From Dreams to Hallucinations: Jean Lhermitte’s Contribution to the Study of Peduncular Hallucinosis and the Dissociation of States
Abstract
Jean Lhermitte (1877–1959) was one of the pioneers of behavioral neurology, including the field of hallucinations. This article focuses on his work concerning the relationship between hallucinations, sleep, and dreams. From 1910, Lhermitte became interested in sleep and its disorders, particularly narcolepsy and its accompanying symptoms. He also reported on sleep disorders and hallucinations occurring in people with lesions of the diencephalic region (“infundibular syndrome”), and later encephalitis lethargica. In 1922, he described a syndrome of complex, predominantly visual hallucinations in patients with vascular damage to the midbrain, known as peduncular hallucinosis. Twelve historical cases of peduncular hallucinosis, including 10 from Lhermitte, are reviewed. He gave a precise phenomenological description of peduncular hallucinosis, and put forward the hypothesis that the lesion disrupted the anatomy and connections of a center regulating wakefulness and sleep, thus enabling a dissociation of the mechanisms of dream and waking states. Although the pathophysiology of peduncular hallucinosis remains to this day partly obscure, the model of a limited subcortical lesion acting through complex mechanisms and ultimately involving the cortex remains valid. Lhermitte was also a pioneer in characterizing what contemporary sleep specialists call dissociation of states.
Jean Lhermitte (1877–1959), a renowned neurologist and a psychiatrist from the Paris school, was one of the pioneers of behavioral neurology and of the neuropsychiatric interface (1, 2). An important part of his work was devoted to hallucinations, about which he published an insightful book in 1951 (3). Here, the focus is specifically on his works concerning hallucinations in their relation to dreaming and sleep. Lhermitte had been interested in sleep and its disorders, particularly narcolepsy and its accompanying symptoms (4, 5), since the 1910s. In 1922, he was the first author to describe a syndrome of complex hallucinations following vascular damage to the midbrain (6), which is still known as peduncular hallucinosis. From this seminal observation, Lhermitte put forward the hypothesis of a dissociation between components of sleep. Subsequently, he published other peduncular hallucinosis cases and remained constant in his interpretation all his life, disputing with some of his contemporaries. In spite of its limitations linked to the knowledge and means of the time, his work remains of great interest for the accuracy of its clinical descriptions and for his pathophysiological hypotheses, which were in many ways innovative. The books written by Lhermitte are well known, but his numerous articles are scattered over many publications. I systematically searched for all articles having Lhermitte as author or coauthor between 1910 and 1955, in Revue Neurologique (Paris) and other medical French journals available online, mainly on the websites of the Bibliothèque Interuniversitaire Santé de Paris (biusante.parisdescartes.fr/histoire/medica/periodiques.php), the Bibliothèque Nationale de France (data.bnf.fr and gallica.bnf.fr), and the Bibliothèque Henri Ey of the Centre Hospitalier Saint-Anne (ghu-paris.fr/fr/bibliotheque-henri-ey). Other articles written by Lhermitte or contemporary authors that he cited in his work were also collected.
BEFORE PEDUNCULAR HALLUCINOSIS: INFUNDIBULAR SYNDROME AND ENCEPHALITIS LETHARGICA
Several observations paved the way for the description of peduncular hallucinosis: the so-called infundibular syndrome, and the hallucinatory phenomena reported by some patients following encephalitis lethargica.
Infundibular Syndrome
In 1917, Henri Claude and Jean Lhermitte (7) published the observation of a 25-year-old man, a submarine mechanic, admitted in 1916 to a military neurology center in Bourges (a city in central France) for deterioration of his general state, insomnia, polyuria with polydipsia, and bitemporal hemianopia. He had a history of syphilitic chancre. Examination of cerebral spinal fluid (CSF) showed unquantified lymphocytosis and albumin at 0.56 g/L. The initial diagnosis was syphilitic meningitis. After clinical fluctuations, “the subject falls into a deep sleep from which it is impossible to wake him. This ‘narcolepsy’ attack, which lasts about five hours, leaves the subject amnesic and astonished when he wakes up.” Thereafter, his state worsened: “The patient is overcome by a confusional delirium in an oneiroid state [“onirism”]. He says his bed is wet from the rain and fog of the sea; he thinks he is in Toulon [a naval base in the south of France] in springtime.” In the following weeks, the patient had new “attacks of narcolepsy,” and he died 6 months after onset, probably from coincidental pulmonary tuberculosis. The autopsy revealed a retrochiasmatic tumor occupying the interpeduncular fossa and compressing the neighboring structures. The tumor thinned the floor of the third ventricle (in particular, the infundibulum and the lamina terminalis), without reaching the pituitary gland. The histological description suggests a craniopharyngioma (Professor Jacques Poirier, personal communication). Based on this observation, the authors described an “infundibular syndrome.” They observed that, “if one does not confuse them with coma, somnolence and sleep, authentic narcoleptic attacks are not exceptional as manifestations of infundibular tumors.” In his 1910 reports (4, 5), Lhermitte had developed a broader conception of narcolepsy as a syndrome with various causes, particularly tumors, alongside idiopathic or essential forms, as described by Jean-Baptiste Gélineau in 1881 (8), which Lhermitte considered rarer.
Encephalitis Lethargica
Lhermitte and de Saint-Martin described two cases of encephalitis lethargica in 1918 under the name of “primitive poliomesocephalitis with narcolepsy,” stressing the sleep disorder that gave the condition its name (9). In the review he later published, Lhermitte (10) noticed that daytime sleepiness was sometimes associated with insomnia and “a certain degree of agitation,” the latter being “associated with a mild and quiet delirium of a confusional and hallucinatory nature,” which the author attributed to “the close relationship between the dreaming associated with confusion and physiological dreaming. . . . In both cases, it is the same sequence of images that projects its disturbing and capricious phantasmagoria onto the sleeping consciousness, the same lack of insight, the same inconsistency, and the same lack of astonishment at the appearance of the most unexpected and disconcerting phenomena.” These images appeared in the evening, were almost exclusively visual, and could, through a series of intermediaries, lead to an acute delusional syndrome. Lhermitte aligned himself with an opinion previously defended in France by Jean Camus (1872–1924), according to which “lesions of the midbrain and the deep gray-matter nuclei can lead to mental disorders independently of cortical lesions,” suggesting the existence of extracortical regulatory centers of the psychic functions (11).
PEDUNCULAR HALLUCINOSIS: THE SEMINAL 1922 ARTICLE AND FOLLOWING OBSERVATIONS
Recent articles have summarized the history of peduncular hallucinosis (12–14). My aim is to analyze more precisely the emergence of this concept in the context of Lhermitte’s time, and then, in the next sections, to integrate peduncular hallucinosis into Lhermitte’s more general theory on the relationship between dreams and hallucinations, and to compare it with contemporary data.
The first observation of peduncular hallucinosis was reported by Lhermitte in 1922 to the Neurological Society of Paris under the title “Syndrome de la calotte du pédoncule cerebral. Les troubles psycho-sensoriels dans les lesions du mésencéphale” (“Syndrome of the peduncular cap [tegmentum]). Psycho-sensory disorders following mesencephalic lesions”) (6). The patient was a 75-year-old woman. She initially had headache and vomiting, and within 2 weeks developed left paralysis of the abducens nerve, then of the oculomotor nerve, and on the right side facial paralysis, a Babinski sign, and kinetic cerebellar syndrome. CSF cell count and albumin concentration were normal. The picture mostly corresponded to Claude’s syndrome (15) with additional pyramidal signs, and suggested involvement of the midbrain tegmentum. Damage to the left abducens nerve, without lateral gaze palsy, is unusual in this setting and could have been due to compression by a vertebrobasilar dolichoectasia (16). Two weeks after completion of the somatic neurological disorders, as depicted by Lhermitte, “the patient spontaneously told us that during the day and especially at nightfall, she sees various animals walking around on the floor of the ward. There are cats, and hens slightly strange in appearance, their dilated pupils possessing a strange luster. In order to verify the reality of these perceptions, the patient tried to touch these animals. She told us that her contact with them resembles that of real animals, but as soon as she touched them, they slowly disappeared through the floor.” The patient “does not think that these are true perceptions, since, when questioned, none of the patients in the same hospital room had experienced them.” In addition, “sleep seems to be strongly disturbed and insomnia at night is associated with some sleepiness during the afternoon.” Three weeks after they began, “the visions are no longer of animals, but of human beings decked out in bizarre and tattered outfits, or children playing with dolls. The patient sees them in the beds of her neighbors.” Finally, insight weakens: “These images are so vivid, she tells us, that they can only correspond to reality,” but “no delusional ideas accompany these visual hallucinations.” In his comments Lhermitte, referring again to Camus (11), stated that “it was not extravagant” to wonder if the mental disorders were related to the peduncular lesion. He insisted on the “striking similarity” between the almost exclusively visual sensory phenomena experienced by the patient and dreams, leading to the statement that hallucinosis was the expression of a dream in a half-asleep subject. Finally, based on his own previous work (7) and that of other authors, Lhermitte concluded that the visual hallucinosis resulted from a “disturbance of the sleep function,” a condition that was “the equivalent of narcolepsy” and was related to the mesencephalic lesion.
In 1924, Ludo van Bogaert (1897–1989), the renowned neurologist and pathologist from Antwerp, Belgium, made the first clinico-anatomical observation of what he was the first to call “hallucinose pédonculaire” (peduncular hallucinosis) (17). The patient, a woman aged 59, had a neurological picture close to Claude’s syndrome, with visual hallucinations, mainly zooptic: “From the very first evening, she saw a dog’s head on her pillow, and the opposite wall frequently bore a picture of a horse on a pink background. . . . On one occasion, she reported the presence of green snakes in her bed for a whole day. She touched them, pushing them away from the bed. They were rough against her hand. . . . None of these hallucinations provoked an emotional reaction in the patient; she shows no surprise . . . and is absolutely convinced of the reality of the animals she sees.” There were also dyschromatopsia and episodes of psychomotor agitation, independent of the hallucinations. A few years later, van Bogaert published additional clinical and autopsy data (18). During the 14 months of follow-up, the patient still experienced “hallucinatory outbursts.” The lesion was “a focal peduncular softening by a syphilitic arteritis of the retromamillary pedicle,” extending to the superior colliculus and the pulvinar. This patient, unlike Lhermitte’s, did not have sleep disorders, leading van Bogaert to argue that “the release of the imaginative automatism can be dissociated from the hypnic state.” He thus diverged from Lhermitte’s interpretation and, referring to the conceptions of the psychiatrist and philosopher Pierre Quercy (19), he stated that “the essential disorder is a momentary weakening of the self which disrupts the function of reality.”
However, for his entire life, Lhermitte remained convinced that peduncular hallucinosis made it possible to approach “the physiological basis of the dream” (20). Following his 1922 article, he published nine other cases of peduncular hallucinosis or similar syndromes that strengthened his conviction. They are summarized in Table 1, together with the first observation and two other historical cases. The next observation (21) was that of a 70-year-old woman who suddenly fell into a “deep sleep,” with bilateral ophthalmoplegia suggestive of bilateral damage to the oculomotor nerves, bilateral pyramidal syndrome, and cerebellar syndrome. When the patient came out of her “lethargy,” she had in the evening “multiple, colorful visions” of animals or people “moving silently.” Sometimes “she thinks she is at the theatre, attending a variety of performances.” These phenomena did not evoke an emotional response. They were associated with memory impairment for recent events and confabulations. The picture retrospectively suggests a bilateral paramedian mesencephalic and thalamic infarction (22).
| Study | Sex | Age at onset (years) | Neurological signs | Hallucinations | Insight and emotional reactions | Sleep disorders | Time to onset after stroke, schedule, and duration | Presumed topography | Anatomical lesions (autopsy) | Presumed cause |
|---|---|---|---|---|---|---|---|---|---|---|
| Lhermitte (6) | Female | 75 | Left: VI, extrinsic III Right: cFP, Babinski sign, and cerebellar syndrome (limbs) | Visual: animals, people Tactile | Yes, later no No | Insomnia, Daytime somnolence | Two weeks Evening ND (>10 days) | Midbrain | NA | Ischemic |
| Lhermitte and Toupet (21); Lhermitte (40) | Female | 70 | Bilateral: ophthalmoplegia (at least both III), Babinski sign, and cerebellar syndrome Memory impairment and confabulations | Visual: animals and people | No No | Coma (“lethargy”) and later daytime somnolence | One month (when coma resolved) Evening NA | Midbrain (probably thalamic, see article text) | NA | Ischemic |
| Van Bogaert (17, 18) | Female | 59 | Right: III Left: III initial, cFP, cerebellar syndrome (limbs), and Babinski sign Vertigo (initially) | Visual: animals Tactile Visual illusions (changed colors) | No No | No (episodes of agitation in the evening) | First day Evening 14 months (until death) | Midbrain: “inferior red nucleus syndrome” | Right: red nucleus, pulvinar, superior colliculus, fibers III, decussation of superior cerebellar peduncles, substantia nigra, periaqueductal gray | Ischemic and syphilitic arteritis |
| Lhermitte and Levy (23); Lhermitte et al. (24) | Male | 60 | Intoxication with hypnotics, coma, then: dysarthria, right III palsy, bilateral AIM, and later left upper-limb “choreo-athetotic” movements | Dream-like (oneiroid) visual: complex scenes Later visual: people Visual illusions | Yes No | ND | Probably several days Evening One month | Midbrain | Tegmentum and nuclei of III, bilateral predominating on the right side | “Toxic” (probably ischemic) |
| Lhermitte and Levy (25) | Female | ND | “Pronation spasm” (presumably dystonia) of the right upper limb | Visual: human head | Yes No | Vivid dreams and shouting while dreaming | Soon after (ND) Evening Five years | Subthalamic (probably thalamic, see article text) | NA | Ischemic |
| Lhermitte and Levy (29) | Female | 42 | Transient right hemiplegia 1 year before Loss of consciousness Left: hemiplegia with partial recovery, Babinski sign, AIM of the lower limb, and thermo-algesic sensory loss | Visual illusions: a man’s red head, moving Visual: skulls | Yes Moderate (fear) | ND | Three weeks Evening About 2 months | Midbrain | NA | Ischemic, syphilitic arteritis |
| de Morsier (30) | Female | 51 | Dysarthria, ataxia Left: cerebellar signs, AIM of the hand, and astereognosia Right: mild ptosis Visual field and visual acuity: normal | Visual: people, animals (bright), once a scene (people on an avenue) “Like a movie” In the left hemifield only | Yes No, or mild (pleasure) | Occasional insomnia | Twenty months Evening At least 1 year | Midbrain (and thalamic) | NA | Vascular |
| Lhermitte et al. (37) | Male | 33 | Right: hemiparesis, VII, VI and X, and myoclonus of the shoulder Left: hemianesthesia (pain, temperature) Dysarthria and dysphagia | Visual: people | Yes Moderate | ND | Two weeks Evening and night “Very ephemeral” | Medulla and pons | NA | Hemorrhage |
| Lhermitte and Bineau (34) | Female | 71 | “Obnubilation,” “lethargy,” bilateral ptosis, limitation of lateral gaze, and diplopia Left: hemiparesis, Babinski sign, and dysmetria | Visual: animals, people, and swastikas Visual illusions (changed colors) | Yes Moderate (fear) | Daytime somnolence | Five days ND Ten days | Midbrain | NA | Ischemic |
| Female | 71 | Loss of consciousness, balance disorder, somnolence, and bilateral dysmetria | Visual: animals Illusion (changed colors) | ND ND | Daytime somnolence | Within days ND “Several months” | Midbrain | NA | Ischemic | |
| Lhermitte (35) | Female | 71 | Progressive left hemiparesis, diplopia; 2 years later, brisk AIM of left upper limb, later of lower limb, small steps, left Babinski sign, lost pupillary reflexes, anisocoria, convergent strabismus, and upward gaze palsy | Visual: people, animals Auditory verbal (concomitant with visual hallucinations of persons) | Delayed Mild | No | About 35 years Five episodes at night, one in daytime Several months | Midbrain and subthalamic area: “contralateral syndrome of the red nucleus“ | NA | Tuberculoma |
| Lhermitte (36) | Male | 66 | Left hemiplegia and sensory loss (abating); bilateral Babinski sign; ophthalmoplegia, including palsy of vertical gaze and skew deviation; right Horner’s syndrome; and later AIM of left upper limb | Auditory: ringtone Visual: objects, people Nocturnal dream-like scenes (or possibly dreams) | ND ND | Daytime somnolence | One day ND and night At least 2 weeks | Midbrain | NA | Hemorrhage (xanthochromic CSF) |
TABLE 1. Cases of peduncular hallucinosis published by Jean Lhermitte and his colleagues and by contemporary investigators referring to Lhermitte’s work (in chronological order)a
A new observation, published with Gabrielle Lévy (1886–1935), differed from the others (23). A 60-year-old man, suffering from long-standing tabes dorsalis, went into a coma after a suicide attempt with hypnotics. On waking, he had a crossed syndrome suggestive of mesencephalic damage (Table 1). For a month, at nightfall, the subject experienced visual hallucinations, which were at first dreamlike: “He thought his room had been transformed into a railway or subway carriage.” Soon after, “since this carriage was in more or less close connection with an aeroplane service . . . he arrived on a platform overlooking a marvelous landscape resembling that of Arabia. A plane descended from the sky and landed on the platform. He climbed aboard. . . . Then he flew over wonderful landscapes for a few moments.” After a few days, the hallucinatory phenomena changed in nature. In the evening, “the walls of the room, the various objects that furnished it, come to life under the astonished gaze of the patient. Two pairs of trousers hung in front of him and a coat rack in the room become two bustling women.” Even the bare wall of his room became animated and lively: “Workers come to nail down tapestries, a modern kind of tapestry, but this tapestry becomes animated in its turn. Characters move around, curtsy to each other, make themselves understood.” The images are colorful and silent, they “are not unpleasant,” and the subject “realizes that he is the plaything of an illusion.” About 3 weeks after its onset, the hallucinosis decreased, with a few visions remaining before sleep: “heads that look at him strangely” on a wall outside. After 1 month, the hallucinatory phenomena subsided. The patient died of a pneumopathy, and a new article provided anatomical findings (24). There were no macroscopic lesions, while histological examination showed “alterations of the peduncular cap (midbrain tegmentum) with metachromatic degeneration associated with an alteration of the ventral nucleus of the third nerve and of the median and intermediate nuclei.” Without excessive conviction, the authors linked these lesions with the drug intoxication. For Professor Françoise Gray (personal communication), the description is compatible with an ischemic lesion dating back several weeks, and leads to the hypothesis of low cerebral blood flow at the time of the intoxication with hypnotics, possibly in arteries with stenotic lesions (atheroma? syphilitic arteritis?).
In 1931, Lhermitte and Gabrielle Lévy (25) published the observation of an elderly patient who 5 years earlier (26) had suffered a “slight stroke” followed by a “pronation spasm of the right arm” (probably dystonia) and sensory disturbances in this limb. Since this accident, the patient “very frequently [saw], at night, a man’s head appear on the wall facing her bed.” The head was “black and grey on a white background . . . the figure [was] very recognizable.” To make it disappear, the patient focused on another object. The vision was rather pleasant, and insight was retained. The patient also suffered from nightmares with screaming. Lhermitte believed that the lesion was located in the subthalamic area and related this observation to those previously published of peduncular hallucinosis. The lesion more likely involved the posterolateral region of the thalamus (27, 28). The same authors published a similar case soon after (29).
An observation by Georges de Morsier (1894–1982), a Swiss neuropsychiatrist, generated new discussions (30). A 54-year-old woman suddenly developed Claude’s syndrome; 18 months later, she reported predominantly evening visions of colorful figures or animals, appearing for a few seconds. The images unfold “like in the cinema.” They “always come from the left and move from left to right until they reach the median line where they disappear.” The visions did not give an impression of reality and did not cause anxiety. They occurred irregularly and could stop for several weeks. Sleep was mostly undisturbed. Although visual fields and visual acuity were normal, de Morsier postulated the existence of a unilateral, retrochiasmatic lesion of the visual pathways. He did not propose any mechanism to explain these hallucinations limited to a visual hemifield, in the absence of detectable damage to the visual field, unlike the hallucinations in a hemianopic field after an ischemic stroke first described in the United States by Seguin in 1886 (31) and in France by Lamy in 1895 (32). No anatomical study was available in this case. Lhermitte stated, at the end of this presentation, that lesions of the optic tract or lateral geniculate bodies never cause hallucinations, which is not certain, even if observations are rare (33). More generally, Lhermitte constantly rejected the hypothesis that an alteration of the visual pathways could contribute to peduncular hallucinosis.
Subsequently, Lhermitte and his collaborators published four more cases of peduncular hallucinosis (34–36), one of them secondary to a peduncular hemorrhage (36), and one observation of hallucinations following a vascular lesion situated in the pons and medulla (37) (Table 1). In addition, five cases presumed to be due to hemorrhage of the peduncular tegmentum were summarized in a review article (38).
PEDUNCULAR HALLUCINOSIS AS A “DISSOCIATION OF THE HYPNIC STATE”
In 1932, Lhermitte summarized his conceptions of peduncular hallucinosis in an article published in L’Encéphale (39). He gave a definition of hallucinosis, in line with that proposed by his contemporaries: “hallucinatory states which do not lead to true delusions”; and of peduncular hallucinosis: “hallucinatory manifestations which appear and develop in patients suffering from lesions limited to the mesodiencephalon, i.e., to the ventral region of the third ventricle and the peduncular cap [tegmentum].” Lhermitte underlined the common features of his cases: “visions of mobile, colorful, and silent animals or animated characters,” occurring in the evening, in the absence of surprise and anxiety, without delusions. Insight, on the other hand, differed from one patient to another; patients might “become caught up in the hallucination when it occurs with crudeness, vividity and naturalness.” Hallucinosis was associated with sleep disturbances. Lhermitte’s views on pathogenesis can be summarized as follows: There is a regulating center for wakefulness and sleep in the mesodiencephalon; sleep has a negative feature, the “suspension of consciousness,” and a positive feature, dreaming; lesions causing sleep alteration can also modify dream activity; and in peduncular hallucinosis observations, there are sleep disorders and visions with “the same attributes as those of dreaming” and “obvious similarities with hypnagogic images.” Lhermitte concluded that a patient with peduncular hallucinosis “is therefore a dreamer awake or insufficiently asleep, a subject whose profoundly disturbed hypnic function has been dissociated by the whim of an anatomical disorganization.” A dissociation of dream mechanisms, however, was not sufficient to explain peduncular hallucinosis. Lhermitte attempted to reconcile his conception with that of van Bogaert, according to whom hallucination was due to “a weakening of the sense of reality . . . causing images and representations to take on an abnormal brilliance.” For Lhermitte, “to sleep and to dream is to slacken one’s attention to reality . . . and to scatter before a drowsy consciousness the images and representations that constitute the capricious and incessantly moving weft of the dream” (23).
In a 1934 review (40), Lhermitte added a few recent cases, including that of Garcin and Renard (41) associating multiple cranial nerve damage, sleep disorders, and visual hallucinations, all transient phenomena attributed to a viral infection affecting the brainstem. The message was that, whatever the cause (vascular, toxic, infectious), the mesodiencephalic region was altered and responsible for the hallucinosis. However, one observation contradicted this assertion (37): A 33-year-old man was suddenly affected by a crossed syndrome (Table 1) presumed to be hemorrhagic and suggestive of a lesion located in the medulla and the pons. Besides somatic neurological symptoms, the patient had, on the one hand, the impression that his legs occupied a position far above the bed plane, and, on the other, visual evening and night hallucinations, in the form of characters moving silently. The first symptom was a postural illusion, similar to phantom limbs, probably related to sensory deafferentation, a symptom rarely reported following brainstem lesions (42). Besides, the hallucinosis suggested that a lesion of the medulla or the pons could generate a syndrome similar to peduncular hallucinosis, as already reported by Stenvers (43) in a patient with a pons tuberculoma who had, in addition to focal signs, visual and auditory hallucinations. Lhermitte, reluctant to admit that a pontine lesion could induce a peduncular hallucinosis, evoked a “disruption in the equilibrium of the organo-vegetative system,” referring to Raoul Mourgue, author of an “organo-vegetative” theory of hallucinations that hardly survived its author (44). In a new review, Lhermitte (45) commented on the dreamlike and hypnagogic phenomena of narcolepsy and the hallucinations associated with sleep paralysis. All these phenomena had in common that they were the positive side of a hypnic function disorder. In his 1951 book (3), Lhermitte devoted 20 pages to “hallucinosis of peduncular origin.” He added some new brief observations, which widened the phenomenological picture: ocular paralysis could be missing; auditory hallucinations, including verbal ones, could occur; and lesions could involve the floor of the third ventricle or the pons. Lhermitte remained true to the concept of a dissociation of the hypnic state and specified that the causal focal lesion only generated hallucinations because it “activated extremely complicated mechanisms, which took place throughout the entire extent of the brain, and especially in the cerebral cortex.” Lhermitte thus refuted the objections of Mourgue, who reproached him for confusing “the anatomical localization with that of the functional syndrome” (44).
PEDUNCULAR HALLUCINOSIS: THE LEGACY OF JEAN LHERMITTE
The aim of this section is to show, through four questions raised by the concept of peduncular hallucinosis, to what extent Lhermitte’s approach, in spite of the limited anatomical and physiological knowledge of the time, was innovative. The detailed history of peduncular hallucinosis from the 1950s to the present time will not be systematically reviewed.
Is Peduncular Hallucinosis a Clinico-Anatomical Clinical Entity?
For Lhermitte, the core features of peduncular hallucinosis were visual hallucinations without delusions, a predominance in the evening, sleep disorders, and an anatomical lesion located in the mesodiencephalic area. However, Lhermitte acknowledged that the syndrome was heterogeneous. Besides visual hallucinations, visual illusions and auditory (including verbal) and tactile hallucinations were reported. Insight into the hallucinatory nature of the experience and the emotional impact varied from one patient to another, or even in the same patient. In most cases, visual hallucinations consisted of people, heads, or animals projected onto an unchanged environment. The patient passively attended the scene (e.g., “she thinks she’s at the theatre attending various performances”) (40). In at least one case (23), the hallucinations were dreamlike, fitting the definitions of onirism (oneiroid syndrome). The patient was acting in a complex and bizarre scenario, moving in an unreal setting along with the hallucinated people. Sleep disorders (e.g., insomnia, daytime sleepiness, or lethargy) were part of the core peduncular hallucinosis syndrome, although van Bogaert (17, 18) had shown that these features could be missing.
Anatomically, Lhermitte postulated that the lesion disrupted a regulatory sleep center located in the midbrain tegmentum or the third ventricle floor, possibly in the pons, but he repeatedly ruled out the possibility of the lesion being localized in the lateral geniculate nucleus or the thalamus. However, the patient reported by van Boagert did have a thalamic lesion (of the pulvinar) associated with those of the midbrain. Interestingly, Lhermitte and Julian de Ajuriaguerra, in an article devoted to hallucinations associated with lesions of the visual pathways (46), reported on patients with ophthalmopathy and visual hallucinations resembling those of peduncular hallucinosis in whom a neuropathological study revealed a thalamic lesion. In Lhermitte’s time, anatomical data were rare, and thalamic lesions could be missed. The possible involvement of thalamic nuclei is now recognized. Galetta et al. (12) collected data on 86 patients with peduncular hallucinosis, among whom 69 had a computerized tomography or MRI brain scan; of these 69 patients, 21 (30%) had a thalamic lesion, isolated or associated with other lesions. In another series of 23 published cases of peduncular hallucinosis, selected on stringent criteria including the exclusion of coexisting lesions of the cortex or visual pathways, Boes et al. (47) found that 16 patients (70%) had a thalamic lesion. It must also be emphasized that peduncular hallucinosis is a rare condition. Peduncular hallucinosis was not recorded in a series of 22 infarcts limited to the midbrain (48), and it was not mentioned (although it is not stated whether it was systematically looked for) in several series totaling 102 patients of infarcts of the midbrain, either isolated (49, 50) or associated with other lesions (51).
In summary, it currently seems relevant to apply the name of peduncular hallucinosis to hallucinations predominating in the visual modality and associated with a demonstrated lesion located in the midbrain, pons, or thalamus, the last of these being commonly involved.
Is Peduncular Hallucinosis a “Waking Dream”?
The first way to address this question is to compare the phenomenological characteristics of dreams and peduncular hallucinosis. Although dreams can occur in all stages of sleep, “it is clear that rapid eye movement (REM) sleep represents the sleep state most conducive to sensorily vivid, motoric and emotionally salient dreams with the most elaborated narrative structure” (52). Lhermitte was not aware of the existence of REM sleep. REMs in sleep were described in 1953 (53), but REM sleep as a specific sleep stage was characterized by Michel Jouvet under the name “paradoxical sleep” after Lhermitte’s death, in the 1960s (54). In his descriptions of dreams, Lhermitte highlighted the features of REM-associated dreams (20, 55).
Hallucinations associated with neurological conditions, including peduncular hallucinosis, are usually quite different from dreams (Table 2). In most cases of complex visual hallucinations of neurological or ophthalmological origin, the hallucinated features, most often people or animals, are projected onto an unchanged environment and do not result in a complex and bizarre scenario (56). There are, however, hallucinatory states close to dreaming within the framework of what Emmanuel Régis (1855–1918), a Bordeaux psychiatrist, called délire de rêve or délire onirique (oneiroid delirium) (57, 58). For Régis, this state fell within the category of mental confusion (i.e., delirium) and was associated with intoxications or infections. The author emphasized the similarities with dream images, as well as the nocturnal predominance and the association with sleep or daytime sleepiness: “They are active, moving sleepers” (58). This gray zone between hallucinations and dreams had already been emphasized by Charles Lasègue in 1881 in the context of delirium tremens in an article with a self-explanatory title: “Alcoholic delirium is not a delirium but a dream” (59). The term onirism (oneiroid state), specific to French nosography, later freed itself from its original link with mental confusion, with, for example, Raoul Benon: “Onirism is characterized by the development in the waking state of illusions and hallucinations which resemble those observed in the normal dream, and which are not accompanied, in pure cases, by delirium or mental confusion” (60). Among the historical cases of peduncular hallucinosis, the term onirism only applies to the patient described by Lhermitte and Lévy (23, 24); more precisely, to the phenomena immediately following his awakening from intoxication, before the appearance of hallucinations of a completely different type. In this case, the two types of hallucinations probably were of different origin: dream-like hallucinations of toxic origin first, then typical peduncular hallucinosis hallucinations secondary to a focal lesion.
| Characteristics | Peduncular hallucinosis | Dreams in REM sleep | Hallucinations in narcolepsy |
|---|---|---|---|
| Sensory modalities | Visual (always), unfrequently auditory, and tactile | Visual (always), commonly auditory, somatic | Visual (most cases), commonly auditory, and somatic |
| Perceptual range | Elements in a scene | Immersive and panoramic scenes | Elements in a scene |
| Superimposed on veridical perceptions | Yes | No | Yes |
| Visual illusions or feeling of presence | Possible (illusions) | No | Possible (illusions and feeling of presence) |
| Case subject is an actor in the scene | No | Yes | Yes |
| Elaborated and bizarre scenario | No | Yes | No |
| Emotional content | No (rarely yes) | Yes | Variable |
| Content linked to memory | No | Yes, frequently | No |
| Content recalled | Yes | Poorly | Yes |
| Insight | Yes, no, or varies according to time | No | Yes, in most cases (during experience or delayed) |
| False belief (i.e., role of an external agent) | No | No | Seldom |
| Relationship with sleep | Yes: occurs in the evening, association with daytime somnolence (according to Jean Lhermitte) | Yes: association with REM | Yes: association with sleep onset and/or offset |
| Somatic changes during perceptual experience | No | Yes: atonia, REM | Possible association with sleep paralysis |
TABLE 2. Comparison of the phenomenological features of peduncular hallucinosis, dreams in rapid eye movement (REM) sleep, and hallucinations in primary narcolepsya
Clearly, then, peduncular hallucinosis is phenomenologically distinct from dreaming, a fact that Lhermitte, probably influenced by his pathophysiological conceptions, was reluctant to accept. He constantly insisted on the similarities between dreams and peduncular hallucinosis, from his first review paper (39) (“Hallucinatory images [of peduncular hallucinosis] possess the same attributes as those of dreams”) until his last article on this topic (61) (“This type of hallucinosis corresponds to a real waking dream”). Even in the posthumous eighth edition of his book about dreams (55), Lhermitte wrote that the images of peduncular hallucinosis “only differ from the images of the physiological dream in that the former are internalized, while the latter are externalized.” At most, Lhermitte referred to “dream fragments,” (34) “dreamlike fragmentation” (45), or images “similar” to those of dreams (3).
The relationship between peduncular hallucinosis and the hallucinations accompanying states of transition between wakefulness and sleep is a more difficult topic. These phenomena were described by Jules Baillarger in 1845 (62), then by Alfred Maury in 1848, who defined them as hypnagogic (when falling asleep) or hypnopompic (when waking up) (63). The very nature of the sensory experience remains debated; notably, the editors of DSM-5 dismissed these phenomena from the realm of hallucinations (64). These phenomena, which for convenience I will call hallucinations, are very common in the general population (about 25%) and even more frequent in younger subjects (65). In primary narcolepsy, these hallucinations are more frequent, more vivid, and have greater emotional repercussions than in healthy subjects. They are also frequently accompanied by sleep paralysis, which makes them more frightening (66). Lhermitte, in his 1927 report (67), emphasized their usually visual character and described them as “images,” or “visions,” differentiating them from hallucinations and dreams. He briefly mentioned that in narcoleptic syndromes, “sometimes, before falling into deep sleep, the patient was subject to auditory or visual phenomena of a hypnagogic nature.” Phenomenologically, the hallucinations of peduncular hallucinosis are closer to those described during sleep–wake transitions than to the dreams associated with REM sleep (Table 2). However, the distinction between “half-sleep visions,” “hallucinosis,” and “authentic hallucinations” was of limited relevance for Lhermitte: “If from the clinical and phenomenological point of view the distinction must be maintained, this no longer holds true from the psycho-physiological point of view” (68).
Recently, it was proposed that in some conditions such as Parkinson’s disease, hallucinations could result from REM dream intrusions into the waking state (69), but this remains controversial (70). It is unknown whether narcoleptic-like sleep-onset REM periods occur in patients with peduncular hallucinosis, which would be the first step in linking hallucinations to REM dream intrusions. The rarity and transient nature of peduncular hallucinosis probably made it impossible to perform polysomnographic studies during the syndrome. However, in two cases, it was possible to show by polysomnography that a REM sleep behavior disorder could be associated with peduncular hallucinosis. The first case involved a hypothetical mechanism (compression of the left subthalamic region by a dilated carotid termination) (71). The second followed a small ischemic lesion of the rostro-dorsal region of the pons (72). The patient reported visual hallucinations and illusions, which persisted in an attenuated form during the years of follow-up. He also developed a REM sleep behavior disorder. This case demonstrates that the same circumscribed pontine lesion can lead to both types of disorders, suggesting an overlap in the mechanisms.
From Brainstem or Thalamic Lesions to Visual Hallucinations: Facts and Missing Links
Lhermitte stated that a lesion of a sleep regulating center would allow the “release” of dreams in a waking subject, through mechanisms involving “the entire extent of the brain, and especially the cerebral cortex” (3). This is not the place to elaborate on the complex mechanisms regulating wakefulness, REM and non-REM sleep, and dreams. Briefly, these phenomena depend on the interaction of a number of neurotransmitter systems in the brainstem, hypothalamus, and basal forebrain, which act by modulating the activity of thalamic nuclei and cortical areas (73). Importantly, mechanisms regulating dreams are at least partially distinct from those regulating sleep stages (74). This complexity precludes the possibility of putting forward a mechanistic model linking a lesion of the upper brainstem to the occurrence of transient episodes of peduncular hallucinosis.
One way to approach the neural basis of peduncular hallucinosis without a prior theoretical framework is to identify the regions functionally affected by lesions producing this syndrome. Indeed, the classical clinico-anatomical method has given way to connectivity studies, where the focus is on the circuitry involved in the symptoms and not the location of lesions in individual cases (47, 75). Boes et al. (47) collected 23 cases of peduncular hallucinosis with identifiable causative brain lesions, mapped these lesions onto a standard template brain, and used normative connectome data and overlapping lesion-associated networks to identify regions common to peduncular hallucinosis syndrome. Although the lesions were heterogeneously distributed, 22 of 23 lesions were negatively correlated with extrastriate visual cortex, a region implicated in visual hallucinations, based on prior functional imaging in patients (75, 76). The authors suggested that sites of anticorrelation (i.e., extrastriate visual cortex) predict sites of postlesion hyperactivity (47). Moreover, the peduncular hallucinosis lesions were positively correlated with the lateral geniculate nucleus. Interestingly, using a similar method, Kim et al. (75) showed that lesions causing visual hallucinations were connected to the lateral geniculate nucleus and that all subcortical lesions were again negatively correlated to extrastriate visual cortex. Thus, a plausible final common pathway of peduncular hallucinosis is the activation of extrastriate visual cortex, possibly secondary to an activation of lateral geniculate nuclei. The missing links between the brainstem lesion and visual cortex hyperactivity have been discussed by Müri (77), who summarized two models. The first one derives from the anatomical description in monkeys of a loop linking the area TE of the inferotemporal cortex (an area involved in object, face, and scene perception) to the basal ganglia (78). The proposed loop starts from TE, then projects successively on the tail of the caudate nucleus, the substantia nigra pars reticulata (SNr), the nigral territory of the thalamus (VAmc in Olszewski’s nomenclature), and finally, back to TE. In this model, a brainstem lesion interrupting the excitatory output from the subthalamic nucleus to the SNr would remove the inhibitory input of SNr to VAmc, leading to increased thalamic input to TE. One major limitation of this model is that SNr lesions are not constantly present in cases with peduncular hallucinosis. The second model proposes that a brainstem lesion produces an imbalance in several transmitter systems, including serotoninergic and cholinergic systems (79). This imbalance would impair the control of inputs to the thalamus and modulate the gating and filtering mechanisms of the thalamus to the visual and other sensory cortices. The key thalamic nuclei in this model are the dorsal lateral geniculate nucleus and the lateral pulvinar. Schematically, “lesions of the raphe nuclei may produce disinhibition of the dorsal lateral geniculate nucleus and impair the fidelity of retino-geniculo-cortical transmission” (79). In line with Lhermitte, Manford and Andermann suggested that hallucinations in narcoleptic syndromes and peduncular hallucinosis were likely to “share a similar pathophysiological basis” (79). Finally, the concept that, in various settings, visual hallucinations are linked to aberrant activity within thalamo-cortical networks remains relevant today (80, 81).
From Peduncular Hallucinosis to State Dissociation
Lhermitte explicitly described a dissociation between normal elements of sleep, waking, and dreaming. Throughout his work, he insisted on this concept with regard to peduncular hallucinosis, writing for example, “Pathological processes can carry out real dissociations of the hypnic function and generate somatic sleep in some cases, psychic sleep in others” (39). Lhermitte also used the term “dissociation” to describe the origin of cataplexy: “We are justified in considering cataplexy as the striking image of a dissociation of the function of sleep, a dissociation which results in inhibition, that is to say the deactivation of muscle tone which contrasts with the conservation of the functions of the mind” (3). He also described sleep paralysis under the term of “awakening cataplexy,” a phenomenon that would seem to “border the physiological state”: “Immediately after waking up in the morning, while his consciousness is perfectly awake, the subject, although he has the desire and the will, finds himself paralyzed. . . . This state is accompanied by an impression of diffuse and extremely unpleasant anguish.” Here again, the phenomenon originates with “the dissociation between psychic awakening and somatic awakening” (67). In short, “the mind watches over a sleeping body in cataplexy” (82). Lhermitte later came back (45) to what Kinnier Wilson had called in the meantime “sleep paralysis” in the context of narcolepsy (83, p. 90). He noticed that sleep paralysis could occur not only on waking, but also on falling asleep or in the middle of the night and that it could be associated with various sensory hallucinations, “psychic hallucinations,” and a “feeling of presence.” In peduncular hallucinosis and narcolepsy, “morbid sleep . . . can . . . manifest a positive side which, contrasting with the inhibition of consciousness or motor activity, allows the deployment of a complete or fragmentary dreamlike activity” (45).
Although described and discussed by Lhermitte, the concept of state dissociation was only recently formalized by Mark Mahowald and Carlos Schenck, two prominent sleep disorder specialists (84, 85). Healthy individuals have three states of being: wakefulness, REM sleep, and non-REM sleep. Either physiologically or in pathological circumstances, some features usually related to one state may occur in another state (Figure 1). For instance, sleepwalking results from a dissociation between wakefulness and non-REM sleep. In summary, “none of the changes occurring with sleep are invariably coupled to sleep” (86). The concept of dissociation of states, or state dissociation, is now widely accepted (87). Lhermitte was interested in and wrote about most of these states, including hypnagogic hallucinations, cataplexy, sleep paralysis, confusional arousals, and somnambulism (20, 61). For him, peduncular hallucinosis hallucinations belonged to this category of phenomena. Interestingly, Mahowald and Schenck (85), in line with Lhermitte, mentioned peduncular hallucinosis and other types of hallucinations as examples of dissociation between wakefulness and REM sleep, an interpretation that is still under discussion. Finally, one of the most studied dissociation of states at present, REM behavior disorder, was not identified by Lhermitte or contemporary authors but was first described by Carlos Schenck in 1986 (88).
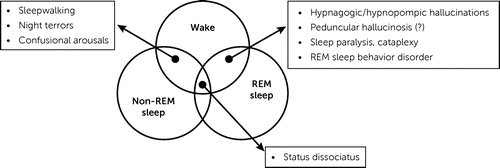
FIGURE 1. State dissociationa
aAdapted from Mahowald and Schenck (85). The diagram shows areas of overlap between states of being and some state dissociation disorders. The inclusion of peduncular hallucinosis in these disorders is in line with Lhermitte’s hypothesis but is debatable. REM=rapid eye movement.
CONCLUSIONS
Jean Lhermitte, in his 1922 article and following works, proposed or anticipated three innovative concepts that still have implications for contemporary clinicians and researchers. The first issue is that a focal lesion, located in the upper part of the brainstem, may cause hallucinations in the visual modality and possibly in others. At a time when psychic phenomena and higher cognitive functions were primarily ascribed to the cortex, Lhermitte claimed that “thought requires, for its normal functioning, the integrity not only of the cortex but of the underlying centers” (6). His model of a limited lesion acting through complex mechanisms and ultimately involving the cortex, possibly inspired by John Hughlings Jackson (1835–1911) (89), remains valid (75). This view also prefigures contemporary advances taking into account the lesion-induced functional alterations in anatomically intact, connected regions, including in the field of hallucinations (47). Secondly, Lhermitte postulated that peduncular hallucinosis was the result of a dissociation of the mechanisms of dream and waking states, the lesion disrupting the anatomy and connections of a center regulating wakefulness and sleep. The definition of peduncular hallucinosis has since been extended to cases involving a thalamic lesion, but the syndrome described by Lhermitte, although rare, remains accepted. His pathophysiological assumptions were limited by a lack of knowledge, at his time, of the complex and still partially obscure mechanisms regulating wakefulness, the different stages of sleep, and dreaming. The attractive but overly simplistic idea that peduncular hallucinosis is due to a mere intrusion of dreaming in a waking subject has not been confirmed, and the precise mechanisms involved in peduncular hallucinosis remain mysterious. However, discussions on the relationship between dreams and hallucinations are still going on (70, 90). Thirdly, Lhermitte identified that a dissociation of states, as conceptualized nowadays, underpinned several phenomena related to sleep, such as hypnagogic hallucinations, cataplexy, sleep paralysis, confusional arousals, and somnambulism.
According to MacDonald Critchley, Jean Lhermitte “was the beau idéal of a neuropsychiatrist” (91). With the description of the peduncular hallucinosis and the discussion of its mechanisms, Lhermitte initiated a broader reflection on hallucinations, which, almost a century later, perfectly illustrates the close relationship and the necessary dialogue between neurology and psychiatry.
Recent studies have examined the relationship between hallucinations and dreams (92, 93), as well as hallucinations in narcolepsy (94–96), building on Jean Lhermitte’s model.
1 : Modern neuropsychology in France: Jean Lhermitte (1877–1959). Cortex 2005; 41:740–741Crossref, Medline, Google Scholar
2 : Physiologie et psychiatrie: le point de vue introductif de Lhermitte en 1923. [Physiology and psychiatry: Lhermitte’s inaugural lecture in 1923]. Rev Neurol (Paris) 2015; 171:329–332Crossref, Medline, Google Scholar
3 : Les hallucinations. Clinique et physiopathologie. Paris, L’Harmattan, 2004Google Scholar
4 : La Maladie du Sommeil et Les Narcolepsies. Brussels, Severeyns, 1910Google Scholar
5 : Les narcolepsies. Rev Neurol (Paris) 1910; 2:203–214Google Scholar
6 : Syndrome de la calotte du pédoncule cérébral: les troubles psycho-sensoriels dans les lésions du mésocéphale. Rev Neurol (Paris) 1922; 38:1359–1365Google Scholar
7 : Le Syndrome Infundibulaire dans un Cas de Tumeur du Troisième Ventricule. Paris, Presse Med 1917; 25:417–418Google Scholar
8 : De la Narcolepsie. Surgères, Tessier et Tessier, 1881Google Scholar
9
10 : L’encéphalite épidémique. Gazette des Hôpitaux Civils et Militaires 1921; 94:37–43Google Scholar
11 : La régulation des fonctions psychiques: troubles mentaux par lésions extra-corticales. Paris Med (Paris) 1922; 45:63–68Google Scholar
12 : Historical trends in the diagnosis of peduncular hallucinosis. J Neuroophthalmol 2018; 38:438–441Crossref, Medline, Google Scholar
13 : Jacques Jean Lhermitte and the syndrome of peduncular hallucinosis. Neurosurg Focus 2019; 47:E9Crossref, Medline, Google Scholar
14 : Peduncular hallucinosis according to Jean Lhermitte. Rev Neurol (Paris) 2019; 175:377–379Crossref, Medline, Google Scholar
15 : Localization of Claude’s syndrome. Neurology 2001; 57:2304–2307Crossref, Medline, Google Scholar
16 : Acute bilateral ophthalmoplegia due to vertebrobasilar dolichoectasia: a report of two cases. Am J Case Rep 2017; 18:1302–1308Crossref, Medline, Google Scholar
17 : Syndrome inférieur du noyau rouge, troubles psycho-sensoriels d’origine mésocéphalique. Rev Neurol (Paris) 1924; 1:417–423Google Scholar
18 : L’hallucinose pédonculaire. Rev Neurol (Paris) 1927; 1:608–617Google Scholar
19 : Remarques sur une théorie bergsonienne de l’hallucination. Ann Med Psychol (Paris) 1925; −:242–259Google Scholar
20 : Le sommeil. Paris, Armand Colin, 1931Google Scholar
21 : Les troubles psycho-sensoriels associés aux ophtalmoplégies centrales. Paris, Bulletin de la Société Ophtalmologique de Paris, 1925Google Scholar
22 : Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology 1988; 38:837–848Crossref, Medline, Google Scholar
23 : L’hallucinose pédonculaire (un nouveau cas de lésion de la calotte pédonculaire provoquée par une intoxication aiguë par divers narcotiques). Rev Neurol (Paris) 1931; 1:312–318Google Scholar
24 : L’hallucinose pédonculaire (étude anatomique d’un cas). Rev Neurol (Paris) 1932; 1:382–388Google Scholar
25 : Phénomènes d’hallucinose chez une malade présentant une torsion et une contracture athétoïde du bras. Rev Neurol (Paris) 1931; 1:609–610Google Scholar
26 : Phénomènes de décérébration, de torsion spasmodique et d’athétose, leurs relations cliniques et pathogéniques. Rev Neurol (Paris) 1926; 2:432–434Google Scholar
27 : Movement disorders and stroke. Rev Neurol (Paris) 2016; 172:483–487Crossref, Medline, Google Scholar
28 : Post-thalamic stroke movement disorders: a systematic review. Eur Neurol 2018; 79:303–314Crossref, Medline, Google Scholar
29 : Hallucinose consécutive à un ictus suivi d’hémiplégie gauche avec troubles de la sensibilité et mouvements involontaires. Rev Neurol (Paris) 1933; 1:67–70Google Scholar
30 : Pathogénie de l’hallucinose pédonculaire: á propos d’un nouveau cas. Rev Neurol (Paris) 1935; 64:606–608Google Scholar
31 : A clinical study of lateral hemianopsia. J Nerv Ment Dis 1886; 13:1–38Crossref, Google Scholar
32 : Hémianopsie avec hallucinations dans la partie abolie du champ de la vision. Rev Neurol (Paris) 1895; 3:129–135Google Scholar
33 : A grid-like hemi-field defect following a lacunar infarct in the lateral geniculate nucleus. Clin Neurol Neurosurg 2012; 114:278–280Crossref, Medline, Google Scholar
34 : Les hallucinations visuelles consécutives aux lésions pédonculaires en foyer. Rev Neurol (Paris) 1937; 2:827–831Google Scholar
35 : Syndrome contro-latéral du noyau rouge avec hallucinations visuelles et auditives. Rev Neurol (Paris) 1938; 70:623–628Google Scholar
36 : Sur une modalité de l’hémorragie des pédoncules cérébraux: syndrome de Parinaud, syndrome oculo-sympathique, iridoplégie, exaltation des réflexes dits de défense: phénomène d’Hertwig-Magendie, hallucinose et onirisme. Rev Neurol (Paris) 1941; 73:114–118Google Scholar
37 : Syndrome bulbaire d’origine hémorragique: distorsion de l’image de soi; hallucinose visuelle. Rev Neurol (Paris) 1937; 1:62–68Google Scholar
38 : Les Hémorragies des Pédoncules Cérébraux. Étude Clinique. Paris, Presse Med 1942; 45:624–626Google Scholar
39 : L’hallucinose pédonculaire. Encéphale (Paris) 1932; 27:422–435Google Scholar
40 : Les hallucinations visuelles au cours des syndromes pédonculaires. Ann Med Psychol (Paris) 1934; 2:556–569Google Scholar
41 : Sur quelques cas de paralysies multiples de nerfs crâniens extensives et curables: polioencéphalites à virus neurotropes probables. Paris Med (Paris) 1934; 1:263–272Google Scholar
42 : Phantom limb after stroke: an underreported phenomenon. Cortex 2010; 46:1114–1122Crossref, Medline, Google Scholar
43 : Tuberkel im Tegmentum Pontis: Beitrag zur Symptomatologie der Ponsherde: Piksche Visionen. Schweiz Arch Neurol Psychiatr 1922; 11:221–229Google Scholar
44 : Neurobiologie de L’hallucination. Essai sur une Variété Particulière de Désintégration de la Fonction. Brussels, Lamertin, 1932Google Scholar
45 : Désordre de la fonction hypnique et hallucinations. Ann Med Psychol (Paris) 1938; 1:1–14Google Scholar
46 : Hallucinations visuelles et lésions de l’appareil visuel. Ann Med Psychol (Paris) 1936; 94:321–351Google Scholar
47 : Network localization of neurological symptoms from focal brain lesions. Brain 2015; 138:3061–3075Crossref, Medline, Google Scholar
48 : Pure midbrain infarction: clinical syndromes, MRI, and etiologic patterns. Neurology 1994; 44:2032–2040Crossref, Medline, Google Scholar
49 : Pure midbrain infarction: clinical, radiologic, and pathophysiologic findings. Neurology 2005; 64:1227–1232Crossref, Medline, Google Scholar
50 : Clinical study of twenty-one patients with pure midbrain infarction. Eur Neurol 2012; 67:81–89Crossref, Medline, Google Scholar
51 : Mesencephalic and associated posterior circulation infarcts. Stroke 2002; 33:2224–2231Crossref, Medline, Google Scholar
52 : Exploring the brain bases of dreaming: commentary on “Beyond the neuropsychology of dreaming: insights into the neural basis of dreaming with new techniques of sleep recording and analysis.” Sleep Med Rev 2017; 35:124–126Crossref, Medline, Google Scholar
53 : Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953; 118:273–274Crossref, Medline, Google Scholar
54 : The mysteries of sleep and waking unveiled by Michel Jouvet. Sleep Med 2018; 49:14–19Crossref, Medline, Google Scholar
55 : Les Rêves. Que Sais-je n 24, 8th ed. Paris, Presses Universitaires de France, 1963Google Scholar
56 :
57 : Des hallucinations oniriques des dégénérés mystiques. Congrès des médecins aliénistes et neurologistes de France et des pays de langue française. Cinquième session. Paris, Masson, 1895Google Scholar
58 : Précis de psychiatrie, 3rd ed. Paris, Doin, 1906Google Scholar
59 : Le délire alcoolique n’est pas un délire mais un rêve. Archives Générales de Médecine, 1881; 7:513–536Google Scholar
60 : Confusion mentale et onirisme. Prog Med (Paris) 1930; 1:1397–1398Google Scholar
61 : Les rêves, le somnanbulisme, l’hypnose et l’onirisme. Rev Prat (Paris) 1954; (4):1563–1569Google Scholar
62 : De l’influence de l’état intermédiaire à la veille et au sommeil sur la production et la marche des hallucinations. Ann Med Psychol (Paris) 1845; 6:1–29Google Scholar
63 : Des hallucinations hypnagogiques, ou des erreurs des sens dans l’état intermédiaire entre la veille et le sommeil. Ann Med Psychol (Paris) 1848; 11:26–40Google Scholar
64
65 : Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000; 97:153–164Crossref, Medline, Google Scholar
66 :
67 : Rapport sur le sommeil normal et pathologique. Rev Neurol (Paris) 1927; 1:750–822Google Scholar
68 : Hypnagogisme, hallucinose et hallucinations. Rev Neurol (Paris) 1941; 73:225–238Google Scholar
69 : Hallucinations, REM sleep, and Parkinson’s disease: a medical hypothesis. Neurology 2000; 55:281–288Crossref, Medline, Google Scholar
70 : What is the link between hallucinations, dreams, and hypnagogic-hypnopompic experiences? Schizophr Bull 2016; 42:1098–1109Crossref, Medline, Google Scholar
71 : Peduncular hallucinosis: a polysomnographic and spect study of a patient and efficacy of serotonergic therapy. Sleep Med 2009; 10:1158–1160Crossref, Medline, Google Scholar
72 : Altered functional connectivity in lesional peduncular hallucinosis with REM sleep behavior disorder. Cortex 2016; 74:96–106Crossref, Medline, Google Scholar
73 : Control of sleep and wakefulness. Physiol Rev 2012; 92:1087–1187Crossref, Medline, Google Scholar
74 : Beyond the neuropsychology of dreaming: Insights into the neural basis of dreaming with new techniques of sleep recording and analysis. Sleep Med Rev 2017; 35:8–20Crossref, Medline, Google Scholar
75 : Lesions causing hallucinations localize to one common brain network. Mol Psychiatry 2021; 26:1299–1309Crossref, Medline, Google Scholar
76 : The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 2016; 69:113–123Crossref, Medline, Google Scholar
77 :
78 : The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA 1996; 93:8683–8687Crossref, Medline, Google Scholar
79 : Complex visual hallucinations: clinical and neurobiological insights. Brain 1998; 121:1819–1840Crossref, Medline, Google Scholar
80 : Visual hallucinations. Wiley Interdiscip Rev Cogn Sci 2010; 1:781–786Crossref, Medline, Google Scholar
81 : On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol 2015; 262:1780–1790Crossref, Medline, Google Scholar
82 : Sur la cataplexie et plus spécialement sur la cataplexie du réveil. L’Encéphale (Paris) 1928; 23:424–434Google Scholar
83 : The narcolepsies. Brain 1928; 51:63–109Crossref, Google Scholar
84 : Status dissociatus: a perspective on states of being. Sleep 1991; 14:69–79Crossref, Medline, Google Scholar
85 : Evolving concepts of human state dissociation. Arch Ital Biol 2001; 139:269–300Medline, Google Scholar
86 : From REM sleep behaviour disorder to status dissociatus: insights into the maze of states of being. Sleep Med 2011; 12(Suppl 2):S68–S71Crossref, Medline, Google Scholar
87 : From state dissociation to status dissociatus. Sleep Med Rev 2016; 28:5–17Crossref, Medline, Google Scholar
88 :
89 : John Hughlings Jackson and the clinico-anatomical correlation method. Cortex 2011; 47:905–907Crossref, Medline, Google Scholar
90 : Dreaming and hallucinations–continuity or discontinuity? Perspectives from dementia with Lewy bodies. Conscious Cogn 2011; 20:1016–1020Crossref, Medline, Google Scholar
91 : Jean Lhermitte, MD. BMJ 1959; 1:652–653Crossref, Google Scholar
92 : Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci 2000; 23:793–842, discussion 904–1121 Crossref, Medline, Google Scholar
93 : Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn Sci 2010; 14:88–100Crossref, Medline, Google Scholar
94 : Psychotic symptoms in narcolepsy: phenomenology and a comparison with schizophrenia. Gen Hosp Psychiatry 2009; 31:146–154Crossref, Medline, Google Scholar
95 : Hallucinations in narcolepsy with and without cataplexy: contrasts with Parkinson’s disease. Sleep Med 2011; 12:497–504Crossref, Medline, Google Scholar
96 :



