EEG Monitoring in Depressed Patients Undergoing Repetitive Transcranial Magnetic Stimulation
Abstract
To date, 33 subjects diagnosed with major depressive disorder have undergone transcranial magnetic stimulation (TMS) in the authors' clinic. Five of these patients showed minimal electroencephalogram (EEG) variants at baseline. The authors describe the course of treatment and serial EEGs in 3 of the 5 patients who did not show progressive EEG changes in association with active rTMS. These three cases suggest that minimal EEG anomalies at baseline need not serve as a contraindication to undergoing rTMS. Two patients with progressive EEG changes in association with sham rTMS in one and active rTMS in the other are also discussed.
Transcranial magnetic stimulation (TMS) is a relatively new experimental technique whose properties, safety, and potential applications in psychiatry have been under active investigation since the mid-1980s.1,2 Evidence to date has generally found the procedure to be well tolerated. A rare, but serious, potential complication of rTMS is seizure induction.3 The impression is current that such risk has been minimized by the careful implementation of, and adherence to, present-day rTMS safety guidelines4 that include real-time monitoring of cortical spread (via EEG, electromyograms, and visual inspection).5 Nevertheless, there are few descriptive accounts of the use of these monitoring techniques in the literature.
We have previously reported that rTMS has no observable effect on the clinical EEGs of depressed patients with normal baseline EEGs.5,6 Our observation lent support to an earlier report showing that rTMS, delivered at 20 Hz frequency and with a 5% to 10% suprathreshold strength, had no ill effects on the EEGs of normal individuals.7 Here we report on our experience to date with EEG monitoring of patients with minimal EEG abnormalities (such as small sharp spikes, mild diffuse slowing, or mild focal slowing) at baseline.
METHODS
The 5 patients included in this report had a history of multiple failed antidepressant medication trials. They all met the DSM-IV criteria for major depressive disorder, along with all other initial screening criteria in the protocol (e.g., no history of traumatic brain injury, epilepsy, seizures, or loss of consciousness). The study was fully explained to the patients and to appropriate family members and treaters prior to the patient's signing an informed consent that was approved by the Yale Human Investigations Committee. Full screening included the following: complete medical and psychiatric history (including review of the medical record), Hamilton Rating Scale for Depression (Ham-D), Structured Clinical Interview for DSM-IV (SCID), physical exam, complete battery of blood tests and urine tests, and electrocardiogram.
A baseline 22-lead EEG was performed. The EEG included a combination of referential and bipolar montages based on the 10–20 international electrode placement system and lasting from 30 to 45 minutes including 3 minutes of hyperventilation. The EEG procedure was extended, if needed, to obtain at least a brief recording during drowsiness or light sleep. During the daily rTMS treatments, EEG tracings were obtained by using a single bipolar montage for 3 minutes prior to rTMS, between trains, and for 2 more minutes at the end of the session. All EEGs were interpreted by a certified electroencephalographer (N.N.B.).
After 2 weeks of medication washout, each patient was randomized and entered into a sham-controlled rTMS protocol. Patients #1 and #2 underwent active rTMS with the following parameters: 20 two-second stimulation trains of 20 Hz each, with 58-seconds intervals between trains, delivered at 80% motor threshold (MT) for 10 treatment days. This protocol used a Cadwell Magnetic Stimulator. Patient #3 underwent the following active rTMS parameters: twenty 1.5-second stimulation trains of 20 Hz each, with 58.5-second intervals between trains, delivered at 100% MT for 10 treatment days. This protocol used a Magstim Rapid Magnetic Stimulator. Patient #4 underwent sham rTMS with parameters similar to the condition for Patient #3; however, the sham condition was achieved by increasing the angulature of the coil from the scalp and by elevating the bottom-most coil margin about 1 cm off the scalp (i.e., the coil surface at the juncture of the double coils was about 2 cm from the scalp). In this way the patient would nevertheless feel the magnetic pulse on the scalp and hear the click.
Patient #5 was randomized to active rTMS and received 8 sessions of forty 5-Hz, 4-second trains with 26 seconds intertrain intervals.
Although mild EEG abnormalities were recorded at baseline and during the treatments in all of the mentioned study subjects, these patients were ultimately admitted to the study because the EEG anomalies are not known to correlate with an increased likelihood of seizure occurrence.8 Moreover, the non-epileptogenic nature of the anomalies was further reinforced by the patients' having no prior seizures, traumatic brain injuries, or events suggestive of aura-like experiences. When a baseline EEG variant was detected, the rTMS team met to review the neurologic history and examination to decide whether a patient would be appropriate for inclusion in the study. The predetermined criterion for terminating the study was worsening of the baseline abnormality (as decided by N.N.B., in a blinded manner. for patients #3 and #4), or the appearance of paroxysmal activity. In the first three subjects, no changes were noted and the rTMS protocol was completed. Treatments were stopped, however, in patients #4 and #5 because of worsening of baseline abnormalities.
CASE REPORTS
Patient #1 was a 42-year-old single white female who presented with a history of episodic nonremitting major depression. She identified this as having started in her mid-thirties, soon after the unexpected and sudden death of a close relative, and having been perpetuated by other serious family stressors. Multiple antidepressant trials with both tricyclic antidepressants and selective serotonin reuptake inhibitors had not provided lasting effects. Initial Ham-D score was 40. Baseline EEG showed transient temporal slowing in the left leads (Figure 1A). The patient was randomized into the sham condition; EEG recordings during this time showed consistent left transient temporal slowing. The EEG continued to show the same location and degree of abnormality through the following 2-week open-label active rTMS (Figure 1B).
Patient #2 was a 38-year-old single white male with a history of chronic major depression with onset in his early teenage years. He had received long periods of psychotherapy without consistent relief. He was also treated with multiple antidepressants without success. He reported a trial of ECT as providing noticeable relief. Initial Ham-D score was 29. The patient was randomized into the active arm of the protocol. Baseline EEG (Figure 2A) showed diffuse slowing in all leads, which continued throughout the active rTMS treatment without changes. (Figure 2B).
Patient #3 was a 69-year-old white married male with a history of intermittent dysthymia, generalized anxiety, and major depression since his childhood years. His first significant episode of clinically disabling depression with suicidal thoughts occurred at the age of 30. Despite having received numerous treatment trials with various antidepressants, he reported modest overall mood improvement. The latest episode of depression had started 10 months prior to the study. Initial Ham-D score was 36. Baseline EEG (Figure 3A) recorded numerous small sharp spikes (SSS) in all leads, which continued to appear consistently and without change in quality or number during the entire duration of the active treatment period (Figure 3B). Hence, daily rTMS treatments using the above-stated parameters did not appear to increase the frequency or change the characteristics of the SSS.
Patient #4 was a 55-year-old married white female with a history of bipolar II disorder diagnosed in her twenties. She described her most recent episode of depression as having started approximately 4 months prior to her screening in our clinic. Multiple failed medication trials and long periods of psychotherapy punctuated her past psychiatric history. Initial Ham-D score was 39. Baseline EEG (Figure 4A) was noteworthy for mild focal slowing (4–5 Hz theta activity) on the right central-temporal region. A slight increase in the sharpness of the theta activity was also noted and was judged not to have an unequivocal epileptogenic potential. The patient was randomized into the sham arm of the protocol. She displayed a noticeable level of anxiety throughout the first and the second treatments. EEG tracings showed increases in high-amplitude theta and delta activity, especially over the frontal regions (Figure 4B). The electroencephalographer, blind to the protocol condition, deemed it necessary to stop the treatment and have the patient worked up neurologically. The blind was opened to the other rTMS team members and to the patient. Subsequent neurologic and seizure workup revealed an increase in spike frequency and amplitude during test hyperventilation. The EEG changes noted during monitoring were therefore attributed to potential anxiety-related hyperventilation during the rTMS sessions.
Patient #5. This 40-year-old single African-American male who was admitted to the rTMS study for treatment-resistant depression. This patient stopped taking his antidepressants approximately 40 days prior to enrolling in the study. His most recent medications included mirtazapine, lithium, and venlafaxine. With the exception of reporting occasional arm twitching (which was not noted by the examiner or any of the researchers involved), he had no history of seizures or any aura-like experiences. A baseline EEG revealed minimal slow transients (occasional theta activity) seen bilaterally, but maximally on the right central region (Figure 5A). Sleep readings were obtained, and no epileptiform activity was detected. Hyperventilation did not produce further exaggeration of the slow-wave activity. Given the minimal nature of his EEG findings, it was decided to admit him to the study. The EEG started showing paroxysmal theta activity during the 6th session, with longer and more frequent bursts noted on the 7th session.
After cessation of stimulation on days 6 and 7, the EEG reverted back to baseline within minutes. The patient had no complaints or any symptoms. During the 8th session the bursts of theta activity, on the right central region (Figure 5B), were more frequent and lasted longer (approximately 3 s, compared with 1 s at baseline). Moreover, the paroxysmal nature of the bursts became more evident. The study was halted at this time. No further epileptic discharges were seen in this subject. Next-day EEG showed a complete return to baseline. Another EEG, 2 days later, was indistinguishable from the baseline EEG showing occasional theta activity on the right central region. The patient elected not to continue follow-up with the rTMS research group and declined an imaging procedure. A follow-up telephone call approximately a month later revealed him not to have developed any new symptoms or complaints.
DISCUSSION
Electroencephalogram recordings are used during rTMS treatments for the purpose of safety monitoring and for assessing potential effects induced by the magnetic pulses. In our studies, the vast majority of patients showed no EEG abnormalities. Such subjects did not develop EEG changes throughout the course of rTMS treatments. Five depressed patients manifested mildly abnormal EEG waveforms at baseline and during treatment. These mild EEG abnormalities include minimal or transient focal slowing, controversial sharp activity like the small sharp spikes9,10 and minimal diffuse slowing. Overall, 15% (5 of 33) of our patients, who were prominently characterized by severe treatment resistance, demonstrated such EEG changes at baseline. This finding is consistent with other reports11,12 that found a 19% to 25% rate of EEG abnormalities in patients with chronic mood disorders.
Case #4 is problematic because it is possible that the patient may have received significant magnetic stimulation. Given that the magnetic field strength would be predicted to decline at a rate that is proportional to the square or cube of the distance of the coil from the cortex, the sham condition (with a 45-degree angulature from the surface of the scalp and using a two-wing coil) should have negligible effect on the underlying neural tissue.13 More recent reports suggest that sham rTMS may be more active than once thought.14,15 It is possible that this patient's tendency to hyperventilate accentuated the effects of the weaker magnetic pulses. The demonstration of the effect of hyperventilation in the absence of rTMS suggests that rTMS did not contribute to the demonstrated worsening of the abnormality during the course of treatment.
Case #5 is similar to case #4 in demonstrating that baseline minimal EEG abnormalities could worsen during the course of rTMS protocols. The progression of a baseline abnormality in 2 out of 5 patients is a significant observation given the lack of such effect in patients with baseline normal EEGs.
The value of obtaining pre–rTMS baseline EEGs rests on the possibility that certain EEG abnormalities may be indicative of an increased likelihood for seizure induction in association with rTMS. The value of EEG monitoring during rTMS rests on the possibility that the emergence of certain EEG patterns will be indicative of an impending seizure. The establishment of the value of either practice should be based on much larger case series, particularly in subjects who did develop seizures during the course of rTMS. It follows that rTMS laboratories capable of obtaining baseline EEGs should include hyperventilation, as well as a period of sleep. Obtaining sleep has been shown to be important in evaluating for possible epileptic activity.16
The data presented in this case series suggest that the presence of baseline EEG abnormalities is an important variable to be further examined in relationship to rTMS efficacy and safety. The data further suggest that the presence of such abnormalities does not preclude safe administration of rTMS, particularly if EEG monitoring is employed.
ACKNOWLEDGMENTS
The authors thank Mr. John Paradise for technical assistance in the implementation of this study and the organization of this manuscript. This work was supported by Veterans Affairs Merit Review Award (N.N.B.) and a Mid-Career Award (R.E.H.) from the National Alliance for Research on Schizophrenia and Depression.
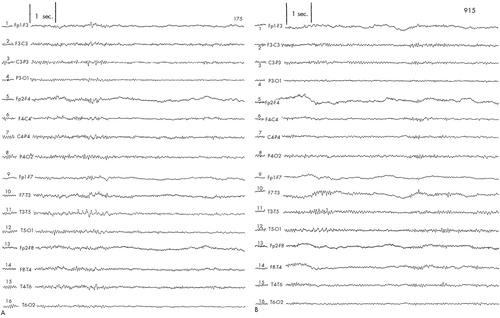
FIGURE 1. EEG tracings obtained from Patient #1A: left temporal slowing at baseline. B: left temporal slowing after completion of rTMS protocol.
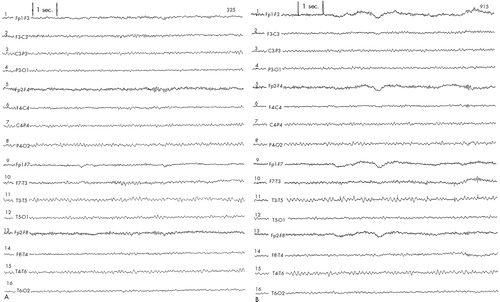
FIGURE 2. EEG tracings obtained from Patient #2A: diffuse slowing at baseline. B: diffuse slowing after completion of rTMS protocol.
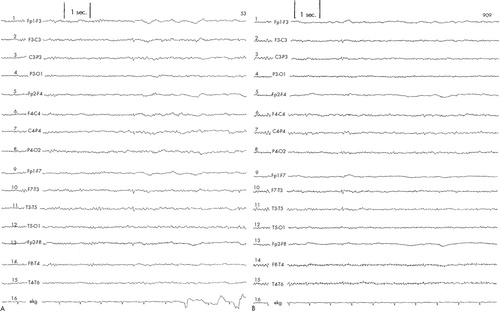
FIGURE 3. EEG tracings obtained from Patient #3A: small sharp spikes at baseline. B: small sharp spikes after completion of rTMS protocol.
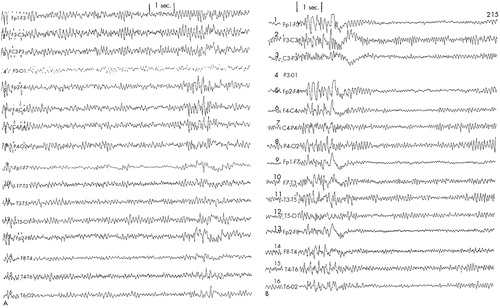
FIGURE 4. EEG tracings obtained from Patient #4A: mild focal slowing (4–5 Hz theta activity) on right central-temporal leads at baseline. B: paroxysmal increases in high-amplitude theta and delta waveforms during second day of sham rTMS.
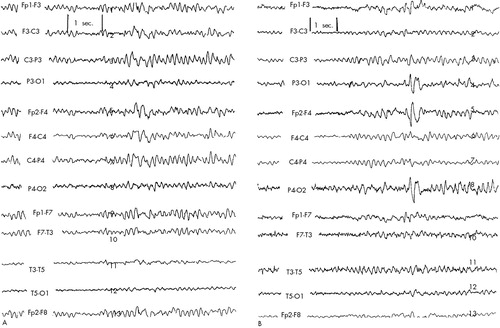
FIGURE 5. EEG tracings obtained from Patient #5A: mild focal slowing (4–5 Hz theta activity) on right central region at baseline. B: EEG tracing taken on day 8 on showing a longer burst of theta activity with a more paroxysmal nature.
1 Barker A, Jalinous R, Freeston IL: Non-invasive magnetic stimulation of human motor cortex. Lancet 1985; i(8437):1106-7Google Scholar
2 Avery D, George M: ISTS Database for studies of TMS in the treatment of depression (abstract). Electroencephalogr Clin Neurophysiol 1998; 107:93PCrossref, Medline, Google Scholar
3 Wassermann EM, Cohen LG, Flitman SS, et al: Seizure in healthy people with repeated safe trains of TMS (letter). Lancet 1996; 347:825Crossref, Medline, Google Scholar
4 Wassermann EM: Risk and safety of rTMS: report and suggested guidelines from the International Workshop on the Safety of rTMS, June 5-7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108:1-16Crossref, Medline, Google Scholar
5 Boutros NN, Berman RM, Hoffman RE, et al: Electroencephalogram and repetitive transcranial magnetic stimulation. Depress Anxiety 2000; 12:166-169Crossref, Medline, Google Scholar
6 Berman RM, Narasimhan M, Miano AP, et al: A randomized controlled trial of repetitive transcranial magnetic stimulation in the treatment of major depression. Biol Psychiatry 2000; 47:332-337Crossref, Medline, Google Scholar
7 Jahanshahi M, Ridding MC, Limousin P, et al: Rapid rate transcranial magnetic stimulation: a safety study. Electroencephalogr Clin Neurophysiol 1997; 105:422-429Crossref, Medline, Google Scholar
8 Boutros NN, Hughes JR, Weiler M: Psychiatric correlates of rhythmic midtemporal discharges and 6/second spike and wave complexes. Biol Psychiatry 1986; 21:94-99Crossref, Medline, Google Scholar
9 Small JG: Small sharp spikes in a psychiatric population: Arch Gen Psychiatry 1970; 22:277-84Google Scholar
10 Gutrecht JA: Clinical implications of benign epileptiform transients of sleep: Electroencephalogr Clin Neurophysiol 1989; 72:486-490Google Scholar
11 Assael M, Winnik HZ: Electroencephalographic findings in affective psychoses: Diseases of the Nervous System 1970; 31:695-702Google Scholar
12 Taylor MA, Abrams R: Gender differences in bipolar affective disorder. J Affect Disord 1981; 3:261-277Crossref, Medline, Google Scholar
13 Mills KR: Magnetic Stimulation of the Human Nervous System. New York, Oxford University Press, 1999Google Scholar
14 Lisanby SH, Luber B, Schroeder C, et al: Intracerebral measurement of rTMS and ECS induced voltage in vivo (abstract). Biol Psychiatry 1998; 43(suppl):100SGoogle Scholar
15 Loo CK, Taylor JL, Gandevia SC, et al: Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiatry 2000; 47:325-331Crossref, Medline, Google Scholar
16 Struve FA, Pike LE: Routine admissions electroencephalograms of adolescents and adults psychiatric patients awake and asleep. Clin Electroencephalogr 1974; 5:56-71Google Scholar



