Scholar
56 : Delta-frequency stimulation of cerebellar projections can compensate for schizophrenia-related medial frontal dysfunction. Mol Psychiatry 2017; 22:647–655Crossref, Medline, Google Scholar
57 : Characterizing the therapeutic response to deep brain stimulation for treatment-resistant depression: a single center long-term perspective. Front Integr Nuerosci 2015; 9:41Crossref, Medline, Google Scholar
58 : Toward electrophysiology-based intelligent adaptive deep brain stimulation for movement disorders. Neurotherapeutics 2019; 16:105–118Crossref, Medline, Google Scholar
59 : DBS programming: an evolving approach for patients with Parkinson’s disease. Parkinsons Dis 2017; 2017:8492619 Google Scholar
60 : Durability of antidepressant response to repetitive transcranial magnetic stimulation: Systematic review and meta-analysis. Brain Stimul 2019; 12:119–128Crossref, Medline, Google Scholar
61 : Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology 2013; 38:2467–2474Crossref, Medline, Google Scholar
62 : Optimizing deep brain stimulation parameters in obsessive-compulsive disorder. Neuromodulation 2020; 13243Google Scholar
63 : Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 2017; 7:CD008497Medline, Google Scholar
64 : Implant site infection and bone flap osteomyelitis associated with the Neuropace responsive neurostimulation system. World Neurosurg 2016; 88:687.e1–687.e6Crossref, Google Scholar
65 : Deep brain stimulation associated gliosis: a post-mortem study. Parkinsonism Relat Disord 2018; 54:51–55Crossref, Medline, Google Scholar
66 : Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul 2013; 6:718–726Crossref, Medline, Google Scholar
67 : Longitudinal follow-up of impedance drift in deep brain stimulation cases. Tremor Other Hyperkinet Mov (N Y) 2018; 8:542 Medline, Google Scholar
68 : Stable long-term chronic brain mapping at the single-neuron level. Nat Methods 2016; 13:875–882Crossref, Medline, Google Scholar
69 : Compulsive thalamic self-stimulation: a case with metabolic, electrophysiologic and behavioral correlates. Pain 1986; 27:277–290Crossref, Medline, Google Scholar
70 : Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatry 2007; 78:517–519Crossref, Medline, Google Scholar
71 : Suicide and suicide attempts after subthalamic nucleus stimulation in Parkinson disease. Neurology 2019; 93:e97–e105Crossref, Medline, Google Scholar
72 , et al: State-dependent responses to intracranial brain stimulation in a patient with depression. Nat Med 2021; 27:229–331Crossref, Medline, Google Scholar



