Occupational Exposure to Solvents: Neuropsychiatric and Imaging Features
Webster’s Dictionary defines a poison as “any substance (natural or synthetic) that, at a certain dosage, damages living tissues and injures or kills.”9 A toxin or venom is a poison “spontaneously produced by living organisms.”9 Poisons and toxins can be inhaled, absorbed percutaneously, injected, or ingested. Within the field of medicine, poisons are generally divided into five categories: heavy metals, gases, organic phosphates/solvents/pesticides, complex metal ions, and psychoactive drugs of abuse. Some poisons fall into two of the above categories because exposure can occur in both abuse and nonabuse settings (e.g., nitrous oxide, solvents). Every individual category of poisons has differing effects on the human body and, in particular, differing effects on areas of the CNS. For example, some poisons destroy axons, others the neuron cell bodies, some impair specific regions and others diffusely destroy myelin. A classic example is the predilection that carbon monoxide has for basal ganglia destruction. In examining the effects of a poison, exposure has to be viewed in terms of both duration and extent, whether one time or on going, pre-existing underlying health of the affected individual, and healthcare received after exposure. Solvent exposure can occur accidentally (e.g., drinking contaminated ground water, percutaneous absorption, or inhalation in an occupational setting) or intentionally (e.g., ingestion orally in a suicide attempt, inhaled as a means to attain an inexpensive “high”). Although much is written about toluene or other solvents in the context of substance abuse, much less is known about the long-term neuropsychiatric course of individuals with occupational or other accidental exposures.
Many organic solvents (e.g., benzene, chloride compounds, chloroethylenes) have been noted by the National Institute for Occupational Safety and Health (NIOSH) and Centers for Disease Control and Prevention (CDC) as carcinogenic or classified as reproductive hazards (see CDC bulletin 87–104; ATSDR bulletin sheets).10,11 CDC Current Intelligence Bulletin 48 estimated that at that time (1987) more than 9.8 million American workers were exposure to solvents in workplace.10 Exposures commonly occurred in degreasing/cleaning agents, paints, glues, printing, agricultural products, and in production of other compounds. The European Nordic countries had been reporting on the long-term effects of occupational solvent exposure since the 1970s.12 As awareness of the dangers of solvent exposure became more evident, occupational exposure rates began to drop in both Europe and the United States (US). Personal protective equipment (PPE), better ventilation of workplaces, and advancing technology all contributed to the declining rates of exposure.12,13
Overview of Solvents
Commonly found organic solvents are classified as hydrocarbons (aliphatic, aromatic, cyclic, or halogenated), ethers, aldehydes, alcohols, ketones, or amines. Many compounds used in industry are combination agents. Blood absorption via skin or lungs occurs quickly after exposure, with blood level dependent on many environmental factors (e.g., solvent concentration in air or on skin, blood solubility of the compound, room ventilation, duration of exposure, and alveolocapillary membrane permeability).10,14 Metabolism in the liver is characterized by oxidation (via cytochrome P-450 enzymes) followed by conjugation (with glucuronic acid, glycine, or glutathione) into water-soluble compounds that can be released in feces or urine. Many solvents can also be retained in organs with high lipid content such as the brain.10 Some solvents are more neurotoxic than others, and some metabolize to more toxic secondary agents than the primary exposing agent (e.g., n-hexane or methyl-butyl ketone).10,15 These secondary agents can bind to DNA, proteins, or RNA. As noted above, there are many complex factors determining an individual’s outcome after exposure to an organic solvent. Acute high level exposures (whether accidental or intentional) can lead to delirium, respiratory depression, and/or death. Chronic low level exposures to the multitude of available solvents have less clearly defined predictable outcomes.
Preclinical Studies
Animal studies have largely focused on providing information on acute effects and lethality rather than chronic toxicity.16 A review of histopathological studies could not identify consistently reproducible CNS deficits that occurred across all 13 commonly encountered occupational solvents.15 Individual solvents did have specific findings (e.g., ototoxicity with trichloroethylene, tremors and reduced numbers of neurons in the granular layer of the cerebellum with methyl chloride, and increases in S-100 protein with a fall in brain DNA with tetrachloroethylene). Of interest, exposure times across the many animal studies varied from a 13-week trial (most common) to 1 year (a few studies), but all had parts per million (ppm) exposures that were known to induce severe peripheral organ toxicity, vastly exceeding human occupational exposure limits.15 In a later study, rats were exposed to 35 times the average human industrial exposure rate to trichloroethylene over 6 weeks, resulting in multiple neuropathological changes (e.g., complex 1 mitochondrial impairment, loss of dopamine neurons, nigrostriatal tract degeneration).17 A recent review of animal studies utilizing chlorinated organic solvents (trichloroethylene, perchlorethylene, and dichloromethane) noted that these substances inhibit excitatory receptors/ion channels and potentiate inhibitory receptors/channels including effects on multiple neurotransmitters.16 Animal studies utilize larger exposures-short time frames to bring possible toxic effects to the forefront early, and therefore, do not replicate common occupational exposures. Such long-term studies in animals would be extremely expensive and difficult to undertake. Thus, ascertaining predictable human clinical course or prognosis after occupational and accidental exposures necessitates review of retrospective case studies, epidemiological data, imaging and neuropsychological testing data in exposed human populations.
Studies in Humans
In considering the long-term effects of chronic low level solvent exposure, there are many controversies in the literature. This is a complex area and multiple variables differ across studies. Upon review, there are reports to consider from solvents as an entire class of agents, individual specific solvent exposures within the class, and many other confounding exposure factors such as those listed above (ventilation, PPE, time exposed, continued exposure versus removal from exposing agent, solvent abuse as a means to get “high”, etc…) as well as differing study/evaluation protocols and time elapsed between exposure and evaluation. Recent reports from the US have examined occupational exposures to ketones, degreasers, benzene, toluene, and xylene for associations with Parkinson’s disease and amyotrophic lateral sclerosis with again conflicting opinions on any causal relationship.18 The largest body of medical literature examining the relationship between occupational solvent exposures and CNS changes comes from the European Nordic countries. The clinical term used for patients experiencing significant subjective clinical symptoms with objective neuropsychological test results believed attributable to long-term occupational solvent exposure is chronic solvent-induced encephalopathy (CSE, also called chronic toxic encephalopathy).19 Two international workshops to classify and stage the diagnosis were held in 1985 [the World Health Organization (WHO) and Raleigh Workshops].20,21 Both identified three stages of impairments (Table 1): the first (Type I/1) has milder subjective symptoms and no objective test findings, symptoms are reversible with removal from exposure; the second (Type II/2A and B) has some clinical and objective test findings that may or may not be reversible; and the third (Type III/3) has strong clinical and objective test results indicating clinically evident encephalopathy and is likely irreversible. Testing in patients with CSE indicates that the most common impairments are in the cognitive domains of attention, memory, processing speed, fine motor performance, and abstract thinking.19 Clinical symptoms can include depression, amotivation, fatigue, personality change, impulsivity, lability of affect, and loss of interest in activities of daily living.19 A recent 12-year retrospective chart review of cases diagnosed in Finland (N=129, all Type II/2B) found CSE was more likely with at least 10 years of daily exposure, but much more likely after 20 years of exposure and in workers over age 45.13 Development of symptoms occurred more slowly with lower rates of exposure, and the highest risks were for workers exposed to aromatic hydrocarbons and spray painting.13 Of significant note, subjects referred for evaluation came largely from workers employed by companies. The authors speculated that if self-employed workers were included, the numbers of cases might be higher. With options for diagnostic classification available, there were and continue to be many unanswered questions. These include time and amount of exposure needed for each stage of diagnosis, whether there are other symptoms not yet identified that should be included, whether there are other genetic, environmental, or psychosocial factors that increase susceptibilities, and whether CSE is progressive. Some recent studies have begun to address aspects of these questions.
| WHO Workshop | Type I: Organic Affective Syndrome | Type II: Mild CTE | Type III: Severe CTE | |
|---|---|---|---|---|
| Course; clinical presentation | Days-weeks, reversible; deficits in mood, energy, behavioral control | Weeks-months, may be reversible; deficits in energy, mood, memory, attention, psychomotor functioning | Years, usually irreversible; impaired energy, mood, memory, attention, psychomotor functioning | |
| Raleigh Workshop | Type 1: Symptoms Only | Type 2A: Sustained Behavioral or Mood Change | Type 2B: Impaired Intellect | Type 3: Dementia |
| Course; clinical presentation | Reversible if no neuropsychiatric dysfunction; deficits in energy, memory, concentration | Clear and sustained deficits in energy, mood, motivation, behavioral control | May be reversible; Subjective and objective deficits including memory, concentration, learning capacity | Usually irreversible but stable if exposure has ceased; global impairments in intellect and memory |
TABLE 1. International Classification Systems for Chronic Solvent Encephalopathy (CSE)19
The WHO criteria exclude patients with evidence of significant psychiatric disorders at time of evaluation from diagnosis of CSE. Following up on smaller studies suggesting increased incidence of some psychiatric disorder with solvent exposure,22,23 a large scale study (The Netherlands) compared DSM-IV criteria based disorders (Structured Clinical Interview, SCID) in CSE confirmed (met all WHO criteria, passed effort testing, N=203) versus suspected (met all WHO criteria, failed effort testing, N=120) subjects and matched (age, gender) community controls (N=3,212).1 This study did not include data on the specific solvents of exposure. Results indicated similarly elevated rates of mood and anxiety disorders in both the confirmed and suspected CSE groups compared with community controls (Figure 1). In contrast, the rate of alcohol-related disorders was lower in both CSE groups. Contrary to expectations, the similar rate of psychiatric disorders in the confirmed and suspected CSE groups did not support presence of symptom exaggeration in the suspected CSE group. The authors interpreted their results to mean that there is a significantly higher presence of DSM-IV moderate-severe mood/anxiety disorders in the CSE population. The group raised the possibility that the same neurotoxicological events that lead to the cognitive deficits in this population may also be leading to formal DMS-IV disorders, and suggested that the WHO criterion excluding anyone with a psychiatric diagnosis from consideration for a CSE diagnosis should be revisited (possibly changing the international rates of confirmed cases). They also noted that the rates of insufficient effort on neuropsychological testing may not be necessarily related to presence or absence of depression/anxiety.1
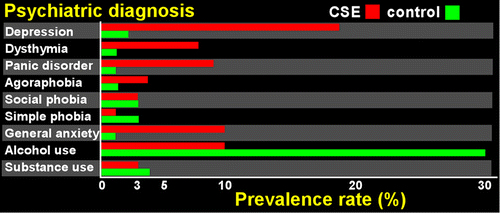
FIGURE 1. A study that compared the prevalence of psychiatric disorders between subjects with chronic solvent encephalopathy (CSE) because of long-term occupational exposure and a matched (age, gender) community sample found that rates were elevated for some disorders and reduced for others, suggesting specificity.1 The prevalence rates for each psychiatric diagnosis in the CSE subjects (red) and the community controls (green) is illustrated. Note that while the prevalence of several mood and anxiety disorders was higher, the prevalence of alcohol-related disorders was much lower in the subjects with CSE. Unexpectedly, the prevalence of the psychiatric disorders did not differ with effort testing (passed versus failed).
One potentially confounding variable to be considered in studies examining CSE is that of alcohol use disorders. For decades, “painters syndrome” and other similar terms have been used in clinical medicine to denote the high rates of alcoholism in painters and other similar occupations.24 In actuality, most studies report normal to lower than community rates of alcohol use disorders in solvent exposed and CSE populations.1,22,25–27 It has been proposed that presence of CSE may lead to alcohol intolerance and more research is needed to clarify the interactions of alcohol and solvents within the metabolic pathways of the liver.1
An additional area of limited knowledge and debate is in progression of CSE. A recent systematic review identified 16 studies (1970–2008) with follow-up of at least 1 year (range 1.3‒7 years).12 Although the reviewed studies were extremely varied in design, methods, specific solvent(s) of exposure, length/amount of exposure, and other factors, some common finding emerged. No study found severe deterioration with the majority of subjects demonstrating improvement or no change in presenting symptoms at follow-up. Better prognosis was found with young age of exposure, lower performance scores in initial testing, and no history of peak exposure. Negative prognostic indicators included involvement in litigation, negative personal perceptions of the subject’s own mental and physical health, older age of exposure, and current use of CNS-active medications. Of note to the authors, none of the follow-up studies reviewed addressed presence or absence of DSM-IV Axis I disorders and no clear solvent dose-response relationship for specific cognitive deficits could be determined.
Studies in multiple populations support the presence of impaired functioning many years after work-related solvent exposure ceased compared with unexposed individuals of a similar age.28–33 One group has completed long-term follow-up studies on two populations of solvent exposed workers without CSE at baseline compared with age-matched, occupationally similar controls.28,29 For one study, floor layers (baseline N=50, retest n=41, some with heavy exposures to alcohol-based glues and contact adhesives with benzene, acetone, toluene, and xylene in the 1950s‒1970s) and carpenters (baseline N=50, retest N=40) were recontacted 18 years after initial testing.28 For the other, rotogravure printers (baseline N=30, retest N=12, median 29 years of toluene exposure) and comparison workers (baseline N=72, retest N=19) were recontacted 20 years after initial testing.29 Both studies reported that the solvent exposed workers had more cognitive deficits than their age-matched, occupationally similar controls. In both groups, the effects were more evident in the older workers and in those with higher cumulative exposures. The authors speculated that solvent exposure decreases cognitive reserve and/or compensation abilities with heavy exposure, even 30 years prior. The importance of cognitive reserve is further supported by a study of retired solvent-exposed workers that reported cognitive deficits were present in the group with less education (did not complete high school) but not in the better educated group.32
One group revitalized an interest in previously reported sleep disorders, particularly sleep disordered breathing, in solvent exposed workers.25 They compared healthy printers (N=21) with long-term (mean 13 years, range 2–34) exposure to solvents to matched (gender, age, education, socioeconomic background, work pattern) military controls (N=26) for evidence of central sleep apnea (polysomnography). The exposures were to toluene and trichloroethylene prior to 1990 and a mixture of aromatic and aliphatic hydrocarbons after that time. The groups did not differ significantly in neurobehavioral symptoms or total sleep time. Sleep-related complaints, impaired hand-eye coordination, and rates of central apnea episodes were positively associated with increasing solvent exposure. The average number of apneic episodes per night was considered “subclinical” and did not rate a formal diagnosis of sleep apnea syndrome.
Although more information is now available regarding diagnostic classification and descriptions of solvent exposed populations of patients in both the short and long terms, clinical and research exam protocols vary widely. In 2012, a consensus conference of European experts met to formulate recommendations on standardized CSE evaluation protocols for research subjects or clinical patients reporting symptoms from solvent exposures.19 The major impairments in CSE were determined to be motor performance, attention, speed of information processing, and memory. Six recommendations for a CSE evaluation were formulated. Included were cognitive domains to be tested and importance of effort testing, method for estimating severity scores, assessment of clinical symptoms, assessment for other comordid conditions/full differential diagnosis, and minimal intervals for retesting.
Imaging Studies
Structural and functional brain imaging studies in solvent-exposed populations can be divided into the studies with subjects who are abusing solvents purposefully and for those nonabuse settings. In both cases, there is limited data. In first turning to poisons as a larger class of agents and to the abuse of solvents as starting references, the literature is more complete than for occupational/accidental exposures. Poisons, as a whole, have a predilection for basal ganglia destruction, as well as small vessel ischemia and iron deposition (examples heavy metals and carbon monoxide).14
Solvent abusers classically demonstrate cerebellar and cerebral white matter demyelination and gliosis on MRI with T2 hyperintensities (which can reduce gray-white discriminability with adjacent gray matter) commonly found in periventricular white matter, centrum semiovale, internal capsule, and pons (Figure 2).4,5,7,34 It is most often irreversible after 4–7 years of abuse, and worsens with level of exposure. Evidence of diffuse atrophy (sulcal widening, callosal thinning, brainstem atrophy) may be present. T2 hypointensities in basal ganglia, and thalamus are present in 30%‒50%, and associated with longer duration of abuse, commonly attributed to increased deposition of iron. However, a case report noting areas hypointense on T2 MRI were of normal appearance on susceptibility weighted MRI suggests iron is not the physical basis for this imaging appearance.35 Other mechanisms have been proposed.36
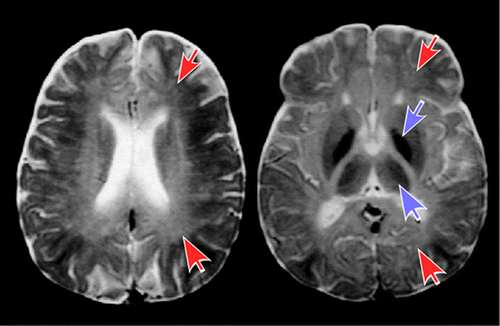
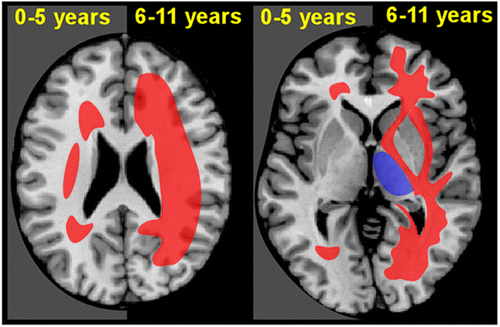
COVER and FIGURE 2. Chronic exposure to solvents primarily causes injury to the white matter of the brain (leukoencephalopathy). Macroscopic changes in solvent abusers include ventricular enlargement and cerebral and cerebellar atrophy. The most common microscopic findings are multifocal myelin loss with preservation of axons, mild gliosis, enlarged perivascular spaces, and presence of macrophages that contain birefringent inclusions.2,3 Based on the similarity of these inclusions to the inclusions found in peroxisomal diseases (e.g., adrenoleukodystrophy), it has been proposed that prolonged solvent exposure might cause an acquired peroxisomal disorder.3 MRI in cases of chronic solvent abuse indicate that solvent-related white matter injury (hyperintense on T2 MRI) is most commonly restricted to the deep periventricular white matter in the early years, extending into adjacent cortical and subcortical white matter if abuse continues.4,5 Some subcortical areas (e.g., basal ganglia, thalamus) may become hypointense on T2 MRI in later stages. Although MRI changes are much less prominent in occupational CSE, when present they are more similar to what is seen in early stage solvent abuse.6Top Row: A visual summary of the results of a cross sectional imaging study of chronic toluene abuse is overlaid on axial MRIs.7 Areas commonly affected in earlier (left side) and later (right side) years of abuse are color-coded by T2 MRI appearance (red, hyperintense; purple, hypointense) Bottom Row: A case example of solvent encephalopathy. Twenty three-year-old woman with 8-year history of extensive solvent abuse presented with encephalopathy and extreme difficulty swallowing. MRI findings included widespread diffuse atrophy and global T2 hyperintensities (red arrows) with diffuse loss of gray matter-white matter contrast. Increased signal suggesting demyelination of cranial nerves was evident in the brainstem (not pictured). T2 hypointensities (purple arrows) were evident in the basal ganglia and thalamus.
A few studies have utilized more advanced neuroimaging techniques in solvent abusing populations. Nuclear imaging (single photon emission computed tomography, SPECT) of presynaptic dopamine transporters (DAT) revealed decreased binding in the putamen in an early 20’s male who had been inhaling paint thinner (toluene) for a decade.35 The authors noted that the SPECT findings and the patient’s susceptibility to neuroleptic malignant syndrome were both consistent with a state of dopamine deficiency, perhaps because of prolonged toluene-induced hyperactivity. An exploratory study that used diffusion tensor imaging (DTI) to assess white matter integrity in adolescent substance abusers predominately using inhalants (N=11) or cannabis (N=11) found areas of reduced fractional anisotropy (FA) in the corpus callosum and adjacent to the hippocampus in the inhalant abuse group compared with drug-naive controls (N=8).37 In addition, reduced FA in the ventral prefrontal white matter correlated with earlier onset of inhalant use. Two studies utilizing proton MR spectroscopy have reported cerebral metabolic changes related to solvent abuse. A study of young (range 15–23 years) toluene abusers (N=22) compared clinical MRI and MR spectroscopy measures (centrum semiovale, thalamus, cerebellum) to age-matched controls (N=22).38 No T2 MRI abnormalities were found in the majority (16/22) of the toluene abuse group. The toluene abuse group had reduced N-acetyl aspartate (NAA)/Creatine (Cr) ratios and increased myo-inositol (mI)/Cr ratios in the cerebellum and centrum semiovale (no differences in Choline (Cho)/Cr ratios). Both changes were significantly associated with duration of abuse. The authors interpreted these results to indicate gliosis (increased mI/Cr) and impaired neuroaxonal integrity (decreased NAA/Cr) with absence of active demyelination (no change in Cho/Cr). The other study compared metabolite measures from the basal ganglia (putamen and globus pallidus) of adult (range 18–41 years) abstinent (mean 4.9 years, range 0.1–14) toluene users (N=12) with no clinical MRI abnormalities to healthy controls.39 NAA/Cr and mI/Cr did not differ between the groups. Cho/Cr ratios were increased in the toluene group and this correlated with severity of psychiatric symptoms. As noted by the authors, these findings suggest presence of abnormal membrane phospholipid metabolism even after prolonged abstinence. These studies must all be considered preliminary, as none included comparisons to adolescents matched for family history of substance use disorders, which has recently been shown to be associated with reduced FA and NAA and increased Cho.40,41
As noted above, imaging of occupationally exposed subjects (with and without CSE) has been more limited. Reviews of early structural imaging studies indicated varied findings, with some studies reporting no differences between controls and exposed subjects and others reporting mild nonspecific atrophy and periventricular hyperintensities and hypointensities of the basal ganglia on T2 MRI in solvent-exposed subjects.15,42 Early functional imaging studies have also been limited with inconsistent results, likely due in part to the wide range of durations and types of toxic exposures.15 These studies provide preliminary evidence for abnormalities in regional cerebral blood flow (multiple areas), increased dopamine synthesis (putamen) in the absence of changes in receptor binding, and altered task-evoked activity in frontal cortex.43–47 In a more recent larger study, clinical MRIs of solvent exposed workers diagnosed with CSE (N=71, all Type II/2B) were visually rated using standardized scales by two experts blinded to exposure data.6 One third (27/71) were classified as abnormal by both raters based on presence of atrophy and/or abnormal white matter T2 hyperintensities. Regression analyses indicated that both cerebral (primarily frontal and parietal) and cerebellar atrophy were related to duration of exposure. The authors noted that the majority of the MRIs were rated as normal, which might be due to the milder CSE stage and/or the stringent criteria utilized. In another study, the volume (manual segmentation) of the corpus callosum was compared between railroad workers (subject N=31) with long-term (average 24.3 years, range 10–35) mixed solvent exposure (chloroethylenes and mineral spirit cleaning agents) and matched (age, education, socioeconomic status) community controls (N=31).48 Both the total volume and the anterior segment (genu) volume of the corpus callosum were smaller in the solvent exposed group, with smaller volume correlating with greater exposure. Smaller anterior segment volume was significantly correlated with worse cognitive task performance in the medically healthy (no hypertension or diabetes) subgroup, but not in the solvent exposed group as a whole.
Two studies assessed cerebral metabolites (proton MR spectroscopy) in solvent exposed populations. A study comparing clinical MRI and MR spectroscopy from mildly symptomatic shoemakers (N=49) recruited from a single factory (daily exposure to toluene and mixed organic solvents) to healthy controls (N=30) reported that the exposed group had significantly increased Cho/Cr ratios in basal ganglia, thalami, and parietal white matter, whereas NAA/Cr did not differ in any region.49 Duration of solvent exposure was positively correlated with Cho/Cr ratios in the basal ganglia. The authors commented that as none of the exposed group had visible white matter changes, their results may indicate demyelination that isn’t visible on standard T2 MRI. Another group completed a more extensive set of neuroimaging measures in a small group of CSE patients (N=10) compared with asymptomatic solvent exposed controls (N=10) and unexposed controls (N=11) using clinical MRI, striatal dopamine D2 receptor binding (SPECT), tissue integrity (DTI) and cerebral metabolites (proton MR spectroscopy).27 White matter hyperintensities were noted on T2 MRI in a higher proportion of the CSE group (6/10, 60%) than the exposed (2/10, 20%) or unexposed (3/11, 27%) control groups. Contrary to expectations, there were no significant group differences in FA for any region (prefrontal white matter, caudate, putamen, thalamus). In contrast to an earlier study that found no difference in D2 receptor binding in the putamen in CSE patients,45 the CSE patients and the exposed controls had similarly reduced D2 receptor binding in the striatum. Regression analyses in the CSE group indicated that psychomotor speed and attention were predicted by striatal D2 binding and FA of the thalamus and caudate. Unlike what has been reported in solvent abuse (see above), there were no significant group differences in the centrum semiovale for any MR spectroscopy measure. NAA was decreased in the frontal gray matter of the CSE (trend) and exposed controls (statistically significant). The authors suggest that their lack of significant findings could be attributed to insufficient power (because of small groups) to detect subtle changes in tissue integrity. They note that these findings suggest the presence of solvent-induced abnormalities in the frontal-striatal circuits, even in asymptomatic exposed workers.
A study utilizing functional MRI (fMRI) compared activations during a working memory task (N-back, sequence of letters) in asymptomatic solvent exposed industrial painters (N=27) and unexposed workers from other building trades (N=27).8 Exclusion criteria included psychiatric disorders, current medications, and presence of white matter abnormalities on T2 MRI. The exposed group performed more slowly (reaction time during the 0-back condition) and less accurately (fewer correct hits, more false hits) than the unexposed group. The exposed group also had less task-evoked activation in several areas of prefrontal cortex (anterior cingulate, dorsolateral) (Figure 3). When differences in task performance were included, the exposed group still had significantly less activation in left dorsolateral prefrontal cortex compared with the unexposed group. Lifetime solvent exposure was negatively correlated with activations in several cortical areas (anterior cingulate, dorsolateral, parietal) that support attention and working memory (Figure 3). As noted by the authors, these results suggest a possible neurobiological basis for impaired executive functioning in solvent exposed populations.
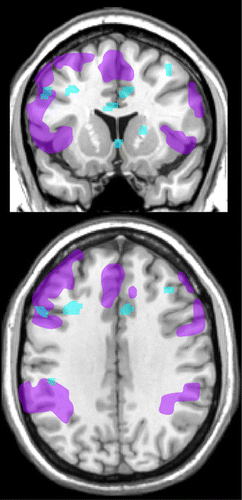
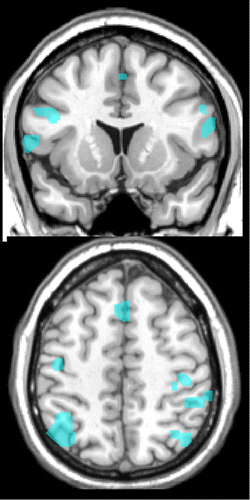
FIGURE 3. Deficits in working memory are one of the most common symptoms of occupational chronic solvent exposure. A functional magnetic resonance imaging (fMRI) study that compared construction workers with occupational chronic solvent exposure to matched (age, education, occupational status) construction workers without solvent exposure during completion of a working memory task reported differences in both performance and regional brain activations.8 Subjects viewed a visual sequence of numbers and pressed a button when a target letter was identified (N-back task, matching to 0, 1, 2, or 3 back in the sequence, depending upon instructions). Left: Approximations of areas activated across groups by the N-back task (purple) and the areas in which the exposed group had lower activation (blue) after correction for verbal IQ and lead exposure are illustrated on coronal (top) and axial (bottom) MRIs. Right: Approximations of areas in which lower activation correlated with higher lifetime solvent exposure (blue) in the exposed group are illustrated on coronal (top) and axial (bottom) MRIs.
Conclusions
Both the substance abuse and occupational health literatures confirm that with extensive exposures, individuals can acquire a solvent-induced encephalopathy. Although predictable CNS deficits for each individual type of solvent exposure and amount of exposure time are not yet clear, common themes have emerged (e.g., cognitive, behavioral, and emotional deficits). To date, occupationally acquired CSE is not thought to be progressive once exposure ceases. However, evidence suggests that heavy exposures even early in life might affect cognitive function decades later. With better PPE in the workplace incident cases are on the decline.13 Nevertheless, it continues to be imperative for clinicians to include questions on poison/toxin exposure, particularly solvents, in initial diagnostic examinations, especially for those patients with cognitive deficits. If solvent induced deficits are a possibility, recent summaries of examination strategies and tools can be referenced.19,50 As additional studies are completed and the neuropathological effects of solvents on CNS structures become clearer, criteria for formal diagnosis of CSE may need to be reexamined. In addition, as both structure and functional neuroimaging techniques become more sophisticated, additional contributions from imaging may further contribute to understanding CSE.
1 : Prevalence of psychiatric disorders in patients with chronic solvent induced encephalopathy (CSE). Neurotoxicology 2011; 32:916–922Crossref, Medline, Google Scholar
2 : Neurotoxicity of solvents in brain of glue abusers. Environ Toxicol Pharmacol 2005; 19:671–675Crossref, Medline, Google Scholar
3 : Brain damage in a large cohort of solvent abusers. Acta Neuropathol 2010; 119:435–445Crossref, Medline, Google Scholar
4 : Addictive illegal drugs: structural neuroimaging. AJNR Am J Neuroradiol 2010; 31:803–808Crossref, Medline, Google Scholar
5 : Your brain on drugs: imaging of drug-related changes in the central nervous system. Radiographics 2012; 32:701–719Crossref, Medline, Google Scholar
6 : Magnetic resonance imaging in occupational chronic solvent encephalopathy. Int Arch Occup Environ Health 2009; 82:595–602Crossref, Medline, Google Scholar
7 : Cranial MR findings in chronic toluene abuse by inhalation. AJNR Am J Neuroradiol 2002; 23:1173–1179Medline, Google Scholar
8 : Occupational solvent exposure and brain function: an fMRI study. Environ Health Perspect 2011; 119:908–913Crossref, Medline, Google Scholar
9 Merriam Webster I: Poison. 2014 [cited 2014 11/21/14]; Available from: http://www.merriam-webster.com/dictionary/poison.Google Scholar
10
11
12 : The course of chronic solvent induced encephalopathy: a systematic review. Neurotoxicology 2009; 30:1172–1186Crossref, Medline, Google Scholar
13 : Occupational chronic solvent encephalopathy in Finland 1995-2007: incidence and exposure. Int Arch Occup Environ Health 2010; 83:703–712Crossref, Medline, Google Scholar
14 : Toxic encephalopathy. Semin Neurol 2011; 31:184–193Crossref, Medline, Google Scholar
15 . Occupational exposure to organic solvents and long-term nervous system damage detectable by brain imaging, neurophysiology or histopathology. Food and chemical toxicology. 2003;41:153-187.Crossref, Medline, Google Scholar
16 : A review of potential neurotoxic mechanisms among three chlorinated organic solvents. Toxicol Appl Pharmacol 2011; 255:113–126Crossref, Medline, Google Scholar
17 : Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol 2008; 63:184–192Crossref, Medline, Google Scholar
18 . The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimer's Dementia. 2014;10(3 Suppl):S213-225.Crossref, Medline, Google Scholar
19 : Chronic solvent-induced encephalopathy: European consensus of neuropsychological characteristics, assessment, and guidelines for diagnostics. Neurotoxicology 2012; 33:710–726Crossref, Medline, Google Scholar
20 : AM. Human aspects of solvent neurobehavioral effects. Report of the workshop session on clinical and epidemiological topics. Neurotoxicolgy 1986; 7:43–56Medline, Google Scholar
21
22 : Mood and behavioral symptoms in individuals with chronic solvent exposure. Psychiatry Res 2000; 97:191–206Crossref, Medline, Google Scholar
23 : Increased incidence of anxiety and depressive disorders in persons with organic solvent exposure. Psychosom Med 2000; 62:746–750Crossref, Medline, Google Scholar
24 : A cohort study of disability pensioning among Norwegian painters, construction workers, and workers in food processing. Epidemiology 1995; 6:132–136Crossref, Medline, Google Scholar
25 : Sleep apnoeas and neurobehavioral effects in solvent exposed workers. Int J Hyg Environ Health 2011; 214:66–70Crossref, Medline, Google Scholar
26 . Alterations in cognitive and psychological functioning after organic solvent exposure. J Occupational Med 1990;32:444-450.Crossref, Medline, Google Scholar
27 : Cerebral impairment in chronic solvent-induced encephalopathy. Ann Neurol 2008; 63:572–580Crossref, Medline, Google Scholar
28 : Influence of solvent exposure and aging on cognitive functioning: an 18 year follow up of formerly exposed floor layers and their controls. Occup Environ Med 2002; 59:49–57Crossref, Medline, Google Scholar
29 : Delayed manifestations of CNS effects in formerly exposed printers—a 20-year follow-up. Neurotoxicol Teratol 2010; 32:620–626Crossref, Medline, Google Scholar
30 : Occupational exposure to solvents and cognitive performance in the GAZEL cohort: preliminary results. Dement Geriatr Cogn Disord 2010; 30:12–19Crossref, Medline, Google Scholar
31 : Solvent exposure and cognitive ability at age 67: a follow-up study of the 1947 Scottish Mental Survey. Occup Environ Med 2010; 67:401–407Crossref, Medline, Google Scholar
32 : Occupational solvent exposure and cognition: does the association vary by level of education? Neurology 2012; 78:1754–1760Crossref, Medline, Google Scholar
33 : Time may not fully attenuate solvent-associated cognitive deficits in highly exposed workers. Neurology 2014; 82:1716–1723Crossref, Medline, Google Scholar
34 : Toluene misuse and long-term harms: a systematic review of the neuropsychological and neuroimaging literature. Neurosci Biobehav Rev 2008; 32:910–926Crossref, Medline, Google Scholar
35 : Severe dopaminergic pathways damage in a case of chronic toluene abuse. Clin Neurol Neurosurg 2009; 111:864–867Crossref, Medline, Google Scholar
36 : Toluene abuse: physical basis for hypointensity of the basal ganglia on T2-weighted MR images. Radiology 1994; 193:473–476Crossref, Medline, Google Scholar
37 : White-matter abnormalities in adolescents with long-term inhalant and cannabis use: a diffusion magnetic resonance imaging study. J Psychiatry Neurosci 2010; 35:409–412Crossref, Medline, Google Scholar
38 : Single-voxel proton MR spectroscopy in toluene abuse. Magn Reson Imaging 2003; 21:777–785Crossref, Medline, Google Scholar
39 : Metabolite alterations in basal ganglia associated with psychiatric symptoms of abstinent toluene users: a proton MRS study. Neuropsychopharmacology 2004; 29:1019–1026Crossref, Medline, Google Scholar
40 : Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum Brain Mapp 2014; 35:5877–5887Crossref, Medline, Google Scholar
41 : Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum Brain Mapp 2014; 35:5401–5413Crossref, Medline, Google Scholar
42 : Brain imaging techniques applied to chronically solvent-exposed workers: current results and clinical evaluation. Environ Res 1993; 61:239–250Crossref, Medline, Google Scholar
43 : Three-dimensional brain metabolic imaging in patients with toxic encephalopathy. Environ Res 1993; 60:295–319Crossref, Medline, Google Scholar
44 : PET and neurobehavioral evidence of tetrabromoethane encephalopathy. J Neuropsychiatry Clin Neurosci 1990; 2:431–435Link, Google Scholar
45 : Do organic solvents induce changes in the dopaminergic system? Positron emission tomography studies of occupationally exposed subjects. Int Arch Occup Environ Health 1997; 70:180–186Crossref, Medline, Google Scholar
46 : Comparison of single photon emission computed tomography findings in cases of healthy adults and solvent-exposed adults: correction of previous results. Am J Ind Med 1997; 32:693–694Crossref, Medline, Google Scholar
47 : Verbal working memory and solvent exposure: a positron emission tomography study. Neuropsychology 2000; 14:551–558Crossref, Medline, Google Scholar
48 . Corpus callosum volume in railroad workers with chronic exposure to solvents. Journal of occupational and environmental medicine. 2006;48:615-624.Crossref, Medline, Google Scholar
49 : Occupational prolonged organic solvent exposure in shoemakers: brain MR spectroscopy findings. Magn Reson Imaging 2004; 22:707–713Crossref, Medline, Google Scholar
50 . Toxic encephalopathy. Safety and health at work. 2012;3:243-256.Crossref, Medline, Google Scholar



