Schizophrenia: What's Under the Microscope?
Dementia praecox, as formally described by Emil Kraepelin in 1898, is a devastating illness affecting every aspect of life for the patient and the patient's family. Over the past century, theories of the cause of schizophrenia have ranged from original sin to in utero viral infection. Heritability patterns support a strong genetic component, yet other factors are clearly involved. Schizophrenia, as this illness is now known, affects 1% of the population worldwide. In 1990, the socioeconomic impact of schizophrenia in the United States alone was $32.5 billion. These patients accounted for 25% of all hospital admissions and occupied 40% of all long-term care beds.3,4
A vital part of fully understanding an illness is identification of the underlying pathology. Initially the search for the biologic basis of schizophrenia focused on changes in gross brain structure (macroscopic neuropathology). Volumetric studies undertaken in recent decades comparing normal subjects and individuals with schizophrenia have shown considerable variability in results.5,6 There is a consensus that the ventricles are commonly enlarged. More subtle abnormalities have been reported for many areas, including prefrontal cortex, basal ganglia, thalamus, and hippocampus, but specific claims are controversial. No single brain region is consistently abnormal in schizophrenia, and no pattern of gross brain abnormalities has been identified that is diagnostic of schizophrenia (see cover for a sagittal T1-weighted magnetic resonance image of a 31-year-old male with chronic paranoid schizophrenia). It may well be, as suggested recently, that schizophrenia results from “the cumulative effect of deviant brain structure.”7 Thus, schizophrenia may be the functional end result of various combinations of brain abnormalities. More recently the focus has shifted to seeking the abnormal brain circuits that could account for the symptoms of schizophrenia. This approach requires measures that can be related to how brain regions function together as parts of the circuits that support complex cognitive functions. Widespread functional circuits can be studied by detailed examination of individual areas and by global examination of entire networks.
Many studies focus on the fine structure (microscopic neuropathology) of brain areas implicated in schizophrenia. Significant decreases in neuronal numbers have been found in the medial dorsal and anterior nuclei of the thalamus (parts of the thalamus with intimate connections to prefrontal and limbic areas) and in the nucleus accumbens (part of the basal forebrain).8 In the anterior nucleus of the thalamus, the reductions are in projection neurons rather than interneurons, suggesting a decrease in thalamocortical transmission.9 Some studies have also reported decreased neuronal numbers in the cingulate cortex, which is reciprocally connected to these thalamic nuclei.10 Most studies have not found decreased numbers of projection neurons (pyramidal cells) in the other major connection area considered important in schizophrenia, prefrontal cortex.11 More subtle differences may exist between individuals with schizophrenia and normal subjects in this area. For instance, several lines of evidence, including those involving increased neuronal density and decreased presynaptic proteins (i.e., synaptophysin), are consistent with a decrease in the density of synapses in prefrontal cortex.12
Alterations have been reported in several neurotransmitter systems in this illness. Immunoreactivity for tyrosine hydroxylase—which is known to mark dopamine (DA)-containing axons—and immunoreactivity for the DA membrane transporter are both decreased in prefrontal cortex (see Figure 1).1 Several measures of the gamma-aminobutyric acid (GABA) system are altered in prefrontal cortex, as well. There may be a decrease in inhibitory (GABAergic) interneurons.11,13 The tissue content of GABA and the cellular expression of the mRNA for the GABA transporter and for glutamic acid decarboxylase (synthetic enzyme for GABA; see Figure 2) are all decreased.2,14 In contrast, cellular expression of the mRNA for the GABAA receptor is increased.14 These changes may be concentrated in a specific subgroup of GABAergic interneurons (chandelier neurons) that provide inhibitory input to the axon initial segment of pyramidal cells, thus exerting control over the excitatory output of the prefrontal cortex.15 Studies of the serotonin (5-HT) system have mixed findings. Postmortem studies have found increased 5-HT receptor density in several cortical areas, including prefrontal and temporal.16 On the other hand, labeling of axons immunoreactive to the serotonin transporter was unchanged in prefrontal cortex.1 The glutamate system has been studied less than other neurotransmitters, but there is evidence for altered expression of some receptor subtypes in hippocampus and some areas of cortex.17
Functional brain imaging is another way of examining neurochemistry. Initial studies focused on imaging DA receptors because the first successful pharmacologic treatment for schizophrenia acted by blockade of dopamine D2 receptors.16 In addition, amphetamines, which can produce a state similar to paranoid schizophrenia, act by increasing presynaptic DA release and inhibiting uptake.18 These findings led to the development of the “dopamine hypothesis” of schizophrenia, in which the symptoms were attributed to excessive DA activity. Many, but not all, patients with schizophrenia have increased D2 receptor density in the striatum. Synaptic concentrations of DA may also be greater in schizophrenia. Dopaminergic responsiveness may also be increased, as indicated by a greater amphetamine-induced release of DA in the striatum. Studies have concentrated on striatum because of its high concentration of DA receptors. The low density of D2 receptors in other areas of brain has precluded their study in vivo, although newly developed ligands may change this in the future.16
The atypical antipsychotics act powerfully on other neurotransmitter systems in addition to DA. In particular, they are potent 5-HT receptor antagonists.19 The balance between 5-HT and DA antagonism may be critical to the lower rate of side effects with these agents.20 Although changes in 5-HT receptor density have been found in postmortem studies of brains from individuals with schizophrenia, two in vivo studies (measuring fluorine-18-labeled setoperone binding) did not find any difference.16 Similarly, in vivo studies of GABA receptor density have not found any abnormalities in individuals with schizophrenia, although alterations have been reported in postmortem studies.16 The basis for this divergence of findings has not yet been established.
Functional imaging can also indirectly observe summed synaptic activity, as mirrored in the metabolic demand of an area. There is sufficient anatomic resolution to observe functional subregions of the brain and thus gain insight into interacting circuits. Many studies have found a decrease in resting metabolism (as measured by regional cerebral blood flow or glucose metabolism) in areas of prefrontal cortex in schizophrenia.21,22 This hypofrontality is most closely associated with the negative symptoms of schizophrenia. Prefrontal cortex is made up of many functional areas, and it is clear that areas of increased and decreased metabolism coexist within individuals, indicating the importance of looking at subregions rather than prefrontal cortex in totality.22 Many other brain regions also show altered resting metabolism, including portions of anterior cingulate, temporal and parietal cortices, striatum, thalamus, and cerebellum.21,22 Correlation analysis has been used to examine functional relationships among many of these areas. The strength of correlation between frontal cortex and related regions is decreased in schizophrenia, suggesting changes in functional connectivity.21 Alterations in neuronal numbers, in the density of synaptic proteins (and therefore of synapses), in the density of transporters for neurotransmitters, and in their synthetic enzymes, found with micropathological studies, all may underlie the findings of changes in connectivity from functional brain imaging studies.
Even more informative, from the perspective of treatment, are the studies examining the effects of antipsychotic agents on regional brain metabolism. Medication-related increases in striatal metabolism have been found by several groups. It has been suggested that this is a “normalization” of function. A low resting striatal metabolic rate may even be predictive of good medication response.21 Similarly, medication-induced increased metabolic rate in striatal areas (caudate, globus pallidus) may predict development of tardive dyskinesia. Regional patterns of antipsychotic-induced metabolic changes vary with the agent. It is possible that careful systematic studies relating these differences to individual patient's medication response may provide a means of predicting responders and nonresponders in future.
In recent years the original DA hypothesis of schizophrenia has been transformed as understanding of interactions among neurotransmitter systems has evolved. It has become evident that considering the DA system in isolation does not provide a full picture of schizophrenia. Many new formulations have been suggested that incorporate other neurotransmitter systems in a wide variety of ways.15,20,23–26 Some emphasize the interactions between DA and 5-HT, others the interactions between DA and glutamate. GABA is central in some theories, glutamate in others. Most of these theories involve multiple brain areas, although not necessarily the same areas. Core similarities among these diverse approaches to understanding schizophrenia are an incorporation of the very complex interactions that exist among neurotransmitter systems at the level of individual synapses and at the level of interconnected brain regions. As a result of this wider view, new medications are being developed that target more neurotransmitter systems and different receptor subtypes than previously. Their success or failure will provide valuable insight into the pathophysiology of schizophrenia, as well as a source for new therapeutic agents.
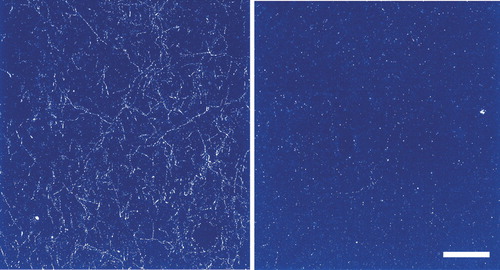
FIGURE 1. Darkfield photomicrograph of dopamine transporter-labeled axons in layer 6 of prefrontal cortex (area 9) from control (left) and schizophrenic (right) subjects (calibration bar=200 μm)Note the clearly diminished density of dopamine axons in the schizophrenic subject. (Adapted from Akil et al.1 Copyrighted 1999 American Psychiatric Association.)
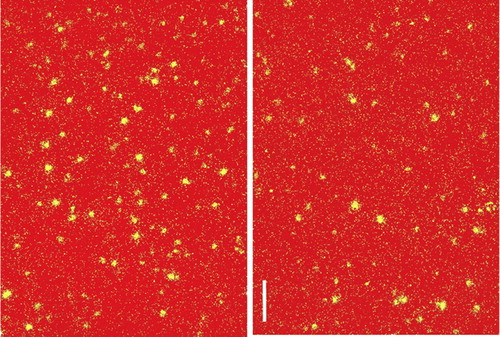
FIGURE 2. Darkfield photomicrographs of the glutamic acid decarboxylase (GAD) mRNA-positive neurons in deep layer 3 of prefrontal cortex (area 9) from control (left) and schizophrenic (right) subjects (calibration bar=150 μm)Note the reduction in labeled GABAergic neurons in the schizophrenic subject. (Adapted from Volk et al.2 Copyrighted 2000 American Medical Association.)
1 Akil M, Pierri JN, Whitehead RE, et al: Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 1999; 156:1580–1589Google Scholar
2 Volk DW, Austin MC, Pierri JN, et al: Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry 2000; 57:237–245Crossref, Medline, Google Scholar
3 Pickar D: Prospects for pharmacotherapy of schizophrenia. Lancet 1995; 345:557–562Crossref, Medline, Google Scholar
4 Rice DP: The economic impact of schizophrenia. J Clin Psychiatry 1999; 60(suppl 1)4–6; discussion 28–30Google Scholar
5 McCarley RW, Wible CG, Frumin M, et al: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Google Scholar
6 Pearlson GD, Marsh L: Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry 1999; 46:627–649Crossref, Medline, Google Scholar
7 Leonard CM, Kuldau JM, Breier JI, et al: Cumulative effect of anatomical risk factors for schizophrenia: an MRI study. Biol Psychiatry 1999; 46:374–382Crossref, Medline, Google Scholar
8 Danos P, Baumann B, Bernstein HG, et al: Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res 1998; 82:1–10Crossref, Medline, Google Scholar
9 Dixon G, Dissanaike S, Harper CG: Parvalbumin-immunoreactive neurons in the human anteroventral thalamic nucleus. Neuroreport 2000; 11:97–101Crossref, Medline, Google Scholar
10 Benes FM: Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev 2000; 31:251–269Crossref, Medline, Google Scholar
11 Thune JJ, Pakkenberg B: Stereological studies of the schizophrenic brain. Brain Res Brain Res Rev 2000; 31:200–204Crossref, Medline, Google Scholar
12 Honer WG, Falkai P, Chen C, et al: Synaptic and plasticity-associated proteins in anterior frontal cortex in severe mental illness. Neuroscience 1999; 91:1247–1255Google Scholar
13 Beasley CL, Reynolds GP: Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res 1997; 24:349–355Crossref, Medline, Google Scholar
14 Ohnuma T, Augood SJ, Arai H, et al: Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABA(A) receptor alpha-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience 1999; 93:441–448Crossref, Medline, Google Scholar
15 Pierri JN, Chaudry AS, Woo TU, et al: Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry 1999; 156:1709–1719Google Scholar
16 Soares JC, Innis RB: Neurochemical brain imaging investigations of schizophrenia. Biol Psychiatry 1999; 46:600–615Crossref, Medline, Google Scholar
17 Meador-Woodruff JH, Healy DJ: Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev 2000; 31:288– 294Crossref, Medline, Google Scholar
18 Vollenweider FX: Advances and pathophysiological models of hallucinogenic drug actions in humans: a preamble to schizophrenia research. Pharmacopsychiatry 1998; 31(suppl):92–103Google Scholar
19 Ichikawa J, Meltzer HY: Relationship between dopaminergic and serotonergic neuronal activity in the frontal cortex and the action of typical and atypical antipsychotic drugs. Eur Arch Psychiatry Clin Neurosci 1999; 249(suppl):90–98Google Scholar
20 Kasper S, Tauscher J, Kufferle B, et al: Dopamine- and serotonin- receptors in schizophrenia: results of imaging-studies and implications for pharmacotherapy in schizophrenia. Eur Arch Psychiatry Clin Neurosci 1999; 249(suppl):83–89Google Scholar
21 Buchsbaum MS, Hazlett EA: Positron emission tomography studies of abnormal glucose metabolism in schizophrenia. Schizophr Bull 1998; 24:343–364Crossref, Medline, Google Scholar
22 Kim JJ, Mohamed S, Andreasen NC, et al: Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry 2000; 157:542–548Crossref, Medline, Google Scholar
23 Carlsson A, Waters N, and Carlsson ML: Neurotransmitter interactions in schizophrenia-therapeutic implications. Eur Arch Psychiatry Clin Neurosci 1999; 249(suppl):37–43Google Scholar
24 Moore H, West AR, Grace AA: The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry 1999; 46:40–55Crossref, Medline, Google Scholar
25 Olney JW, Newcomer JW, Farber NB: NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 1999; 33:523– 533Crossref, Medline, Google Scholar
26 Aghajanian GK, Marek GJ: Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Res Rev 2000; 31:302–312Crossref, Medline, Google Scholar



