Characteristics of Psychotic Disorder Due to Traumatic Brain Injury
Abstract
The authors analyzed data from 69 published case studies of Psychotic Disorder Due to Traumatic Brain Injury (PDTBI) in order to describe its common characteristics and assist in its diagnosis and differentiation from schizophrenia. The majority of these PDTBI patients were males with onset of symptoms occurring within the first 2 years after moderate to severe head injury. A majority showed abnormalities on MRI/CT and EEG with localization in the frontal and temporal areas. The general presentation included delusions and hallucinations without co-occurring negative symptoms. The findings demonstrate that patients with PDTBI have a profile that distinguishes itself from schizophrenia.
Psychotic Disorder Due to Traumatic Brain Injury (PDTBI) is the current DSM-IV diagnosis given to individuals who develop a psychosis after a traumatic brain injury (TBI).1 Diagnostic criteria include 1) presence of hallucinations or delusions; 2) evidence (history, physical, or laboratory) that the psychosis is a direct physiological consequence of TBI; 3) psychosis is not better accounted for by another mental disorder; and 4) psychosis does not occur exclusively during a state of delirium.
Ahmed and Fujii2 argue that PDTBI is often difficult to diagnose because criteria are vague. This contention is supported by the wide range of incidence rates for PDTBI cited in the literature. For example, retrospective chart reviews of World War II soldiers have yielded incidence rates of 0.7%, 7.5%, and 8.9%.3–5 By contrast, a study with closed head injury medical patients reported a 20% incidence rate.6
According to Ahmed and Fujii, diagnostic difficulties hinge on two aspects of the criteria: 1) establishing that the psychosis is a direct physiological consequence of TBI and 2) determining that the psychosis is not due to another mental disorder.2
As to the first difficulty, there are several key issues in establishing TBI as the etiology of a psychotic condition. 1) What severity of TBI is significant enough to cause a psychosis? 2) How long after an injury is the TBI considered to be etiologically relevant to the development of a psychosis? and 3) What laboratory findings are associated with psychosis?
Reports vary as to the severity of TBI required to trigger a psychosis. Some studies suggest that individuals who develop a psychosis after TBI had generally sustained moderate to severe head injuries.5,7 By contrast, many case studies report development of a psychosis after mild brain injuries with no loss of consciousness8–11
Another issue in determining the etiological significance of TBI in the development of psychosis is the latency between the injury and onset of symptoms. One study reported latencies ranging from 2 days to 48 years.4 Another reported latencies between 3 months to 19 years.7 In many cases, the combination of frequently mild severity of injury and long latency in developing PDTBI can raise doubts about whether the TBI was a contributory factor in developing a psychosis.
The question of associated laboratory findings in determining PDTBI is probably less controversial. Preliminary evidence reports a preponderance of temporal lobe abnormalities on CT/MRI and EEG.7,12 Frontal lobe abnormalities on CT/MRI are also common but are less consistently found.7 The data, however, are sparse, and studies are limited by small sample sizes. Still, the localization of findings is generally consistent with earlier large sample studies that determined lesion site by area of missile wound.4,5,13
The second difficulty in diagnosis of PDTBI is ruling out other mental disorders. Perhaps the most common differential diagnosis would be with schizophrenia. Many schizophrenics sustain TBI during their lifetime.14 In people with schizophrenia it is often difficult to determine if a psychotic condition is a direct physiological consequence of TBI or even if TBI contributes to or exacerbates the psychotic process.14
Another difficult differential is with Psychosis Due to Seizure Disorder (PDSD). Schizophrenia-like psychosis occurs 6 to 12 times more frequently in patients with seizure disorder than in the general population.15,16 At the same time, seizure disorder is also a common sequela in patients with posttraumatic psychosis. Studies have found that 33% to 58% of patients with PDTBI also experienced seizures.7,13 In addition, EEG abnormalities in the temporal lobes have been reported in patients without a documented history of seizure disorder. It is also possible that these patients experienced seizures that were not reported.13 This high comorbidity between psychotic symptoms, head injury, and seizure disorder has led some researchers to speculate that the subsequent psychosis in PDTBI patients may actually result from the secondary seizures. Thus the secondary seizure disorder would be the intervening variable between TBI and the development of psychosis.17 In such cases the differential between PDTBI and PDSD is difficult to determine.
In addition to the differential diagnostic problems with the aforementioned mental disorders, there may be multiple factors contributing to psychotic symptoms—for example, genetic risk, substance abuse, or other neurological problems.18
So far, the literature examining PDTBI is sparse. Many case reports of PDTBI include neurological investigations such as brain imaging and EEG, but there are only a few studies describing such data,7,12 and these are limited by small sample sizes. Thus the utility of the findings is questionable. Studies with large sample sizes exist, but the injuries are primarily missile wounds and the lesion analysis is based on the location of the wound.3–5 Given that the overwhelming majority of current TBI patients have sustained closed head injuries, the applicability of these finding to many of today's patients may be limited.
The present study examines characteristics of PDTBI through an analysis of case studies in the literature. The purpose of the study is to provide descriptive information to assist in diagnosis of PDTBI. This information would be useful in ruling out other mental conditions such as schizophrenia or seizure disorder when formulating a diagnosis for a psychotic patient who has a history of TBI. Specifically, we focus on three questions: 1) What are common characteristics of PDTBI? 2) What historical, physical, or laboratory findings are associated with PDTBI? and 3) What are some characteristics that discriminate between PDTBI and schizophrenia, and PDTBI and seizure disorder?
METHODS
A comprehensive literature search of psychiatric, psychological, neurological, and medical journals written in English and listed in the PubMed data base was made from the year 1971 to 1997. The literature search focused on cases in which a psychosis developed after a traumatic brain injury and which also reported results of neurological studies such as computed tomography (CT), magnetic resonance imaging (MRI), and electroencephalography (EEG). Specific criteria for inclusion were based on DSM-IV criteria for PDTBI.1 In addition, cases reporting a family history of mental disorder were excluded.
A total of 39 articles were included, yielding 69 cases. The authors of articles are listed in Table 1.7,9–12,19–52 The following data were taken for each case: 1) gender, 2) etiology of TBI, 3) loss of consciousness, 4) age when TBI was sustained, 5) age at onset of psychotic symptoms, 6) presence of seizure disorder, 7) family history of mental illness, 8) medications, 9) clinical outcome, 10) presence of negative symptoms, 11) inpatient/outpatient status, 12) diagnosis, 13) presence and type of delusions, 14) presence and type of hallucinations, 15) EEG findings, 16) MRI/CT findings, 17) presence of neurological signs, and 18) neuropsychological test findings.
Severity of TBI was based on criteria set by the Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine (ACRM).53 According to their criteria, a head injury is considered mild if the duration of loss of consciousness (LOC) is 30 minutes or less and moderate to severe if LOC is longer than 30 minutes.
Psychotic symptoms were rated in the following manner. The presence of negative symptoms was determined by reports of affective flattening, alogia, and avolition. The absence of these behavioral descriptions resulted in the rating of “no” negative symptoms. Positive symptoms of delusions and hallucinations were based on reports and case descriptions. In several cases, more than one type of delusion or hallucination was described; thus the total number of positive symptoms exceeds the total number of subjects. Clinical outcome was based on rating case descriptions of outcome. A three-point system was used to rate outcomes as follows: 1) improved, 2) no improvement, or 3) decline in status.
Localization on EEG and CT/MRI was calculated by the system used by Fujii and Ahmed.7 In this system, each occurrence of abnormalities is tallied individually. Thus the total number of abnormalities exceeds the total number of subjects.
In many cases, there were missing data because of differences in reporting. All data points for each variable were included in individual analyses despite missing data for the case. Thus for individual variables, the number of cases is often less than the sample size of 69. Given that most data were nominal in nature, analyses were primarily chi-squares. When base rates were known or appropriate, these figures were used in the chi-square calculations. All other calculations are based on chance differences. We also conducted t-tests when appropriate. Because of the exploratory nature of the study and relatively lower power of nonparametric (versus parametric) statistics to detect significant differences between groups, a nonconservative significance level was set at 0.05.
RESULTS
Demographics
Known demographic data are presented in Table 2. The sample was composed of 49 males and 11 females. The proportion of males to females was significantly different from the expected (2:1) base rate for TBI (χ2=6.07, df=1, P<0.02). The significant majority of our subjects sustained TBI from motor vehicles accidents (67%; χ2=116.20, df=4, P<0.001). The second most frequent etiology of TBI was assault (13%), followed by gunshot wounds (4%) and falls (4%). The etiology in 13% of the cases was unspecified. Of the known cases, 92% sustained closed head injuries and 8% open head injuries (χ2=36.25, df=1, P<0.001).
A significant majority of the sample (89%) sustained TBI with loss of consciousness (χ2=6.55, df=1, P<0.01). Of the known cases, a significant proportion of subjects sustained moderate to severe head injuries (χ2=7.75, df=1, P<0.01). Most of the subjects (87%) were seen in inpatient settings (χ2=27.60, df=1, P<0.001). No differences were found for whether subjects came from psychiatric or medical settings (χ2=3.37, df=1, P<0.10).
The mean sample age for sustaining a TBI was 29.1±17.6 years (means and standard deviations reported). The mean age for onset of psychosis after head trauma was 33.4±15.4 years. The distribution of scores for mean delay for onset of psychotic symptoms was highly skewed, with a range of 0 to 34 years. The mean number of years after trauma for onset of psychosis was 4.1±6.6. The mode was less than 1 year (38%), and the median was 1 year. Seventy-two percent of the cases reported an onset of psychosis before the mean.
Clinical Presentation and Course
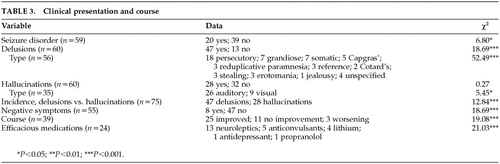
Data for the clinical presentation and course of psychosis are presented in Table 3. Of the 59 known cases, 20 reported a history of seizure disorder. The resulting proportion of patients sustaining secondary seizures is significantly smaller if compared with chance (χ2=6.80, df=1, P<0.01). However, if compared with the highest base rate reported in the TBI literature (12%), the proportion of patients with diagnosed seizure disorder is significantly higher (χ2=21.03, df=1, P<0.001).16
A significant proportion of subjects experienced delusions (47/60; χ2=18.69, df=1, P<0.001). By far the most common type of delusion was persecutory (n=18), followed by grandiose and somatic (7 each); Capgras (5); reduplicative paramnesia, religious, reference, stealing, and erotomanic (3 each); Cotard's (2); and jealousy (1). In 4 cases the delusions were unspecified. The proportion of persecutory delusions was significantly higher than chance (χ2=52.49, df=10, P<0.001).
Roughly half the subjects experienced hallucinations (28/60). The proportion of subjects demonstrating hallucinations was not significantly greater than chance (χ2=0.27, df=1, P>0.10). The most common hallucination was auditory (26) followed by visual (9). There was a significantly higher proportion of auditory versus visual hallucinations reported (χ2=5.45, df=1, P<0.02). A comparison of the incidence of delusions versus hallucinations indicates a significantly higher incidence of delusions in the sample (χ2=12.84, df=1, P<0.001).
Of 55 subjects, only 8 reported negative symptoms. The proportion negative symptoms is significantly lower than chance (χ2=18.69, df=1, P<0.001).
In the cases that described the course of psychosis, 25 patients were reported to be improved, 11 reported no improvement, and 3 reported a progressive worsening of symptoms. The proportion of improved outcome was significantly different from chance (χ2=19.08, df=1, P<0.001). In the cases that reported efficacious medications, neuroleptics were cited in 13 cases, anticonvulsants in 5 cases, lithium in 4 cases, antidepressants in 1 case, and propranolol in 1 case. The proportion of efficacious medications was significantly different from chance (χ2=21.03, df=4, P<0.001).
Neurological Investigations
Data for neurological investigations are listed in Table 4. EEG data were reported in 41 cases. Of these cases, 29 (about 70%) reported positive findings, which is significantly greater than chance (χ2=5.2, df=1, P<0.05). No differences were found in hemisphere location of EEG findings (left=17, right=18; χ2=0.03, df=1, P>0.10). Localization was as follows: 16 in the temporal areas, 5 frontal, 2 parietal, 5 occipital, 1 central, and 6 diffuse. The proportion of localized findings for specific cortical areas was greater than chance (χ2=12.66, df=5, P<0.05). In the cases that described the specific types of EEG abnormalities, 10 reported spiking, 24 slowing, and 2 dysrhythmia. The proportion of each abnormality type was greater than chance (χ2=25.1, df=2, P<0.001).
Data for CT and MRI were combined because both examine structural abnormalities. Of the 55 cases that included CT or MRI data, 36 reported positive findings, which is significantly greater than chance (χ2=5.15, df=1, P<0.05). A trend was found in hemisphere location of abnormalities (left=30, right=46; χ2=3.37, df=1, P<0.10). The frequency in localization of findings was as follows; 23 frontal, 15 temporal, 11 ventricular enlargement, 10 diffuse cortical, 8 parietal, 6 subcortical, 5 cerebellar, 3 central, and 3 brainstem. The proportion of localized findings was significantly greater than chance (χ2=40.28, df=9, P<0.001). In cases that described specific types of abnormalities, 34 reported focal lesions and 33 reported atrophy. No differences were found in type of lesion (χ2=0.00, df=1, P<0.10).
In 24 cases, either results from a neurological examination were reported or neurological symptoms were described. Of these cases, 14 reported positive findings, which was not different from chance (χ2=1.78, df=1, P>0.10). Neuropsychological test data were described in 17 cases, 15 of these reported impairments. The proportion of findings of impairment on neuropsychological test data was significantly greater than chance (χ2=9.94, df=1, P<0.001). Specific areas of impairment on neuropsychological test data were as follows: 10 memory, 7 executive functions, 7 visuospatial abilities, 2 language, and 2 attention. The proportion of impaired cognitive areas in comparison to each other approached significance when compared with chance (χ2=8.95, df=4, P<0.10).
Overall, when data from EEG, CT/MRI, neurological examination, and neuropsychological test data were combined, 57/63 or roughly 90% demonstrated at least one abnormality or impaired cognitive function. This proportion was significantly greater than chance (χ2=41.28, df=1, P<0.001).
Gender Differences
Gender differences in PDTBI were examined. There were trends for females to have sustained TBI at a later age than males (t=–1.81, df=58, P=0.10), and to have developed psychosis at a later age (t=–1.89, df=58, P=0.08). No differences were found for time of delayed onset in developing a psychosis (t=0.38, df=58, P=0.71) or for severity of TBI (χ2=0.37, df=1, P>0.05).
In terms of clinical presentation, no differences were found for proportion of seizure disorder (χ2=0.51, df=1, P>0.05), delusions (χ2=0.15, df=1, P>0.05), hallucinations (χ2=1.98, df=1, P>0.05), or negative symptoms (χ2=0.48, df=1, P>0.05). No differences were found on laboratory findings for EEG (χ2=1.22, df=1, P>0.05), CT/MRI (χ2=0.44, df=1, P>0.05), or neurological signs (χ2=0.01, df=1, P>0.05).
Delay in Onset of Psychotic Symptoms
Comparisons were also made for patients with a short versus long delay in onset of psychotic symptoms. Patients were divided into two groups based on scores in relation to the median (1 year). Those with onset of symptoms 1 year and less after TBI were compared to those with onset of symptoms of more than 1 year. Results are presented in Table 5.
Analyses reveal several significant differences between the groups. Differences were found in the setting at the time of evaluation, with the short-delay group having a higher proportion of inpatient medical versus psychiatry setting than the long-delay group (χ2=16.17, df=1, P<0.001). Significant differences were found for proportion of mild versus moderate/severe TBI, with the brief-delay group having more mild brain injury (χ2=4.79, df=1, P<0.05). The long-delay group showed a higher proportion of hallucinations than the short-delay group (χ2=4.07, df=1, P<0.05). By contrast, a trend was found for the short-delay group to show a higher proportion of visual hallucinations (χ2=3.78, df=1, P<0.10).
No significant differences were found for gender (χ2=0.11, df=1, P>0.10), clinical outcome (χ2=3.03, df=2, P>0.10), proportion demonstrating delusions (χ2=0.30, df=1, P>0.10), seizure disorder (χ2=0.94, df=1, P>0.10), negative symptoms (χ2=1.59, df=1, P>0.10), successful medications (χ2=3.47, df=3, P>0.10), positive EEG findings (χ2=0.31, df=1, P>0.10), EEG hemispheric localization (χ2=0.05, df=1, P>0.10), EEG lobe localization (χ2=7.12, df=4, P>0.10), CT/MRI positive findings (χ2=0.13, df=1, P>0.10), CT/MRI hemispheric localization (χ2=2.57, df=1, P>0.10), temporal lobe abnormalities (χ2=2.57, df=1, P>0.10), ventricular abnormalities (χ2=2.27, df=1, P>1.0), neurological signs (χ2=0.02, df=1, P>0.10), or neuropsychological testing (χ2=0.08, df=1, P>0.10).
Seizure Disorder
Specific analyses were also conducted for subjects with a diagnosis of seizure disorder versus those without a diagnosis. Data are presented in Table 5. No differences were found in age at time of TBI (t=0.35, P>0.10), age at onset of psychosis (t=0.56, P>0.10), or delay in onset of psychosis (t=0.52, P>0.10). In terms of clinical presentation, there were no differences in proportion of subjects presenting with hallucinations (χ2=1.88, df=1, P>0.10); however, nonseizure patients demonstrated a significantly higher proportion of delusions (χ2=4.15, df=1, P>0.05). On laboratory tests, no differences were found in proportion of positive findings on EEG (χ2=0.0, df=1, P>0.10) or type of finding on EEG (χ2=4.02, df=2, P>0.05). No differences were found in proportion of positive CT/MRI findings (χ2=0.45, df=1, P>0.10).
DISCUSSION
What are typical characteristics of PDTBI?
Our data indicate that males are significantly over-represented in cases of PDTBI even after correcting for the base rate of males to females in TBI (2:1). This result is consistent with other studies reporting gender differences.7,15,54 The robustness of this finding may suggest that males are at higher risk for developing PDTBI. Males are reported to have a higher incidence of other neurodevelopmental disorders such as learning disabilities.1,55 They are also believed to have poorer prognosis for recovery from aphasia after a cerebral vascular accident, because of the increased hemispheric lateralization in organization of the male brain.56–59 In addition, males generally develop schizophrenia at an earlier age than females.1
In terms of clinical presentation, our data indicated that delusions appear to be more common than hallucinations. The most common delusion is the persecutory type, although there was a wide variety of subtypes. The most common hallucination was auditory. The presence and type of hallucinations appeared to be associated with delay in onset of psychosis after TBI. Patients with later onset of symptoms (2 years or longer after the trauma) were more likely to have hallucinations than early-onset patients (onset less than 2 years after trauma). However, early-onset patients were more likely than late-onset patients to have visual hallucinations. This occurrence would be consistent with the presence of possible delirium and/or types of hallucinations that are secondary to neurological and medical causes.60 Negative symptoms such as alogia, affective flattening, and avolition are uncommon sequelae.
Our results are very similar to findings in three of four previous studies that examined different data sources, including a general psychiatric sample, head-injured war veterans, and a previous meta-analysis of the literature.4,15,54 These studies reported a preponderance of paranoid delusions and schizophreniform disorders. The lone study in which the presentation was not highly consistent was based at a state hospital.7 Results from the state hospital study reported slightly more hallucinations. Paranoid delusions were, however, still the most prevalent subtype. Negative symptoms were not reported. It is possible that the greater frequency of hallucinations in the state hospital study may be due to the likely greater severity of illness or injury in that population.
The course of illness for a significant majority of subjects in our analysis was good. Prognosis did not appear to be affected by variables such as gender, delay in onset of psychosis after TBI, or diagnosis of a seizure disorder. The most common class of efficacious medication appears to be neuroleptics. Neuroleptics appeared to be efficacious in these patients regardless of diagnosis of seizure disorder.
Results from other PDTBI studies reporting data on course of illness are not as favorable. A study with World War II veterans reported a relatively poor prognosis: 40% of patients with delusional disorders and 63% of schizophrenic patients demonstrated a chronic course.5 Another study with general psychiatric patients reported an uncertain course that was not related to severity of TBI.54
What historical, physical, or laboratory findings are associated with PDTBI?
What severity of TBI is significant enough to cause a psychosis?
A significant proportion of known cases reported moderate to severe TBI as defined by ACRM criteria. However, the majority of cases reported LOC of unspecific duration, and thus severity could not be determined. There were cases in which PDTBI occurred without LOC. For these subjects, the onset of psychosis was generally early, within the first year after trauma. Our results are similar to other findings that the majority of patients who develop a PDTBI sustained a moderate to severe head injury.4,7,15,61 Still, in all of these studies there were patients who developed PDTBI after a mild TBI, including some who did not lose consciousness.
How long after an injury is the TBI considered to be etiologically relevant to the development of a psychosis?
In general, our results indicate a wide range in time between TBI and the onset of psychosis (range 0–34 years). The mean age for sustaining TBI was 29.1±17.6 years, whereas the mean age for developing psychosis was 33.4±15.4 years. The majority of subjects had a delayed onset, the mean length of delay being 4.1±6.6 years. Half of the subjects demonstrated psychotic symptoms before the second year after TBI, and 72% reported an onset of psychosis before 4 years—that is, before the mean length of delay.
Our results are similar to those of all other studies reviewed in finding a wide range of delay in the onset of psychotic symptoms.4,5,7,15 The studies differ, however, in terms of tendencies for onset of symptoms. Our results are very similar to those in a previous meta-analysis of the literature, which reported that most closed head injury patients who demonstrate psychotic symptoms do so within the first year (52%) and the majority within 5 years (85%), while the majority of moderate to severe TBI patients who report psychotic symptoms do so within the first year (67%).15 Another study with predominantly closed head injury patients reported a mean onset of 5.9 years.7 Contradictory findings were reported for two studies consisting of World War II veterans, many of whom sustained open head injuries.4,5 One of these studies reported 60% who developed a psychosis had onsets after 5 years; the other reported a majority of delusional psychoses occurring between 15 and 19 years post injury. It is possible that differences in onset after TBI may be due to type of head injury.
What laboratory findings are associated with psychosis?
Abnormal EEGs were reported in about 70% of the cases. A significant majority of abnormalities were found in the temporal lobes. The most common abnormality was slowing followed by spiking. There were no differences in laterality of positive findings.
About 65% of the cases reported positive findings on MRI/CT. The most common location of findings was the frontal lobes, followed by the temporal lobes and ventricles. There were no differences in laterality of findings. Among the cases that reported type of abnormalities, focal lesions and atrophy were about equally represented.
About 88% of cases reported impairments on neuropsychological testing. The most common impaired function was memory, followed by executive and visuospatial functions. About 58% of cases reported positive neurological symptoms, which was not significantly different from chance. Aggregating data from different sources, we found that temporal and frontal lobes appear to be the most commonly affected areas in PDTBI. Findings from MRI/CT indicated enlarged ventricles were also common.
As we mentioned above, research with neurological studies on patients with PDTBI is scarce. Data from the two such studies, both with small sample sizes, are incorporated in our findings.7,12 Three other studies with different data bases (general psychiatry, meta-analysis of the literature, and World War II veterans) consistently reported temporal lobes abnormalities in their patients.4,15,54 The meta-analytic study also reported a preponderance of left-sided lesions, which contradicts our findings indicating equal occurrence of left and right hemispheric lesions.15 The study on the war veterans reported 23.8% frontal lesions, 20% temporal, 15.3% parietal, and 14.3% basal lesions.4
What are some characteristics that may discriminate between PDTBI and schizophrenia?
A comparison of our data and the literature on schizophrenia suggests that several characteristics distinguish between the two disorders (Table 6).
First, PDTBI patients appear less likely to demonstrate negative symptoms than schizophrenic patients. In our sample, only 14% reported the presence of negative symptoms, whereas the prevalence rate of negative symptoms in schizophrenia has been reported to range from 25% to 84%.62,63
Patients with PDTBI and schizophrenia also appear to demonstrate differences on neurological studies. On MRI/CT, a higher percentage of PDTBI than schizophrenic patients demonstrate positive findings, and the types of findings appear to be qualitatively different. In our study, positive findings were found in 65% of the PDTBI sample. This percentage is much larger than in studies on schizophrenia that report rate of abnormalities (12.5%–35%).64–68 For PDTBI patients, there appeared to be an equal distribution of focal signs (62%) and general atrophy (60%). Focal lesions were most common in the frontal (42%) and temporal lobes (27%). About 20% of PDTBI patients demonstrated enlarged ventricles. By contrast, atrophy/volume loss is the most common type of MRI/CT finding for schizophrenia patients,69,70 whereas unspecified focal findings are reported in only 6% to 9% of patients.71,72 The most common findings in schizophrenic patients are enlarged ventricles (22%–35%)64–70 and atrophy in the temporal lobes.69,70
In addition, 70% of the PDTBI patients in our study demonstrated EEG abnormalities. In studies with schizophrenic patients, EEG abnormalities were found in 20% to 60% of the subjects.73 The percentage of EEG abnormalities in our sample resembles that of nonfamilial schizophrenic patients (72.3%).74 This higher percentage of EEG findings may suggest that TBI or seizure disorder may be a factor for developing a psychosis in nonfamilial schizophrenics.
Roughly half (55%) of the EEG abnormalities in our sample were localized findings, with the modal abnormality being temporal slowing. The most common findings across studies of schizophrenia are delta and theta waves in the frontal areas, a decreased mean frequency in alpha, and increased beta power.75
What are some characteristics that may discriminate between PDTBI patients with or without a diagnosed seizure disorder?
About 34% of the subjects had received diagnoses of a seizure disorder. This prevalence rate is similar to the rates in other studies on posttraumatic psychosis7 and is significantly higher than the highest estimate of seizure disorder after all closed head injuries (12%).16 This higher incidence provides some corroborative support for an association between seizure disorder and PDTBI.7,15,16,17
The presence of seizure disorder did not appear to be a strong discriminating factor in our sample. In comparing subjects with and without a seizure disorder diagnosis, we found no differences in proportion of positive findings or type of findings on EEG, proportion of positive MRI/CT findings, age at time of TBI, age at onset of psychosis, or delay in onset of psychosis. The lone difference between the two groups was that nonseizure subjects reported a higher proportion of delusions (95%) than seizure patients (63%).
Limitations
Our study is exploratory and descriptive, and thus the findings should be viewed as preliminary. Its major limitation is the use of archival data that are subject to biases in data collection. For example, there could have been a selection bias for more early-onset PDTBI cases because it would be easier to justify the relationship between TBI and the onset of psychosis. We also looked only at PDTBI cases in which there were neurological and neuropsychological investigations. Likewise, there could have been a bias toward positive findings on imaging data because negative findings are less likely to be submitted or selected for publication. Another problem is with the comparability of findings between cases because of differences in researcher ratings and observations. A specific problem in our data set involved missing data points due to differences in reporting. To address these weaknesses, future studies should use prospective data and address potential problems of interrater reliability.
ACKNOWLEDGMENTS
The authors thank Lisa Anne Matsumoto, Casey Pinter, and Mary Church for their assistance in obtaining articles and in data preparation.
 |
 |
 |
 |
 |
 |
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC, American Psychiatric Association, 1994Google Scholar
2 Ahmed I, Fujii DE: Posttraumatic psychosis. Semin Clin Neuropsychiatry 1998; 3:23-33Medline, Google Scholar
3 Lishman WA: The psychiatric sequelae of head injury: a review. Psychol Med 1973; 3:304-318Crossref, Medline, Google Scholar
4 Achte KA, Hillbom E, Aalberg V: Psychoses following war injuries. Acta Psychiatr Scand 1969; 45:1-18Crossref, Medline, Google Scholar
5 Achte K, Jarho L, Kyykka T, et al: Paranoid disorders following war brain damage. Psychopathology 1991; 24:309-315Crossref, Medline, Google Scholar
6 Koufen DI, Hagel KH: Systematic EEG follow-up study of traumatic psychosis. Eur Arch Psychiatry 1987; 237:2-7Crossref, Medline, Google Scholar
7 Fujii DE, Ahmed I: Psychosis secondary to traumatic brain injury. Neuropsychiatry Neuropsychol Behav Neurol 1996; 9:133-138Google Scholar
8 Will RG, Young JPR, Thomas DJ: Kleine-Levin syndrome: report of two cases with onset of symptoms precipitated by head trauma. Br J Psychiatry 1988; 152:410-412Crossref, Medline, Google Scholar
9 Katz DI, Alexander MP, Seliger GM, et al: Traumatic basal ganglia hemorrhage: clinicopathologic features and outcome. Neurology 1989; 39:897-904Crossref, Medline, Google Scholar
10 Silva JA, Leong GB, Thi Luong M: Split body and self: an unusual case of misidentification. Can J Psychiatry 1989; 34:728-730Crossref, Medline, Google Scholar
11 Marshall JC, Halligan PW, Wade DT: Reduplication of an event after head injury? A cautionary case report. Cortex 1995; 31:183-190Crossref, Medline, Google Scholar
12 Feinstein A, Ron M: Psychosis associated with demonstrable brain disease. Psychol Med 1990; 20:793-803Crossref, Medline, Google Scholar
13 Hillbom E: After-effects of brain injuries. Acta Psychiatr Neurol Scand Suppl 1960; 142:1-195Google Scholar
14 Burg JS, McGuire LM, Burright RG, et al: Prevalence of traumatic brain injury in an inpatient psychiatric population. Journal of Clinical Psychology in Medical Settings 1996; 3:245-251Crossref, Google Scholar
15 Davison K, Bagley CR: Schizophrenia-like psychoses associated with organic disorders of the central nervous system: a review of the literature. Br J Psychiatry 1969 (suppl); 114:113-162Google Scholar
16 McKenna Kane JM, Parrish K: Psychotic syndromes in epilepsy. Am J Psychiatry 1985; 142:895-904Crossref, Medline, Google Scholar
17 Annegers JF, Gradow JD, Kurland LT, et al: The incidence, causes, and secular trends of head trauma in Olmstead County, Minnesota 1935-1974. Neurol 1980; 30:912-919Crossref, Medline, Google Scholar
18 Nasrallah HA: The neuropsychiatry of schizophrenia, in Textbook of Neuropsychiatry, 2nd edition, edited by Yudofsky S, Hales R. Washington DC, American Psychiatric Press, 1992, pp 621-638Google Scholar
19 Bamrah JS, Johnson J: Bipolar affective disorder following head injury. Br J Psychiatry 1991; 153:117-119Crossref, Google Scholar
20 Barnhill LJ, Gualteri CT: Two cases of late-onset psychosis after closed head injury. Neuropsychiatry Neuropsychol Behav Neurol 1989; 2:211-217Google Scholar
21 Benson DF, Stuss DT: Frontal lobe influences of delusions: a clinical perspective. Schizophr Bull 1990; 16:403-412Crossref, Medline, Google Scholar
22 Bienenfeld D, Brott T: Capgras' syndrome following minor head trauma. J Clin Psychiatry 1989; 2:68-69Google Scholar
23 Bouvy PF, van de Wetering JD, Meerwaldt JD, et al: A case of organic brain syndrome following head injury successfully treated with carbamazepine. Acta Psychiatr Scand 1988; 77:361-363Crossref, Medline, Google Scholar
24 Buckley P, Stack JP, Madigan C, et al: Magnetic resonance imaging of schizophrenia-like psychoses associated with cerebral trauma: clinicopathological correlates. Am J Psychiatry 1993; 150:146-148Crossref, Medline, Google Scholar
25 Cohen MA, Alfonso CA, Haque MM: Lilliputian hallucinations and medical illness. Gen Hosp Psychiatry 1994; 16:141-143Crossref, Medline, Google Scholar
26 Dalby JT, Arboleda-Florez J, Seland TP: Somatic delusions following left parietal lobe injury. Neuropsychiatry Neuropsychol Behav Neurol 1989; 2:306-311Google Scholar
27 Dolinar LJ: Delusions in a patient with post-traumatic headache. Psychosomatics 1991; 32:460-462Crossref, Medline, Google Scholar
28 Drake ME: Cotard's syndrome and temporal lobe epilepsy. Psychiatry J Univ Ottawa 1988; 13:36-39Medline, Google Scholar
29 Filley CM, Jarvis PE: Delayed reduplicative paramnesia. Neurol 1987; 37:701-703Crossref, Medline, Google Scholar
30 Hayman MA, Abrams R: Capgras' syndrome and cerebral dysfunction. Br J Psychiatry 1977; 130:68-71Crossref, Medline, Google Scholar
31 Ikemura Y, Akena H, Iida M, et al: Psychiatry of diencephalon damages: a case report. Funct Neurol 1987; 11:87-91Google Scholar
32 Levine DN, Finklestein S: Delayed psychosis after right temporoparietal stroke or trauma: relation to epilepsy. Neurology 1982; 32:267-273Crossref, Medline, Google Scholar
33 Lloyd DW, Tsuang MT: A snake lady: post-concussion syndrome manifesting visual hallucinations of snakes. J Clin Psychiatry 1981; 42:246-247Medline, Google Scholar
34 Murai T, Toichi M, Sengoku A, et al: Reduplicative paramnesia in patients with focal brain damage. Neuropsychiatry Neuropsychol Behav Neurol 1997; 10:190-196Medline, Google Scholar
35 Nasrallah HA, Fowler RC, Judd LL: Schizophrenia-like illness following head injury. Psychosomatics 1981; 22:359-361Crossref, Medline, Google Scholar
36 O'Callaghan E: Early onset schizophrenia after teenage head injury. Br J Psychiatry 1988; 153:394-396Crossref, Medline, Google Scholar
37 O'Connor M, Wallbridge M, Sandson T, et al: A neuropsychological analysis of Capgras syndrome. Neuropsychiatry Neuropsychol Behav Neurol 1996; 4:265-271Google Scholar
38 Prigatano GP, O'Brien KP, Klonoff PS: The clinical management of paranoid delusions in postacute traumatic brain-injured patients. J Head Trauma Rehabil 1988; 3:23-32Crossref, Google Scholar
39 Puri BK, El-Dosoky A, Barrett JS: Self-inflicted intracranial injury. Br J Psychiatry 1994; 164:841-842Crossref, Medline, Google Scholar
40 Rogers MJC, Franzen MD: Delusional reduplication following closed head injury. Brain Inj 1992; 6:469-476Crossref, Medline, Google Scholar
41 Sabhesan S, Arumugan R, Natarajan M: Paraamnesic delusions following head injury. Indian Journal of Psychiatry 1988; 30:177-181Medline, Google Scholar
42 Sabhesan S, Natarajan M: Delusional disorders after head injury. Indian Journal of Psychiatry 1988; 30:39-45Medline, Google Scholar
43 Sandel ME, Olive DA, Rader MA: Chlorpromazine-induced psychosis after brain injury. Brain Inj 1993; 7:77-83Crossref, Medline, Google Scholar
44 Signer SF, Cummings JL: De Clerambault's syndrome in organic affective disorder: two cases. Br J Psychiatry 1987; 151:404-407Crossref, Medline, Google Scholar
45 Silva JA, Leong GB, Wine DB: Misidentification delusions, facial misrecognition, and right brain injury. Can J Psychiatry 1993; 38:239-241Crossref, Medline, Google Scholar
46 Staton RD, Brumback RA, Wilson H: Reduplicative paramnesia: a disconnection syndrome in memory. Cortex 1982; 18:23-35Crossref, Medline, Google Scholar
47 Tisher PW, Holzer JC, Greenberg M, et al: Psychiatry presentations of epilepsy. Harv Rev Psychiatry 1993; 1:219-228Crossref, Medline, Google Scholar
48 Waldfogel S, Field HL, Wu L: Oedipism in a patient with frontal lobe encephalomalacia. Brain Inj 1994; 8:377-381Crossref, Medline, Google Scholar
49 Weston MJ, Whitlock FA: The Capgras syndrome following head injury. Br J Psychiatry 1971; 119:25-31Crossref, Medline, Google Scholar
50 White AC, Armstrong D, Rowan D: Compensation psychosis. Br J Psychiatry 1987; 150:692-694Crossref, Medline, Google Scholar
51 Will RG, Young JPR: Kleine-Levin syndrome: report of two cases with onset of symptoms precipitated by head injury. Br J Psychiatry 1988; 152:410-412Crossref, Medline, Google Scholar
52 Young AW, Robertson IH, Helawell DJ, et al: Cotard delusion after brain injury. Psychol Med 1992; 22:799-804Crossref, Medline, Google Scholar
53 Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine: Definition of mild traumatic brain injury. J Head Trauma Rehabil 1993; 8:86-87Crossref, Google Scholar
54 Violin A, Violin A, De Mol J: Psychological sequelae after head traumas in adults. Acta Neurochir (Wien) 1987; 85:96-102Crossref, Medline, Google Scholar
55 Geschwind N, Galaburda AM: Cerebral lateralization, biological mechanisms, associations, and pathology. Arch Neurol 1985; 42:428-459Crossref, Medline, Google Scholar
56 Gur RC, Gur RE, Obrist WD, et al: Sex and handedness differences in cerebral blood flow during test and cognitive activity. Science 1982; 217:659-660Crossref, Medline, Google Scholar
57 Levy J, Heller W: Gender differences in human neuropsychological functioning. in Handbook of Behavioral Neurobiology, vol 11, Sexual Differentiation, edited by Geralm AA, Ward IL. New York, Plenum, 1992, pp 245-274Google Scholar
58 Pizzamiglio L, Mammucari A, Razzano C: Evidence for sex differences in brain organization in recovery in aphasia. Brain Lang 1985; 25:213-223Crossref, Medline, Google Scholar
59 Basso A, Capitani E, Moraschini S: Sex differences in recovery from aphasia. Cortex 1982; 18:469-475Crossref, Medline, Google Scholar
60 Cummings JL: Clinical Neuropsychiatry. New York, Grune and Stratton, 1985Google Scholar
61 Sabhesan S, Arumughan R, Natarajan M: Neuroanatomical correlates of delusions in head injury. Indian Journal of Psychiatry 1990; 32:180-184Medline, Google Scholar
62 Fenton WS: Longitudinal course and outcome of schizophrenia, in Handbook of Mental Health Economics and Health Policy, vol 1, edited by Moscarelli M, Rupp A, Sartorius N. New York, Wiley, 1996, pp 79-91Google Scholar
63 Breier A, Schrieber JL, Dyer J, et al: National Institute of Mental Health longitudinal study of chronic schizophrenia. Arch Gen Psychiatry 1991; 48:239-246Crossref, Medline, Google Scholar
64 Cazzullo CL, Vita A, Sacchetti E, et al: Brain morphology in schizophrenic disorder: prevalence and correlates of diffuse (cortical and subcortical) brain atrophy. Psychiatry Res 1989; 29:257-259Crossref, Medline, Google Scholar
65 Vita A, Sacchetti E, Calzeroni A, et al: Cortical atrophy in schizophrenia: prevalence and associated features. Schizophr Res 1988; 1:329-337Crossref, Medline, Google Scholar
66 Sacchetti E, Calzeroni A, Vita C, et al: The brain damage hypothesis of the seasonality of births in schizophrenia and major affective disorders: evidence from computerised tomography. Br J Psychiatry 1992; 160:390-39Crossref, Medline, Google Scholar
67 Nasrallah HA, Kuperman S, Hamra BJ, et al: Clinical differences between schizophrenic patients with and without large cerebral ventricles. J Clin Psychiatry 1983; 44:407-410Medline, Google Scholar
68 Vita A, Dieci M, Giobbio GM, et al: CT scan abnormalities and outcome in chronic schizophrenia. Am J Psychiatry 1991; 148:1577-1579Crossref, Medline, Google Scholar
69 Pearlson GD, Marsh L: Structural brain imaging in schizophrenia: a selective review. Biol Psychiatry 1999; 46:627-649Crossref, Medline, Google Scholar
70 McCarley RW, Wible CG, Frumin M, et al: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099-1119Crossref, Medline, Google Scholar
71 Cunningham-Owen DG, Johnstone EC, Bydder GM, et al: Unsuspected organic disease in chronic schizophrenia demonstrated by computed tomography. J Neurol Neurosurg Psychiatry 1980; 43:1065-1069Crossref, Medline, Google Scholar
72 Lewis SW: Computerised tomography in schizophrenia 15 years on. Br J Psychiatry 1990; 157(suppl):16-24Google Scholar
73 Gerez M, Tello A: Selected quantitative EEG (QEEG) and event-related potential (ERP) variables as discriminators for positive and negative schizophrenia. Biol Psychiatry 1995; 38:34-49Crossref, Medline, Google Scholar
74 Kendler KS, Hays P: Familial and sporadic schizophrenia: a symptomatic, prognostic, and EEG comparison. Am J Psychiatry 1982; 139:1557-1562Crossref, Medline, Google Scholar
75 Hughes JR, John ER: Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci 1999; 11:190-208Link, Google Scholar



