Surgical Treatment of Mental Illness: Impact of Imaging
A. Axial computed tomographic scans from two patients who underwent frontal leukotomy (transection of the fiber tracts leading from the frontal gray matter) are shown to the left. A third case is shown on the cover. Early surgeries were necessarily performed freehand, resulting in considerable variability in actual lesion location and size. Note the dramatic differences among these three cases.
B. The original emotion and memory circuit proposed by Papez is diagrammed below in black. Current theories emphasize the presence of parallel processing of different aspects of behavior. The orbitofrontal circuit is thought to mediate socially appropriate behavior, impulse control, and empathy (blue). The dorsolateral circuit is thought to accomplish organization, planning, and attention (pink). The anterior cingulate circuit is thought to produce motivation by balancing the inhibitory input of the supplemental motor area with its own stimulus that supports wakefulness and arousal (green). The three circuits are color-coded at the level of the leukotomy on a coronal myelin-stained brain slice on the cover. Note that these pathways run close together. Subcortical injury is likely to affect more than one.
The accidental brain injury suffered by Phineas Gage in 1848 planted the seeds for a later formulation of the influence of the frontal lobes on personality and behavior. In the late 19th century, personality changes were also seen in patients following resection of frontal lobe tumors. Early studies correlating frontal lobe lesions (resulting from head wounds sustained in World War I) and changes in personality, affect, and behavior added to the preliminary theories of frontal lobe function. This knowledge was expanded with experimental studies in primates.1,2 Since then, intensive study of both brain-injured patients and animals has broadened and deepened our understanding of the brain circuits that underlie many aspects of normal cognition and personality, as well as mental illnesses.
The application of the neurosurgical approach to treatment of mental illness began in earnest with Moniz in 1936. He was convinced that lesions in the frontal lobes, by interrupting the fibers connecting the frontal lobes with other brain regions, would provide relief from the devastating symptoms of severe mental illness. His work came at a time in which the only treatments available for mental illness (other than heavy sedation or physical restraint) were hydrotherapy or convulsive treatment (by induction of hypoglycemic coma or metrazol seizures). Although these somatic therapies were sometimes effective, the remission of symptoms was usually transitory. The possibility of a treatment approach that could provide more permanent relief was thus of great interest, although it was recognized early on that there was a high likelihood of unwanted personality changes.
Surgical treatment of mental illness was prevalent prior to the development of antipsychotic medications in the 1950s. The white matter connecting the orbitofrontal and/or cingulate cortices with other cortical and subcortical structures was a common surgical target. The choice of this target was not primarily based on knowledge of the underlying brain circuits, but rather was based on symptom relief and better surgical outcome. Thus, surgical treatment brought passivity and apathy in many cases where before there was agony and aggression. It was also recognized from tumor surgeries that white matter lesions bled less than gray matter and had fewer postoperative complications.
During this early period, surgery was necessarily performed freehand, resulting in considerable variability in actual lesion location and size (see Figure A) as well as surgical complications. Although therapeutic improvements were reported in many patients, there were also clear changes in personality and drive. Little was done to correlate lesion size and/or location with therapeutic effect. Such studies were difficult because the areas affected could only be assessed postmortem. Limited autopsy studies from 1947 to 1950 documented great differences in the lesions.2 The refinement of lesion size and placement was then attempted, based on experimental studies in primates, in an effort to diminish the occurrence of personality changes (still without a cohesive knowledge of circuitry). For a more complete account of the history of neurosurgical treatment of mental illness in the early years, see Dorman1 or Swayze.2
Parallel to this era, a better understanding of brain circuitry was taking form. Near the turn of the century, Eduard Hitzig had proposed the concept of cortical localization and the motor cortex from his work with voltage studies in canine brains. After getting no motor response from frontal lobe stimulation, Hitzig deduced that “the frontal lobes have abstract thought.”1 Papez formally introduced the idea of the limbic circuit in 1937, naming specific brain structures associated with emotion and memory (see Figure B). This initial circuit was expanded by McLean in 1952 to include, notably, the frontal and temporal lobes.3 The concept of specific brain circuits mediating cognition, emotion, and memory gained wide acceptance in neuropsychiatry. As the 20th century passed, knowledge of these circuits greatly expanded—as did their clinical applications. Later refinements emphasized the presence of parallel processing of different aspects of behavior (see Figure B).
Development of pathophysiologic models of psychiatric disease has been greatly enhanced by the explosive increase in the techniques available to study the living brain that has occurred during the past three decades. Improvements in our understanding of the functional circuitry underlying both normal cognitive functions and mental illness have benefited enormously from the availability of methods for probing, in vivo, both structure (computed tomography [CT], magnetic resonance imaging [MRI]) and function (positron emission tomography [PET], single-proton emission computed tomography [SPECT], functional magnetic resonance imaging [fMRI], magnetoencephalography [MEG], tomographic electroencephalography [tEEG]).
Just as these imaging techniques expanded the knowledge of normal circuitry and pathophysiology, so did they influence treatment of disease.
Surgical treatment of mental illness is critically dependent upon a solid understanding of these circuits and the locations of the pathways connecting them.4–7 Currently neurosurgery for mental illnesses is done, very infrequently, in expert centers for intractable mood or anxiety disorders. However, all stages of this treatment have benefited from these new imaging techniques. Imaging now allows preoperative localization of target areas at high resolution and has improved the placement of lesions significantly.8,9 Although primarily used for treatment of “neurological” conditions (e.g., intractable Parkinson's disease, central pain, uncontrollable seizures), stereotactic planning with MRI or CT is done to localize the exact target for radiofrequency lesions with electrodes in cingulotomies (accurate within 1–3 mm).5 Oblique coronal T1-weighted MR images currently are done as well as the traditional sagittal T1- weighted images.10 MRI localization is also used for anterior capsulotomy for intractable obsessive-compulsive disorder.11
There are reports that now address the three-dimensional differences in target localization between CT and MRI, as well as which MRI sequence is best for visualizing small anatomical landmarks needed for stereotactic planning.8,9,12 CT is less prone to image distortion but has lower anatomic resolution. MRI provides much better visualization of anatomy, although image distortion may be present, which is highly dependent on scanner and pulse sequence. Overall, the accuracy of stereotactic localizations with CT and MRI appears to be equivalent.12,13 The greater anatomic resolution of MRI is now being exploited to provide direct visualization of target structures. For example, a heavily T1-weighted fast spin echo inversion recovery MRI sequence (FSE-IR) has been used to directly localize the internal globus pallidus.9 This approach allows individual variations in anatomy to be taken into account in surgical planning, something not possible with the previously used gradient echo sequence—thus allowing more accurate ablation of the internal globus pallidus in intractable Parkinson's disease. The FSE-IR images are acquired in both the axial and coronal planes of section, providing localization of the target in all three dimensions.8,9
Postoperatively, imaging has an important role in many arenas. The gross extent and location of ablation can be easily evaluated by using CT or (preferably) MRI. Studies correlating lesion location and size with differential outcome are beginning to provide valuable insights. For instance, there is an ongoing controversy as to whether cingulotomy or anterior capsulotomy provides better symptom resolution in obsessive-compulsive disorder patients.10,14,15 In the immediate postoperative period (first few months), fatigue and loss of initiative and mental drive have been correlated with MRI-based visualization of edema surrounding the lesion site.11 In addition, imaging allows postoperative comparison of different surgical techniques (e.g., thermocapsulotomy with electrodes versus gamma capsulotomy with irradiation; thermo-controlled electrocoagulation versus radioactive yttrium-induced lesions).11,16
Remote changes and degeneration of major pathways subsequent to surgical intervention can also be studied by using standard clinical MRI.17–19 In addition, newer MRI techniques such as magnetization transfer (MT) and diffusion tensor imaging (DTI) may provide more sensitive ways to study these changes, allowing a more accurate delineation of the pathways disrupted by the lesion.20–22 MT imaging is sensitive to interactions between free protons (unbound water in tissue) and bound protons (water bound to macromolecules such as those in myelin membranes). The amount of magnetization transfer correlates with the degree of myelination. Thus, MT imaging is a promising method for studying both normal development and injury-induced pathway degeneration.20,23,24 DTI is a more complicated version of diffusion-weighted (DW) MRI. DW MRI is sensitive to the speed of water diffusion. In DTI, a set of at least six DW images is collected, each sensitized to a different anatomic direction. From this set of images several measures can be derived, including the principal direction of diffusion within each voxel of the image. Diffusion within white matter occurs much faster along the length of axons than transaxonally, so the principal direction of diffusion within white matter reflects the average direction of the fibers and may be useful in tracing pathways.21 In areas of pathway degeneration this directionality is diminished or lost.22 The greater sensitivity of these methods to injury-related changes may well provide a better correlation with differential outcome.23,24
Metabolic imaging provides a way to ascertain functional alterations remote from the lesion site. Only a few studies have been done comparing metabolic measures before and after surgery.25,26 A case report of bifrontal leukotomy for refractory obsessive-compulsive disorder found that regional glucose metabolism (as measured by PET) was decreased toward normal in the orbital frontal cortex in concert with clinical improvement. Another study reported a correlation between symptom reduction and decreased cerebral blood flow (as measured by SPECT) in anterior frontal and cingulate cortex following subcaudate tractotomy for refractory depression. These findings are consistent with the present understanding of the brain circuits involved.
Imaging has changed the face of neurosurgical interventions for psychiatric diseases. It has made possible refined and carefully controlled procedures. The future holds yet more promise with the applications of functional MRI and other techniques such as three-dimensional spiral CT and intraoperative MRI.27–29 Ultimately these should lead to better treatments for severe and intractable mood and anxiety disorders.
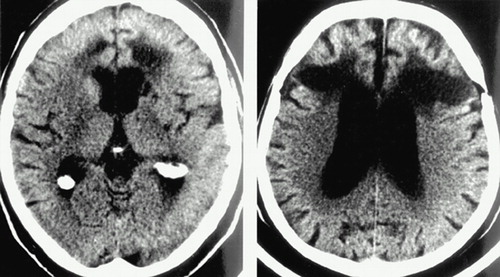
A Axial computed tomographic scans from two patients who underwent frontal leukotomy (transection of the fiber tracts leading from the frontal gray matter) are shown to the left. A third case is shown on the cover. Early surgeries were necessarily performed freehand, resulting in considerable variability in actual lesion location and size. Note the dramatic differences among these three cases.
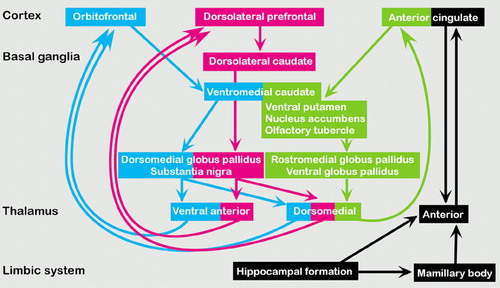
B The original emotion and memory circuit proposed by Papez is diagrammed below in black. Current theories emphasize the presence of parallel processing of different aspects of behavior. The orbitofrontal circuit is thought to mediate socially appropriate behavior, impulse control, and empathy (blue). The dorsolateral circuit is thought to accomplish organization, planning, and attention (pink). The anterior cingulate circuit is thought to produce motivation by balancing the inhibitory input of the supplemental motor area with its own stimulus that supports wakefulness and arousal (green). The three circuits are color-coded at the level of the leukotomy on a coronal myelin-stained brain slice on the cover. Note that these pathways run close together. Subcortical injury is likely to affect more than one.
1 Dorman J: The history of psychosurgery. Tex Med 1995; 91:54–61Medline, Google Scholar
2 Swayze VW: Frontal leukotomy and related psychosurgical procedures in the era before antipsychotics (1935–1954): a historical overview. Am J Psychiatry 1995; 152:505–515Crossref, Medline, Google Scholar
3 Cosgrove GR, Rauch SL: Psychosurgery. Neurosurg Clin N Am 1995; 6:167–176Crossref, Medline, Google Scholar
4 Trivedi MH: Functional neuroanatomy of obsessive-compulsive disorder. J Clin Psychiatry 1996; 57(suppl):26–35Google Scholar
5 Marino R Jr, Cosgrove GR: Neurosurgical treatment of neuropsychiatric illness. Psychiatr Clin North Am 1997; 20:933–943Crossref, Medline, Google Scholar
6 Trimble MR, Mendez MF, Cummings JL: Neuropsychiatric symptoms from the temporolimbic lobes. J Neuropsychiatry Clin Neurosci 1997; 9:429–438Link, Google Scholar
7 Burruss JW, Hurley RA, Taber KH, et al: Functional neuroanatomy of the frontal lobe circuits. Radiology 2000; 214:227–230Crossref, Medline, Google Scholar
8 Starr PA, Vitek JL, DeLong M, Bakay RA: Magnetic resonance imaging-based stereotactic localization of the globus pallidus and subthalamic nucleus. Neurosurgery 1999; 44:303–313Crossref, Medline, Google Scholar
9 Reich CA, Hudgins PA, Sheppard SK, et al: A high-resolution fast spin-echo inversion-recovery sequence for preoperative localization of the internal globus pallidus. AJNR Am J Neuroradiol 2000; 21:928–931Medline, Google Scholar
10 Spangler WJ, Cosgrove GR, Ballantine HT Jr, et al: Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery 1996; 38:1071–1076Google Scholar
11 Mindus P, Rasmussen SA, Lindquist C: Neurosurgical treatment for refractory obsessive-compulsive disorder: implications for understanding frontal lobe function. J Neuropsychiatry Clin Neurosci 1994; 6:467–477Link, Google Scholar
12 Holtzheimer PE, Roberts DW, Darcey TM: Magnetic resonance imaging versus computed tomography for target localization in functional stereotactic neurosurgery. Neurosurgery 1999; 45:290–297Crossref, Medline, Google Scholar
13 Bednarz G, Downes MB, Corn BW, et al: Evaluation of the spatial accuracy of magnetic resonance imaging-based stereotactic target localization for gamma knife radiosurgery of functional disorders. Neurosurgery 1999; 45:1156–1161Google Scholar
14 Sachdev P, Hay P: Site and size of lesion and psychosurgical outcome in obsessive-compulsive disorder: a magnetic resonance imaging study. Biol Psychiatry 1996; 39:739–742Crossref, Medline, Google Scholar
15 Irle E, Exner C, Thielen K, et al: Obsessive-compulsive disorder and ventromedial frontal lesions: clinical and neuropsychological findings. Am J Psychiatry 1998; 155:255–263Medline, Google Scholar
16 Malhi GS, Bartlett JR: A new lesion for the psychosurgical operation of stereotactic subcaudate tractotomy (SST). Br J Neurosurg 1998; 12:335–339Crossref, Medline, Google Scholar
17 Sawlani V, Gupta RK, Singh MK, et al: MRI demonstration of Wallerian degeneration in various intracranial lesions and its clinical implications. J Neurol Sci 1997; 146:103–108Crossref, Medline, Google Scholar
18 Yamada K, Shrier DA, Rubio A, et al: MR imaging of the mamillothalamic tract. Radiology 1998; 207:593–598Crossref, Medline, Google Scholar
19 Khurana DS, Strawsburg RH, Robertson RL, et al: MRI signal changes in the white matter after corpus callosotomy. Pediatr Neurol 1999; 21:691–695Crossref, Medline, Google Scholar
20 Rademacher J, Engelbrecht V, Burgel U, et al: Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer MR. Neuroimage 1999; 9:393–406Crossref, Medline, Google Scholar
21 Jones DK, Simmons A, Williams SC, et al: Noninvasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 1999; 42:37–41Crossref, Medline, Google Scholar
22 Wieshmann UC, Symms MR, Clark CA, et al: Wallerian degeneration in the optic radiation after temporal lobectomy demonstrated in vivo with diffusion tensor imaging. Epilepsia 1999; 40:1155–1158Google Scholar
23 Bagley LJ, McGowan JC, Grossman RI, et al: Magnetization transfer imaging of traumatic brain injury. J Magn Reson Imaging 2000; 11:1–8Crossref, Medline, Google Scholar
24 McGowan JC, Yang JH, Plotkin RC, et al: Magnetization transfer imaging in the detection of injury associated with mild head trauma. AJNR Am J Neuroradiol 2000; 21:875–880Medline, Google Scholar
25 Malizia AL, Allen SJ, Maisey MN, et al: Changes in low frontal cerebral blood flow correlate with outcome in stereotactic subcaudate tractotomy carried out for refractory depression, in Refractory Depression: Current Strategies and Future Directions, edited by Nolen W et al. New York, Wiley, 1994, pp 163–167Google Scholar
26 Biver F, Goldman S, Francois A, et al: Changes in metabolism of cerebral glucose after stereotactic leukotomy for refractory obsessive-compulsive disorder: a case report. J Neurol Neurosurg Psychiatry 1995; 58:502–505Crossref, Medline, Google Scholar
27 Moringlane JR, Bartylla K, Hagen T, et al: Stereotactic neurosurgery planning with 3-D spiral CT-angiography. Minim Invasive Neurosurg 1997; 40:83–86Crossref, Medline, Google Scholar
28 Rubino GJ, Farahani K, McGill D, et al: Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery 2000; 46:643–653Crossref, Medline, Google Scholar
29 Hall WA, Liu H, Martin AJ, et al: Safety, efficacy, and functionality of high-field strength interventional magnetic resonance imaging for neurosurgery. Neurosurgery 2000; 46:632–641Crossref, Medline, Google Scholar



