Ketone Bodies and Brain Metabolism: New Insights and Perspectives for Neurological Diseases
The brain accounts for only approximately 2% of total body weight. However, it consumes about 20% of the body’s energy, among the highest of all organs (1). Brain energy metabolism (neuroenergetics) is an essential process for neural function and higher brain functions, such as memory and cognition (6). The neuroenergetic requirements to sustain neural and brain function depend primarily on glucose consumption via glycolysis or mitochondrial respiration processes via oxidative phosphorylation. Glycolysis does not require oxygen, occurs in the cytosol, and results in two adenosine triphosphate (ATP) molecules. Mitochondrial respiration requires oxygen and generates a much higher energy yield of 30–36 ATP molecules (1, 7, 8).
Glucose and oxygen are the primary energy substrates for the brain (8). However, under specific circumstances (e.g., breastfeeding, adult ketosis, and diabetes), ketone bodies (KBs), lactate, and pyruvate act as alternative substrates for neurons (1). These monocarboxylate substrates can sustain normal brain activity (e.g., neuronal function and synaptic activity) during glucose deprivation (9). However, when systemic blood glucose levels drop, low endogenous carbohydrate levels cannot meet the body’s energy requirements, causing ketogenesis (2, 3) (Figure 1A–B).
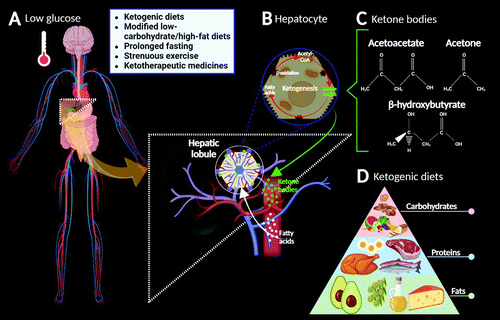
FIGURE 1. Ketogenesis (simplified).
A. When systemic blood glucose levels drop, the low endogenous carbohydrate levels are unable to sustain the energy requirements of the body, thereby ketogenesis begins. In these circumstances, the body responds by decreasing insulin secretion, increasing glucagon secretion, and mobilizing fats from adipocytes to the liver cells (hepatocytes) for the breakdown of fatty acid molecules. B. Dietary fatty acids undergo β-oxidation within hepatocytes in the hepatic lobules of the liver, resulting in the formation of ketone bodies (1). C. Ketone bodies (acetoacetate, acetone, and β-hydroxybutyrate) can substitute for glucose as the primary source of energy in the body, particularly in the heart and the brain (2). D. Ketogenic diets are high-fat, high-protein, low-carbohydrate diets that result in the modulation of glycemia, elevated fatty acid levels, and relative caloric restriction (2, 3).
KBs such as acetoacetate, acetone, and β-hydroxybutyrate can substitute for glucose as the primary source of energy for the body, particularly in the heart and the brain (2) (Figure 1C). Neonatally, KBs act as precursors for production of biological molecules, such as fats (especially cholesterol) and amino acids (10). KBs enter nerve cells via monocarboxylate transporters and enter the mitochondrial metabolic pathway, resulting in the production of ATP (Figure 2A–B). In the central nervous system (CNS), brain cells can use KBs as respiratory substrates for oxidative metabolic processes (4). KB metabolic activity in the brain is regulated by the permeability of the blood-brain barrier, which depends on the abundance of cerebral monocarboxylate transporters, brain enzymatic processes, and other factors (e.g., diet, fasting, and exercise) (10).
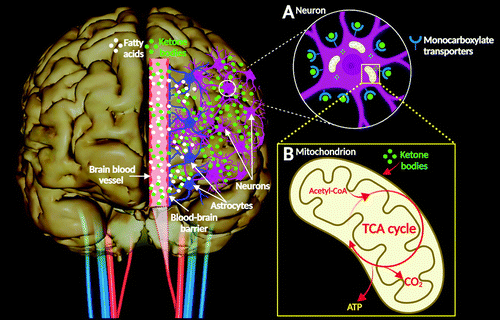
FIGURE 2 and COVER. Brain energy metabolism (simplified).
A. Ketone bodies are transported in blood vessels. They cross the blood-brain barrier and access nervous tissue via monocarboxylate transporters located on the plasmalemma of vascular endothelial cells and brain cells (1). In the central nervous system, brain cells can use ketone bodies as respiratory substrates for oxidative metabolic processes (4). Astrocytes can perform fatty acid oxidation to produce ketone bodies, which are then transferred to neurons as their main energy substrate. Neurons express all degradation enzymes (e.g., d-β-hydroxybutyrate dehydrogenase, acetoacetate-succinyl-CoA transferase, and acetoacetyl CoA-thiolase) required for ketolysis (ketone body metabolism). B. Within the mitochondria of neurons, ketone bodies are converted into acetyl coenzyme A (acetyl-CoA), which enters the tricarboxylic acid cycle (TCA cycle), yielding adenosine triphosphate (ATP) molecules (1, 5).
All images created under the terms of the Creative Commons Attribution License. Created with VH Dissector and BioRender.com.
Ketogenic diets (KDs) are high-fat, high-protein, low-carbohydrate diets that result in the modulation of glycemia, elevated fatty acid levels, and relative caloric restriction (2, 3) (Figure 1D). Nutritionally, such diets increase the production of KBs, a process called ketosis (2). However, ketosis can also occur in people consuming a low-calorie diet or a modified low-carbohydrate, high-fat diet and in individuals undergoing prolonged fasting periods and strenuous exercise (although the last two do not induce nutritional ketosis) (11, 12). In addition, ketotherapeutic medicines (e.g., medium-chain triglycerides and ketone esters) are bioenergetic supplements that can induce ketosis and regulate energy metabolism (12). These interventions are considered safe and well-tolerated treatments, potentially serving as excellent metabolic alternatives that prevent, slow, halt, or even reverse the development of some neurodegenerative diseases (13, 14) (Figure 1A–D).
Effects of Ketone Bodies in Neurological Diseases
KBs can provide neuronal protection during conditions of glucose deficiency, such as hypoglycemia (1). Thus, multiple reports have confirmed some potential therapeutic benefits of KDs for various neurological conditions (15–18). A meta-analysis of 170 animal studies concluded that KDs provide multiple benefits involving aspects of epigenetics; neurotransmitter function, neuroinflammation, neuroprotection, and neuroplasticity; nociception; signaling pathways and synaptic transmission; and vascular supply (18). Similarly, a systematic study summarizing trials of therapeutic use of KDs in traumatic brain injury (TBI) and other neurological disturbances (including aggressive brain tumors, ischemic stroke, and status epilepticus) concluded that KDs can be supportive in clinical management (2). Neurological conditions with preliminary evidence showing the benefits of KDs are discussed below.
Traumatic Brain Injury
TBI is linked to a significant increase (∼85%) in the number of monocarboxylate transporter channels that facilitate the transport of KBs into neurons and is accompanied by a surge of β-hydroxybutyrate–metabolizing enzymes in these cells (19–22). These findings suggest that upon injury, the brain shifts to the energetic pathway involving the metabolism of KBs (2). In line with this concept, the β-hydroxybutyrate components of KBs have two well-known neuroprotective properties: supporting the biochemical reconstruction of the respiratory chain and providing at least some energy from KB metabolism when the first complex of the respiratory chain via ATP-sensitive potassium channels is disrupted (21, 22).
Results from rodent studies of TBI indicate that KDs improve cerebral metabolism, neuroprotection, and behavioral outcomes; protect myelin-forming oligodendrocytes; and reduce axonal damage while mitigating cerebral edema and apoptosis (23–26). A preliminary clinical trial revealed that a KD could be effective and feasible in adults with TBI (26). However, clinical trial data supporting the widespread use of ancillary KDs in the clinical management of TBI remain limited (27).
Epilepsy
KDs have been associated with positive outcomes in patients with medication-resistant epilepsy and febrile infection–related epilepsy syndrome (3, 28, 29). KDs may be effective in treating infants and children with medication-resistant epilepsy by increasing levels of KBs, which in turn mechanistically contribute (at least in part) to seizure control, possibly due to anticonvulsant effects (30–32). Some of the anticonvulsant characteristics of KBs result from the amplification of brain messengers and neuroactive substances, such as gamma-aminobutyric acid, agmatine, and monoamines, thereby reducing neuronal hypersensitivity (33). Anticonvulsive effects also result from the regulation of glutamate, possibly by altering the behavior of vesicular glutamate transporters, regulation of the neuronal membrane potential via ATP-sensitive potassium channels (activated during conditions of low ATP), and optimization of the tricarboxylic acid cycle and the electron transport chain cellular energy systems (34).
Alzheimer’s Disease
The success of ketogenic therapies in epilepsy has increased interest in testing their use for other neurological disorders, such as Alzheimer’s disease (AD) (12). Comorbid AD and epilepsy is being increasingly recognized in people of advanced age. Patients with comorbid epilepsy and AD may experience seizures and epileptiform discharges at any stage of AD (35). In AD, mitochondrial dysfunction and decreased respiratory chain function alter processing of the amyloid precursor protein, which leads to increased production and deposition of beta-amyloid fragments in the brain (36). In addition, high-sugar diets are linked to increased deposition of beta-amyloid fragments, suggesting that the brain’s insulin resistance may contribute to AD (37, 38).
The synthesis of KBs may lead to specific neurological benefits, including reducing inflammatory and apoptotic mediators and improving mitochondrial functionality (39). Furthermore, KBs generated from KD consumption decrease deposition and plaque formation of beta-amyloid fragments by reversing beta-amyloid neurotoxicity. Thus, KDs (including low-carbohydrate diets) might be useful in the clinical management of AD (40).
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common progressive neurodegenerative disorder of the CNS, affecting over 1% of people over 60 years of age (41, 42). In addition to reductions in dopamine synthesis, the pathogenesis of PD seems to involve other contributing factors, such as abnormal glucose metabolism in the brain, inflammation in the CNS, mitochondrial dysfunction, and metabolic disturbances (42, 43). Approximately 50%–80% of patients with a PD diagnosis also have impaired carbohydrate metabolism. In addition, peripheral insulin resistance, which is typically linked to brain insulin signaling and neuronal bioenergetic issues, is often seen in early PD (43, 44).
Two important factors should be considered regarding KDs as a treatment for PD. First, high-carbohydrate, low-fat diets facilitate the availability of tyrosine, a dopamine precursor, in the cerebrospinal fluid, thereby increasing brain dopamine (insulin induced) (45). This change improves one of the most important pathophysiologic hallmarks of PD, deficits in the neurotransmitter dopamine. Second, increased KBs (via KDs) may improve mitochondrial oxidative phosphorylation in the brain and bolster energy metabolism in central and peripheral neurons through mechanistic stimulation of mitochondrial biogenesis (22, 46). These changes may contribute to attenuating the substantia nigra and frontal cortex deficits in respiratory chain complex I activity that have been reported in patients with PD (42, 43, 47).
Levodopa (L-DOPA) is considered the primary medication for PD (48). L-DOPA ameliorates PD motor symptoms but does not seem to have any neuroprotective effects (49) and, paradoxically, may promote aggregation of alpha-synuclein (via the metabolite 5-S-cysteinyldopamine), inducing oxidative stress that can further deplete dopamine in the brain (50). Some studies indicate that KDs improve the bioavailability of L-DOPA (49, 51). Other studies suggest that simultaneous use of L-DOPA and a KD may halt the progression of PD symptoms (49, 52, 53).
Animal and human studies indicate that KDs and ketotherapeutic supplements have other benefits, including protection of dopaminergic neurons from degeneration and improvements in motor function (22, 51, 54). In a recent randomized controlled trial, 47 patients with PD were assigned to either a KD (high-fat diet) group or a low-fat diet group for 8 weeks. Both groups demonstrated significantly improved motor and nonmotor skills. However, the KD group showed greater improvements in nonmotor symptoms. In addition, the KD was confirmed to be a safe and reasonable intervention for patients with PD (55). Although these results are promising, more investigation is required (56).
Gliomas
Gliomas are highly heterogeneous brain tumors, and glioblastoma is the most aggressive type of glioma in adults. Glioblastomas have an extremely poor prognosis of approximately 12–15 months from the time of diagnosis, and the 5-year survival rate is below 5%. These tumors are characterized by a poor response to treatment (57–59). Glioma cells survive mainly on glucose and cannot function without it, suggesting that KDs could potentiate apoptosis. In a clinical trial, ketosis occurred in patients with glioblastoma who consumed a liquid KD 2 weeks before beginning chemoradiation therapy. These results suggested that a KD had some therapeutic effects and was both feasible and safe in combination with standard chemoradiation therapy (59). In addition, a recent systematic analysis indicated that KDs are beneficial for patients with malignant gliomas, mostly based on higher rates of survival (60).
Migraine
Migraine is a chronic disease, resulting from both genetic and environmental factors. Two systematic studies reported therapeutic potential for KDs, with one study noting that KD interventions reduced the number of attacks and the intensity of headaches among participants with migraine (2, 61).
Multiple Sclerosis
Multiple sclerosis (MS) is a neurodegenerative and inflammatory condition of the CNS with an autoimmune origin (2, 62). A growing body of evidence suggests that a KD is beneficial for those with MS and is both safe and feasible (63–65). Additionally, the clinical evidence suggests that patients with relapsing MS who follow a KD over a 6-month period typically experienced neuroprotective and desirable disease-modifying effects, such as reduced fatigue, depression, neurological disability, and adipose-related inflammation (65).
Limitations of KETOGENIC Diets
KD and ketotherapeutic interventions have some potential adverse effects and contraindications. Adults who consume a KD often report weight loss; gastrointestinal side effects, such as constipation, diarrhea, nausea, and vomiting; and a transient increase in lipids. Although rare, headaches, abdominal pain, irregular menstruation, drowsiness, nephrolithiasis, and pancreatitis also have been associated with KDs (2, 66, 67). Consuming low-carbohydrate diets may also lead to reduced appetite and suppressed hunger. Older people, especially those with dementia, typically have a lack of appetite and dysphagia. The use of KDs in such individuals may cause them to omit meals, resulting in malnutrition and other nutrient deficiencies, which may worsen their condition (68). In a study of patients with PD, KDs were associated with periodic tremors or stiffness, increased irritability, and exacerbated hunger or thirst (55). Long-term use of KDs may also cause more serious side effects, including hyperuricemia, proteinuria, and metabolic acidosis, especially among individuals with coexisting diabetes and inadequate insulin management. In addition, increased aminotransferase and other liver enzyme activity has been reported, suggesting a temporary increase in hepatic enzyme activity possibly associated with a KD-induced hypercholesteremia (67, 69).
Finally, there is the so-called “keto flu,” a cluster of symptoms and side effects that appear within the first few weeks of KD initiation. Symptoms may include “brain fog,” headache, nausea, dizziness, fatigue, gastrointestinal discomfort, constipation, arrhythmias, or low energy (70). Overall, these symptoms are transient and seem to be mild (2, 70). Serious adverse effects of KDs seem rare and usually result from a lack of or inadequate clinical supervision. Thus, use of KDs and ketotherapeutic approaches should be individualized for each patient (2).
Conclusions
In summary, nutritional regimens (KDs and ketotherapeutic supplements) that generate increased KBs in plasma and the brain (ketosis) appear to have substantial potential to improve neuronal processes, such as mitochondrial metabolism, cell signaling, and neurotransmitter function. Additionally, KDs can reduce oxidative stress, inflammation, and toxicity, which can increase neural network stability and thereby improve cognitive function. There is a growing body of evidence supporting the benefits of KBs for some neurological conditions. However, there remains a lack of data from randomized, blinded trials in large populations and relevant subpopulations to determine the feasibility, sustainability, and long-term effects of ketotherapeutic interventions, either alone or as adjuvant treatments for CNS disorders.
1. : Brain energy metabolism; in Fundamental Neuroscience. 4th ed. Edited by Squire LR, Berg D, Bloom FE, . San Diego, Academic Press, 2013, pp 261–284 Google Scholar
2. : The role of ketogenic diet in the treatment of neurological diseases. Nutrients 2022; 14:5003Crossref, Medline, Google Scholar
3. : Ketogenic diet and neuroinflammation. Epilepsy Res 2020; 167:106454Crossref, Medline, Google Scholar
4. : Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 1987; 18:551–561Crossref, Medline, Google Scholar
5. : Effects of intermittent fasting on brain metabolism. Nutrients 2022; 14:1275Crossref, Medline, Google Scholar
6. : A cellular perspective on brain energy metabolism and functional imaging. Neuron 2015; 86:883–901Crossref, Medline, Google Scholar
7. : Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci 2018; 19:63–80Crossref, Medline, Google Scholar
8. : Neuroenergetics and “general intelligence”: a systems biology perspective. J Intell 2020; 8:31Crossref, Medline, Google Scholar
9. : Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 2006; 26:142–152Crossref, Medline, Google Scholar
10. : Cerebral ketone body metabolism. J Inherit Metab Dis 2005; 28:109–121.Crossref, Medline, Google Scholar
11. : Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017; 25:262–284Crossref, Medline, Google Scholar
12. : Neuroketotherapeutics: a modern review of a century-old therapy. Neurochem Int 2018; 117:114–125Crossref, Medline, Google Scholar
13. : Multi-loop model of Alzheimer disease: an integrated perspective on the Wnt/GSK3β, α-synuclein, and type 3 diabetes hypotheses. Front Aging Neurosci 2019; 11:184Crossref, Medline, Google Scholar
14. : Ketotherapeutics for neurodegenerative diseases. Int Rev Neurobiol 2020; 155:141–168Crossref, Medline, Google Scholar
15. : The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 2012; 3:59Crossref, Medline, Google Scholar
16. : The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol 2008; 10:410–419Crossref, Medline, Google Scholar
17. : Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain 2016; 17:58Crossref, Medline, Google Scholar
18. : Ketogenic diets and the nervous system: a scoping review of neurological outcomes from nutritional ketosis in animal studies. Nutr Res Rev 2022; 35:268–281Crossref, Medline, Google Scholar
19. : Induction of monocarboxylate transporter 2 expression and ketone transport following traumatic brain injury in juvenile and adult rats. Dev Neurosci 2006; 28:447–456Crossref, Medline, Google Scholar
20. : The molecular pathophysiology of concussive brain injury. Clin Sports Med 2011; 30:33–48Crossref, Medline, Google Scholar
21. : Beta-hydroxybutyrate, a cerebral function improving agent, protects rat brain against ischemic damage caused by permanent and transient focal cerebral ischemia. Jpn J Pharmacol 2002; 89:36–43Crossref, Medline, Google Scholar
22. : D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 2003; 112:892–901Crossref, Medline, Google Scholar
23. : The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Inj 2018; 32:416–422Crossref, Medline, Google Scholar
24. : Ketogenic diet as a potential treatment for traumatic brain injury in mice. Sci Rep 2021; 11:23559Crossref, Medline, Google Scholar
25. : Ketogenic diet protects myelin and axons in diffuse axonal injury. Nutr Neurosci 2022; 25:1534–1547Crossref, Medline, Google Scholar
26. : Phase I single center trial of ketogenic diet for adults with traumatic brain injury. Clin Nutr ESPEN 2022; 47:339–345Crossref, Medline, Google Scholar
27. : Role of the ketogenic diet in acute neurological diseases. Clin Neurol Neurosurg 2020; 192:105727Crossref, Medline, Google Scholar
28. : Extinguishing febrile infection-related epilepsy syndrome: pipe dream or reality? Semin Neurol 2020; 40:263–272Crossref, Medline, Google Scholar
29. : Ketogenic diets for drug-resistant epilepsy. Am Fam Physician 2021; 103:524–525Medline, Google Scholar
30. : Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst Rev 2020; 6:CD001903Medline, Google Scholar
31. : Long-term effectiveness and adverse effects of ketogenic diet therapy in infants with drug-resistant epilepsy treated at a single center in Argentina. Epilepsy Res 2021; 178:106793Crossref, Medline, Google Scholar
32. : Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology 2018; 133:233–241Crossref, Medline, Google Scholar
33. : The metabolic role of ketogenic diets in treating epilepsy. Nutrients 2022; 14:5074Crossref, Medline, Google Scholar
34. : Ketone bodies in epilepsy. J Neurochem 2012; 121:28–35Crossref, Medline, Google Scholar
35. : Alzheimer’s disease and epilepsy: an increasingly recognized comorbidity. Front Aging Neurosci 2022; 14:940515Crossref, Medline, Google Scholar
36. : Amyloid precursor protein processing and bioenergetics. Brain Res Bull 2017; 133:71–79Crossref, Medline, Google Scholar
37. : A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr 2017; 106:1463–1470Crossref, Medline, Google Scholar
38. : Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs 2017; 77:47–65Crossref, Medline, Google Scholar
39. : Ketogenic diet in Alzheimer’s disease. Int J Mol Sci 2019; 20:3892Crossref, Medline, Google Scholar
40. : The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019; 60:118–121Crossref, Medline, Google Scholar
41. : Effects of ketogenic diet on neuroinflammation in neurodegenerative diseases. Aging Dis 2022; 13:1146–1165Crossref, Medline, Google Scholar
42. : The effect of the ketogenic diet on the therapy of neurodegenerative diseases and its impact on improving cognitive functions. Dement Geriatr Cogn Dis Extra 2022; 12:100–106Crossref, Medline, Google Scholar
43. : Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: a controlled pilot trial. Clin Park Relat Disord 2019; 1:41–47Medline, Google Scholar
44. : Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol 2008; 7:97–109Crossref, Medline, Google Scholar
45. : Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr 2003; 77:128–132Crossref, Medline, Google Scholar
46. : Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 2006; 60:223–235Crossref, Medline, Google Scholar
47. : Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res 2008; 1189:215–218Crossref, Medline, Google Scholar
48. : Levodopa (L-Dopa). Treasure Island, Fla., StatPearls, 2022Google Scholar
49. : The therapeutic role of ketogenic diet in neurological disorders. Nutrients 2022; 14:1952Crossref, Medline, Google Scholar
50. : 5-S-cysteinyldopamine neurotoxicity: influence on the expression of α-synuclein and ERp57 in cellular and animal models of Parkinson’s disease. J Neurosci Res 2014; 92:347–358Crossref, Medline, Google Scholar
51. : Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology 2005; 64:728–730Crossref, Medline, Google Scholar
52. : Microbiome-gut-brain axis and Toll-like receptors in Parkinson’s disease. Int J Mol Sci 2018; 19:1689Crossref, Medline, Google Scholar
53. : Dietary approaches to improve efficacy and control side effects of levodopa therapy in Parkinson’s disease: a systematic review. Adv Nutr 2021; 12:2265–2287Crossref, Medline, Google Scholar
54. : Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res 2009; 1286:25–31Crossref, Medline, Google Scholar
55. : Low-fat versus ketogenic diet in Parkinson’s disease: a pilot randomized controlled trial. Mov Disord 2018; 33:1306–1314Crossref, Medline, Google Scholar
56. : Ketogenic therapy for Parkinson’s disease: a systematic review and synthesis without meta-analysis of animal and human trials. Maturitas 2022; 163:46–61Crossref, Medline, Google Scholar
57. : Malignant gliomas in adults. N Engl J Med 2008; 359:492–507Crossref, Medline, Google Scholar
58. : Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med 2014; 6:1359–1370Crossref, Medline, Google Scholar
59. : Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: a feasibility and safety study. Ther Adv Med Oncol 2019; 11:1758835919853958Crossref, Google Scholar
60. : Effects of the ketogenic diet in the treatment of gliomas: a systematic review. Nutrients 2022; 14:1007Crossref, Medline, Google Scholar
61. : Efficacy and tolerability of the ketogenic diet and its variations for preventing migraine in adolescents and adults: a systematic review. Nutr Rev 2022; 80:1634–1647Crossref, Medline, Google Scholar
62. : Self-management of falls in people with multiple sclerosis: a scoping review. Clin Rehabil 2023; 37:162–176Crossref, Medline, Google Scholar
63. : Role of ketogenic diets in multiple sclerosis and related animal models: an updated review. Adv Nutr 2022; 13:2002–2014Crossref, Medline, Google Scholar
64. : Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2019; 6:e565Crossref, Medline, Google Scholar
65. : Phase II study of ketogenic diets in relapsing multiple sclerosis: safety, tolerability and potential clinical benefits. J Neurol Neurosurg Psychiatry 2022; 93:637–644Crossref, Medline, Google Scholar
66. : Ketogenic diets for adult neurological disorders. Neurotherapeutics 2018; 15:1018–1031Crossref, Medline, Google Scholar
67. : Safety and effectiveness of the prolonged treatment of children with a ketogenic diet. Nutrients 2020; 12:306Crossref, Medline, Google Scholar
68. : Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes Rev 2015; 16:64–76Crossref, Medline, Google Scholar
69. : Ketogenic diet-induced elevated cholesterol, elevated liver enzymes and potential non-alcoholic fatty liver disease. Cureus 2020; 12:e6605Medline, Google Scholar
70. : Consumer reports of “keto flu” associated with the ketogenic diet. Front Nutr 2020; 7:20Crossref, Medline, Google Scholar



