Application of Magnetoencephalography to the Study of Autism
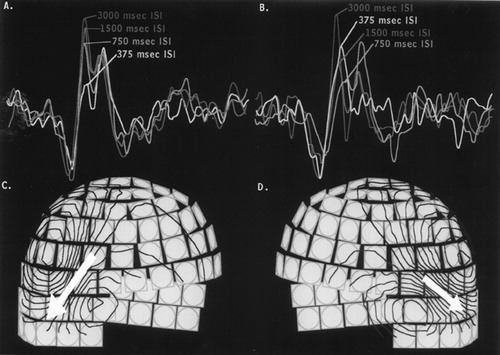
Whole-head magnetoencephalographic (MEG) data were collected from a 33-year-old male who was diagnosed at 6 years of age with autism. Childhood symptoms included acquired aphasia, self-stimulatory behavior, poor eye contact, and social withdrawal. By parental report, the subject was highly agitated by a variety of sounds during his childhood years, although his level of sound sensitivity has decreased in adulthood. MEG involves noninvasive measurement of the magnetic signal generated by the brain's neuroelectric activity. The upper set of graphs (A, B) show the tracings from single representative sensors over the right (A) and left (B) temporal lobes. These data were collected during an auditory task where tones were presented with varying interstimulus intervals (ISI). The lower set of drawings (C, D) represent magnetic field flux lines (dark blue represents magnetic flux flowing into the head; red represents magnetic flux flowing out of the head) and the mathematically derived magnetic field generators (>yellow arrows) that are calculated from the sensor data (representative sensors from the full array of 306 sensors are shown in gray). The activity localizes to auditory cortex (indicated by triangles in the MR on the journal cover). Note that over the right hemisphere (A) there is the normal progressive decrease in the response at 100 ms as a function of decreasing ISI. In contrast, over the left hemisphere (B), the response at 100 ms in the shortest ISI condition (375 ms; light blue) is actually larger than that seen for longer ISI conditions (750 and 1,500 ms; medium and dark blue). There is also evidence of abnormal waveform morphology over the left hemisphere, especially for the 750-ms ISI condition. This demonstrates that patients with autism may have altered patterns in the decay of auditory memory traces and dysfunction of auditory sensory gating mechanisms.
Autistic disorder or infantile autism is a pervasive developmental disorder that occurs in 1 to 2 children per 1,000 live births, more commonly in males than females (2–4:1).1–3 Its reported incidence has increased dramatically with the recent recognition of higher functioning individuals. Autism was first described by Kanner in 1943.4 Since that time, it has been a disorder of extensive scientific interest and controversy.5
Current diagnostic standards require symptom presentation before three years of age. These children (and adults) display problems in three major areas: 1) social interaction, 2) communication, and 3) range of interests and activities.2,3,5 Impairments in social interaction may include lack of person-to-person bonding (expressed as a lack of attachment to the primary caregiver or failure to develop appropriate peer relationships) and inability to interact socially and to share with others. Impairments in communication may include delay in, lack of, or loss of development of receptive or expressive language; difficulty with initiating and sustaining conversation; stereotyped or repetitive use of language; and lack of spontaneous make-believe play. Restrictions in the range of interests and activities may include preoccupation with repetitive or stereotyped patterns of interest (often with abnormally intense focus), inflexible adherence to exact schedules or routines, and stereotyped or repetitive movements (head banging, rocking, hand or finger flapping or twisting). Executive dysfunction, decreased central coherence (the ability to integrate information into a whole versus individual pieces) and abnormal sensory sensitivity are also commonly seen.6–8
Prognosis in autism is generally poor. Longitudinal studies indicate that 90% of individuals with autism have clinical deficits that persist throughout adulthood.9 Approximately 60% to 70% of autistic individuals have an IQ below 70, and symptom severity is inversely related to IQ.1,5,9 In one longitudinal study, 27% of autistic adults were employed, primarily in sheltered workshops or menial jobs. Almost all required assisted living, either with family members (47%) or in residential placements (53%).9,10
Whereas autism was once considered to be the result of poor parenting, it is now generally accepted that it is a biologically based neurodevelopmental disorder. Several lines of evidence suggest serotonergic abnormalities in autism. Autism is likely to have a polygenetic component, although concordance rates in twin studies indicate that other factors must also be important. A difficult challenge in both genetic and neurobiological research in autism is the possibility that there are many different core etiologies, each ultimately generating a similar cognitive and behavioral profile through a common final pathway. For example, there are several very different disease conditions (e.g., fragile X syndrome, congenital rubella, herpes simplex encephalitis, tuberous sclerosis, and fetal exposure to toxins) that are often associated with autism.5,11
Recent reviews of imaging studies in autism indicate that no focal brain injury has yet been consistently demonstrated, although a number of potentially important differences have been reported.3,12 In brief, autopsy studies have found cerebellar pathology, including a decreased number of Purkinje and granular cells in posterior–inferior cerebellar cortex. Limbic structures were also abnormal, including increased neuronal density with small cell bodies and stunted dendrites. Some magnetic resonance imaging (MRI) studies have reported significantly smaller lobules VI and VII of the cerebellar vermis in autism, but this finding has not been replicated consistently. Generalized increases in brain volume have been reported from studies of head circumference and MRI-based measurements, but not from an autopsy study that measured brain weight.3,12,13
More promising are the results from various methods of functional brain imaging, which may provide insight into abnormalities in processing and functional organization. These include initial reports of globally elevated glucose utilization as well as focal metabolic or blood flow abnormalities in the anterior cingulate gyrus, temporal and parietal lobes, basal ganglia, thalamus, and cerebellum. There are also indications of atypical language dominance and decreased hemispheric functional specialization.3,12,14,15
Neuronal functioning can be recorded more directly by electroencephalography (EEG) and magnetoencephalography (MEG). Abnormalities in the spontaneous EEG are common in autism, and several studies have demonstrated that epilepsy develops in 20% to 30% of autistic individuals.10,16A general limitation of EEG studies is a difficulty in relating EEG abnormalities to specific brain regions. This is because electrical conductivity barriers between the brain, cerebrospinal fluid, skull, and scalp distort the neuroelectric pattern recorded at the scalp. MEG uses superconducting sensors to noninvasively measure the neuromagnetic fields generated by the brain's electrical activity. It offers an attractive alternative to EEG because the above-described conductivity barriers cause only minimal neuromagnetic distortions. Thus, relatively simple mathematical models can be used to infer the spatiotemporal pattern of brain activities that generated specific neuromagnetic signals of interest, allowing good localization of the active brain regions.17 In autism, MEG has recently shown a high incidence of sleep epileptiform activity in peri-Sylvian brain regions, even in patients who have never experienced a clinical seizure.10,16 MEG is also used to measure changes in brain activity in response to functional activation. In the case presented here, an auditory task was used to demonstrate abnormal auditory information processing.
As is the case with other neurodevelopmental disorders, there is no cure for autism. However, there have been recent advances and recommendations for the reduction of specifically targeted symptoms through behavioral and pharmacological strategies. The most successful advances have been achieved with behavior modification programs that incorporate therapist-trainers and parents in home-based programs such as TEACCH (Treatment and Education of Autistic and Related Communication Handicapped Children) and educational day centers focused on activities of daily living, social skills, and communication (both auditory and visual).18 Pharmacological interventions have also proved useful for reducing specific symptoms. Neuroleptics and mood stabilizers may decrease aggression and hyperactivity. Selective serotonin reuptake inhibitors such as fluoxetine and paroxetine have been reported to lessen ritualistic obsessive activities.19,20 Use of other agents, such as vitamin B6, immunoglobulin injections, clomipramine, naltrexone, and clonidine has been more controversial, with some inconsistencies in the reproducibility of effects.5,21,22 Medications such as sodium valproate can help to reduce autistic features, consistent with EEG and MEG observations of increased incidence of epileptiform activity in autism.23 Through definition of specific cortical processing abnormalities in autism, functional brain imaging strategies may soon lead to the development of new therapeutic approaches to the pervasive developmental disorders.
ACKNOWLEDGMENTS
This work was supported by grants from the Cure Autism Now Foundation (G.M.J.), the March of Dimes Birth Defects Foundation (J.D.L.), the National Alliance for Research in Schizophrenia and Depression (J.D.L.), and Picker International (J.D.L. and W.W.O.).
1 Honda H, Shimizu Y, Misumi K, et al: Cumulative incidence and prevalence of childhood autism in children in Japan. Br J Psychiatry 1996; 169:228–235Crossref, Medline, Google Scholar
2 Rapin I: Autism. N Engl J Med 1997; 337:97–104Crossref, Medline, Google Scholar
3 Rapin I, Katzman R: Neurobiology of autism. Ann Neurol 1998; 43:7–14Crossref, Medline, Google Scholar
4 Kanner L: Autistic disturbances of affective contact. Nervous Child 1943; 2:217–250Medline, Google Scholar
5 Rutter M: The Emanuel Miller Memorial Lecture 1998. Autism: two-way interplay between research and clinical work. J Child Psychol Psychiatry 1999; 40:169–188Crossref, Medline, Google Scholar
6 Bailey A, Phillip W, Rutter M: Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiatry 1996; 37:89–126Crossref, Medline, Google Scholar
7 Pring L, Hermelin B, Heavey L: Savants, segments, art and autism. J Child Psychol Psychiatry 1995; 36:1065–1076 Google Scholar
8 Frith U, Happe F: Language and communication in autistic disorders. Philos Trans R Soc Lond B Biol Sci 1994; 346:97–104Crossref, Medline, Google Scholar
9 Ballaban-Gil K, Rapin I, Tuchman R, et al: Longitudinal examination of the behavioral, language, and social changes in a population of adolescents and young adults with autistic disorder. Pediatr Neurol 1996; 15:217–223Crossref, Medline, Google Scholar
10 Nordin V, Gillberg C: The long-term course of autistic disorders: update on follow-up studies. Acta Psychiatr Scand 1998; 97:99–108Crossref, Medline, Google Scholar
11 DeLong GR: Autism: new data suggest a new hypothesis. Neurology 1999; 52:911–916Crossref, Medline, Google Scholar
12 Deb S, Thompson B: Neuroimaging in autism. Br J Psychiatry 1998; 173:299–302Crossref, Medline, Google Scholar
13 Courchesne E, Muller RA, Saitoh O: Brain weight in autism: normal in the majority of cases, megalencephalic in rare cases. Neurology 1999; 52:1057–1059 Google Scholar
14 Ryu YH, Lee JD, Yoon PH, et al: Perfusion impairments in infantile autism on technetium-99m ethyl cysteinate dimer brain single-photon emission tomography: comparison with findings on magnetic resonance imaging. Eur J Nucl Med 1999; 26:253–259Crossref, Medline, Google Scholar
15 Muller RA, Behen ME, Rothermel RD, et al: Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord 1999; 29:19–31Crossref, Medline, Google Scholar
16 Lewine JD, Andrews R, Chez M, et al: Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics 1999; 104:405–418Crossref, Medline, Google Scholar
17 Lewine JD, Orrison WW: Clinical electroencephalography and event-related potentials, in Functional Brain Imaging, edited by Orrison WW, Lewine JD, Sanders JA, et al. St. Louis, MO, Mosby-Year Book, 1995, pp 327–368Google Scholar
18 Campbell M, Schopler E, Cueva JE, et al: Treatment of autistic disorder. J Am Acad Child Adolesc Psychiatry 1996; 35:134–143Crossref, Medline, Google Scholar
19 Posey DI, Litwiller M, Koburn A, et al: Paroxetine in autism (letter). J Am Acad Child Adolesc Psychiatry 1999; 38:111–112Crossref, Medline, Google Scholar
20 DeLong GR, Teague LA, McSwain KM: Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998; 40:551–562Crossref, Medline, Google Scholar
21 Gupta S: Treatment of children with autism with intravenous immunoglobulin (letter). J Child Neurol 1999; 14:203–205Crossref, Medline, Google Scholar
22 Sanchez LE, Campbell M, Small AM, et al: A pilot study of clomipramine in young autistic children. J Am Acad Child Adolesc Psychiatry 1996; 35:537–544Crossref, Medline, Google Scholar
23 Childs JA, Blair JL: Valproic acid treatment of epilepsy in autistic twins. J Neurosci Nurs 1997; 29:244–248Crossref, Medline, Google Scholar



