Traumatic Brain Injury and Atrophy of the Cingulate Gyrus
Abstract
The medial surface areas of the cingulate gyrus (CG) and other midline structures (corpus callosum, thalamus, lateral ventricle) were examined in 27 traumatically brain injured (TBI) and 12 age- and gender-matched control subjects from an established TBI data base. Significant atrophy, primarily in the posterior CG, was found in TBI patients. Degree of atrophy was related to severity of injury. TBI subjects also had significantly reduced corpus callosum and thalamic cross-sectional surface areas with associated increased lateral ventricular volume, as well as reduced brain volume and increased ventricle-to-brain ratio. Despite significant atrophy of the posterior CG, neuropsychological performance was not related to changes in CG cross-sectional surface area in the TBI subjects. This apparent discrepancy is discussed.
The most common neuropsychological deficits associated with traumatic brain injury (TBI) are alterations in memory and in executive and emotional functioning.1,2 Typically these sequelae have been discussed in terms of selective damage to frontotemporal regions, including limbic structures, or similar indirect effects from phenomena such as transneuronal degeneration.3–6 The largest and most recently evolved of the limbic structures, located medially in each hemisphere, is the cingulate gyrus (CG). The cingulate plays an important role in such diverse processes as integration of cognition and emotion, modulation of emotion-related autonomic activity, and emotional responses to pain.7–9 Additionally, the cingulate has been shown to be involved in attention, executive processes, word generation, and memory.10,11 Despite the functional significance of the cingulate gyrus in TBI, few studies have examined the anatomical and behavioral effects of acquired damage to this region.
A significant body of literature has suggested regional differences in the functional organization of the CG.9 In particular, damage to the cingulate has been shown to affect a variety of neurobehavioral functions, producing affective disruption (anterior lesions) and memory and visuospatial dysfunction (anterior and posterior lesions).7,9,12–16 Fontaine et al.,17 using positron emission tomography (PET), reported significant relationships between reduced CG regional metabolic activity and neurobehavioral functioning in a group of 13 TBI subjects. In a functional magnetic resonance imaging (fMRI) study, McAllister et al.18 reported that compared with more discrete bifrontal, biparietal, and cingulate activation in control subjects,19 mild TBI subjects showed more widespread activation. Furthermore, McCrory and Berkovic20 have asserted that in TBI subjects the “well-recognized behavioral and memory dysfunction is probably due to basal forebrain and limbic insults.” (p. 1491) It is noteworthy, however, that none of these investigations specifically examined cingulate morphology as a function of brain injury.
From a brain injury perspective, the cingulate may be protected because it lies deep in the cerebrum and the medial surface of each CG is supported by the falx cerebri during lateral movement. However, the position of the CG may be misleading, since it is vulnerable to herniation in cases of midline shift.21 Moreover, damage to the cingulum following TBI may be caused by transneuronal degeneration rather than direct injury.9 In light of these ambiguities, systematic investigation is needed to elucidate whether the cingulate shows structural changes in TBI and, if it is damaged, what the neurobehavioral sequelae are. Accordingly, in the present study we examined the relationships between cross-sectional CG surface area on magnetic resonance (MR) imaging and neurobehavioral status and injury severity in diffuse moderate to severe TBI.
METHODS
Subjects
Subjects were selected from the LDS Hospital–Brigham Young University TBI database.22,23 Each subject met criteria for significant TBI as defined by the TBI Model Systems definition24 and received inpatient rehabilitation services. To be included in this study, the TBI subjects had to have received neuropsychological testing and MR imaging studies, appropriate for quantitative image analysis, where equidistant midsagittal views had been obtained. Subjects with obvious focal lesions or infarction of the CG were excluded. A total of 27 TBI subjects were included in the study: 18 males and 9 females. The mean age (±SD) for the TBI subjects was 26±8.0 years (range 17–32 years), and their mean educational level was 12.6±2.1 years (range 9–18 years). The mean Glasgow Coma Scale (GCS) score for the TBI patients, based on the highest GCS score recorded within 24 hours of hospital admission, was 8.33±3.17 (range 5–14). Posttraumatic amnesia (PTA) was recorded as none, <24 hours, 24–48 hours, 49–96 hours, or >96 hours. Under this scheme, the average PTA was >96 hours. Neuroimaging studies were performed at an average of 22.8 months (3.07 to 83.97 months) postinjury, and neuropsychological testing occurred an average of 29.1 months (3.2 to 81.37 months) postinjury. Both neuroimaging and neuropsychological findings can thus be considered reflective of stable deficits.25 The 2:1 male-to-female ratio was maintained in the selection and matching of the control subjects without head injury. The 12 control subjects, selected from the normative database of Blatter et al.,26 were 4 women and 8 men, group matched to the mean age and total intracranial volume (TICV) of the TBI subjects.
Magnetic Resonance Imaging
MR images were obtained on a 1.5-tesla GE Signa Scanner (General Electric, Milwaukee, WI).23 Sagittal scans were T1-weighted, 500/11/2 (repetition time/echo time/excitations), with a 256×192 pixel acquisition matrix, field of view 2 cm, and slice thickness 5 mm with a 1-mm interspace gap. Axial intermediate and T2-weighted (3,000/31, 90/1) spin-echo images were acquired with a 256×192 acquisition matrix, field of view 22 cm, and slice thickness 5 mm with a 2-mm interspace gap. Imaging data remained in digital form throughout the data analysis, following a well-established protocol.22,23,26,27
Volumetric Image Analysis
The axial intermediate and T2-weighted images were processed by use of ANALYZE segmentation routines (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN). Quantitative or volumetric analyses of the cerebral structures obtained were then performed on all subjects, by methods described previously,22,26 for total intracranial volume including lateral ventricular volume; total brain volume; and ventricle-to-brain ratio (VBR). All brain measures from the TBI subjects were converted to z scores by using the mean and standard deviation from the control subjects. There were no significant differences between TBI subjects and control subjects for TICV because this was one of the matching factors. Accordingly, no correction for head size was used in any measure.
Cross-Sectional Surface Area Measurements
Using the procedure outlined by Killiany et al.,28 the midsagittal slice at the level of the aqueduct of Sylvius was used to define midline (Figure 1 and Figure 2). Parasagittal cuts were made 5 mm from each side of midline so that left and right side measures could be obtained (Figure 1). Cross-sectional surface areas of the thalamus, CG, and lateral ventricle were measured for right and left hemispheres by using the IMAGE software program.29 In addition, the corpus callosum (CC) was measured in the midsagittal slice. As depicted in Figure 2, each image was rotated to maintain identical anterior–posterior (A–P) plane orientation. Two perpendicular lines were drawn so that the anterior aspect of the genu and the posterior aspect of the splenium of the CC were intersected. The cingulate was then divided into three regions. Two CG regions were defined by an equidistant bisection of the A–P plane spanning the length of the CC, with the CG area posterior to the bisection constituting the posterior cingulate (roughly Brodmann area 23) and from the bisection to the line perpendicular to the anterior CC constituting the caudal anterior cingulate. The rostral cingulate was defined as the area anterior to the CC and anterior cingulate as defined above (Figure 2). Preliminary analysis comparing caudal anterior and rostral anterior cingulate demonstrated no unique contribution of either, and so these two aspects of the anterior cingulate were combined to form a single index of the anterior cingulate (roughly Brodmann area 24).
In each case, the cingulate area was defined superiorly by the cingulate sulcus and inferiorly by the callosal sulcus. The boundaries of the thalamus were defined superiorly by the lateral ventricle and the fornix, and inferiorly by the superior border of the midbrain. All cross-sectional surface areas were then converted to z scores, once again using the mean and standard deviation of the control subjects. Within each boundary, only brain parenchyma was included, with the IMAGE threshold technique being used to eliminate CSF. Several atlases were used to assist in defining CG boundaries, particularly with respect to the two distinct morphological appearances we identified in the anterior cingulate.30–32 These anatomical variants, which were designated as either “single gyrus” or “double parallel gyrus,” are discussed in later sections.
Three raters provided the tracing for image quantification. A single rater traced each structure twice, and the average was recorded. A second rater repeated the same procedure on the same subject, and the average from the two raters was then used as the cross-sectional surface area for the structure of interest. Each rater worked independently and was blind to the identities of the brain injury and control groups and to the findings of other raters. Interrater reliabilities for the TBI and control subjects were 0.88 and 0.93, respectively. Intrarater reliabilities comparing the two tracings of each subject all exceeded 0.90.
Neuropsychological Testing
The following neuropsychological tests had been administered according to standardized testing protocols as part of the overall neuropsychological workup: Wechsler Memory Scale–Revised,33 Rey Auditory Verbal Learning Test,34,35 Rey-Osterrieth Complex Figure,35,36 Trail Making Test,37 Beck Depression Inventory,38 and Beck Anxiety Inventory.39
Statistical Analyses
A multivariate analysis of variance (MANOVA) was performed for all comparisons of brain structures between TBI subjects and normal control subjects because of the multiple dependent variables. Correlations were used to evaluate the relationship between brain morphology and measures of injury severity (GCS, PTA) and neuropsychological outcome. Because right and left values were obtained, the first analysis assessed for laterality effects, which were not found. Accordingly, for the structures where left and right surface areas were obtained, these were combined into a single measure (average of left and right), and all analyses reported below are based on a single score representing the average cross-sectional surface area of both hemispheres.
RESULTS
Results are summarized in Figure 3. The MANOVA demonstrated several significant differences between control subjects and TBI subjects (Figure 3). Total CG was smaller in TBI subjects compared with control subjects, although the difference was not statistically significant. However, significant atrophy was found in the posterior CG of TBI compared with control subjects (F=6.17, df=1,27, P=0.02). Decreased thalamic cross-sectional surface area was also found in the TBI subjects (F=4.13, df=1,27, P=0.05). As expected, the cross-sectional surface area of the CC was significantly reduced in TBI subjects (F=6.52, df=1,27, P=0.02). Similarly, lateral ventricular volume was significantly larger in TBI subjects compared with control subjects (F=9.30, df=1,27, P=0.004). TBI subjects were also significantly different from normal control subjects (results not shown) on whole brain volume (F=18.15, df=1,27, P< 0.001) and VBR (F=10.79, df=1,27, P=0.002).
The TBI group was further divided into severe (GCS 3 to 6), moderate-to-severe (GCS 7 to 12), and mild (GCS 13 to 15) TBI, and the subgroups were compared for atrophic changes in brain structure (Figure 4). In the severe and moderate TBI subgroups (combined) compared with the control group, significant atrophy was observed in posterior CG (F=13.66, df=1,27, P=0.001), thalamus (F=7.93, df=1,27, P=0.008), and corpus callosum (F=10.67, df=1,27, P=0.003), as well as significant enlargement of the lateral ventricles (F=13.79, df=1,27, P=0.001). For both the severe and moderate TBI subgroups compared with the mild TBI group, there was significant atrophy of the posterior CG (F=5.16, df=1,27, P=0.03), anterior CG (F=5.88, df=1,27, P=0.02), and thalamus (F=4.87, df=1,27, P=0.04), and significant enlargement of the lateral ventricles (F=4.62, df=1,27, P=0.04). In contrast, the mild TBI group showed much smaller and nonsignificant changes in brain morphology compared with the control group.
Greater impairment on neuropsychological test findings was associated with greater severity on GCS, but there were no significant correlative relationships between CG morphology and neuropsychological outcome. Similarly, the results of the Beck Depression and Anxiety Scales did not significantly correlate with CG morphology in the TBI subjects.
DISCUSSION
Traumatic brain injury has typically been conceptualized as an injury affecting diffuse neural systems that extend beyond the site of injury.1–3,6,22,23,40 The assertion of diffuse injury is supported by the current findings, which show atrophic changes of the posterior CG, in the presence of generalized atrophy of the whole brain (i.e., increased VBR and decreased brain volume), CC, and thalamus, in TBI subjects. CG atrophy was associated with injury severity (GCS) but not with neuropsychological outcome. Several possible explanations for the lack of relationship between the CG atrophy and neuropsychological performance are discussed below.
First, it is important to note that atrophy of the posterior CG was primarily responsible for the overall reduction of CG surface area in TBI subjects. Unlike the CC, where front-to-back organization of fiber tracks corresponds with frontal-to-occipital anatomy, this organization is more distributed in the CG.7,9,15,16,41,42 There are important frontotemporal connections with the CG that are organized posteriorly, including hippocampal input. Since the frontal and temporal areas are more likely to be damaged in trauma,43 including hippocampal atrophy,44 we would speculate that secondary neuronal cell loss from these regions may be a factor in the reduction of posterior CG surface area. In no case were lesions due to direct trauma or infarction of the CG, subjects with such lesions having been excluded from analysis. However, in the cases of severe TBI it is particularly plausible that direct mechanical deformation of the medial surface of the cingulate could strike the falx, causing injury. Accordingly, the significant posterior CG atrophy may have resulted from transneuronal degeneration secondary to frontotemporal damage, in the context of generalized, diffuse injury and resultant atrophy of the entire brain and/or direct mechanical contact with the falx.
Second, the lack of relationship between cingulate morphology and neuropsychological functioning may be due to the more indirect role that the CG plays in cognitive and emotional function and the fact that the cingulum is several synaptic connections away from the more direct cortical influence over behavior.45,46 It is also possible that even though significant damage did occur in the cingulate, a threshold for impairment was not reached and compensatory recruitment of functionally related regions prevented direct neurobehavioral sequelae.18 It may also be the case that more sensitive physiological measures such as PET imaging or fMRI are needed to show relationships between CG atrophy and behavior. Another factor is that left and right cingulate morphology were not separately examined because of sample size constraints. Lateralized cingulate function may relate more to neuropsychological function than an average measure of the cingulate. Also, we did not use traditional executive function tests, which often show impairment in TBI patients, so the relationship between traditionally measured executive functions and atrophy of the cingulate gyrus was not available for analysis. This is an area that should be examined in future research.
Third, the absence of anterior CG atrophy may be related to the greater anatomical variability of the more anterior aspect of the CG. As Ono et al.30 demonstrated through detailed sulcal analyses, only 58% of their postmortem subjects had uninterrupted continuous cingulate sulci. This creates a potential boundary problem in precisely defining the cingulate, even with the anatomic atlases used in this study. For instance, one-half of the subjects in this study (3 with mild injury, 8 with moderate to severe injury, and 3 with severe injury) possessed “double parallel” or split gyri in the anterior cingulate that tended to have larger surface area than those with a “single” gyrus. Statistical comparisons of the double versus single anterior CG in predicting neuropsychological outcome were not significant. However, issues of sample size and statistical power may have affected the results of our analyses.
In a previous study, Connor47 did not find a significant reduction in CG in TBI, but his measurement of the CG was taken in the coronal plane in a region analogous to the caudal anterior cingulate of the current study. In addition, he did not examine the single versus double phenotypes or the posterior cingulate. Nonetheless, this observation provides further support for the lack of atrophic changes in the anterior CG following TBI. It is apparent, however, that without a clear method for delineating anatomical variants, measurement error may obscure potential anterior CG–behavior relationships. We are not aware of any study that has systematically examined the neurobehavioral significance of single versus double-parallel cingulate gyrus morphology. Furthermore, the subjects with moderate to severe TBI in the present study did have significantly smaller anterior CG than the mild group, who actually had an anterior CG that was three-quarters of a standard deviation larger than control subjects (see Figure 4). This finding further underscores the need to account for variability in CG morphology if CG–behavior relationships are to be understood.
The present study found posterior CG atrophy in TBI to be associated with severity of injury. Neuropsychological functioning was not significantly related to CG atrophy in the three regions examined, even when severity of injury was taken into consideration. Our study is limited in that we examined the CG in relation to structures commonly damaged in TBI, but we did not measure other limbic and frontotemporal neocortical structures such as the hippocampus and amygdala. These regions are vulnerable in TBI as well, and they are functionally related to the CG. It may be that other limbic structures need to be investigated to clarify relationships between CG atrophy and neurobehavioral outcome in TBI. Functional imaging studies of the CG have demonstrated relationships with executive and memory ability in TBI, so it is likely that CG atrophy does relate to the spectrum of dysfunction that accompanies TBI, although not through a simple linear anatomic connection. Indeed, even when the anterior cingulum is ablated, subjects may remain cognitively intact and experience a reduction of psychiatric symptoms.48 In light of these observations, in several prospective studies, we are currently designing a line of research to elucidate the role of the cingulate gyrus in relation to functionally related systems in TBI and other disorders to more fully assess the relationships between CG morphology and neurobehavioral status.
ACKNOWLEDGMENTS
This study was funded in part by a National Science Foundation Neuroscience Research Experience for Undergraduates Grant (DBI 9912126), the Deseret Foundation, and the Ira Fulton Foundation.
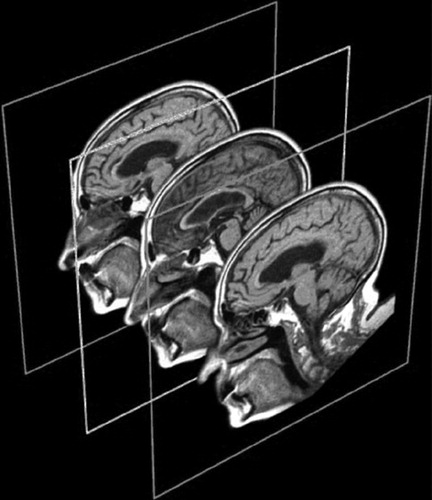
FIGURE 1. Magnetic resonance imaging scans of the midsagittal and right and left parasagittal slices showing the scans used for the cross-sectional surface measures of the cingulate gyrus, corpus callosum, lateral ventricle, and thalamus.
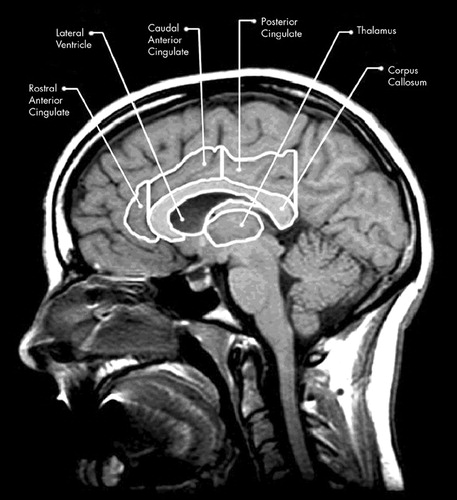
FIGURE 2. Parasagittal magnetic resonance imaging view of the brain showing structural outlines used for the cross-sectional surface measurements of the corpus callosum, cingulate gyrus (rostral, anterior, and posterior regions), and thalamus, using the method of Killiany et al.28
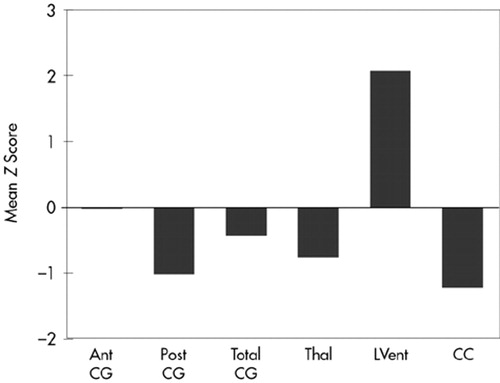
FIGURE 3. Mean z scores for the TBI subjects for morphological measures showing atrophy of the posterior cingulate, thalamus, and corpus callosum and significant enlargement of the lateral ventricles. Ant CG=anterior cingulate gyrus; Post CG=posterior cingulate gyrus; Total CG=total cingulate gyrus; Thal=thalamus; LVent=lateral ventricles; CC=corpus callosum. All differences were significant except for the Ant CG and Total CG measures. The z scores were derived from the normative values of the control subjects.
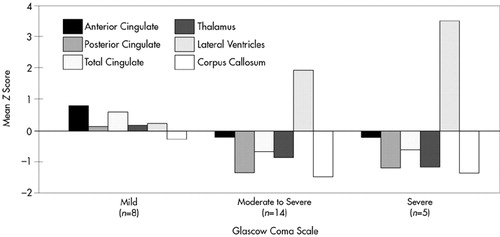
FIGURE 4. Mean z scores for the TBI subjects for mild, moderate, and severe subgroups. The moderate and severe subgroups show atrophy of all aspects of the cingulate, thalamus, and corpus callosum and significant enlargement of the lateral ventricles.
1 Hinnant DW: Neurobehavioral consequences: assessment, treatment, and outcome, in Traumatic Brain Injury, edited by Marion DW. New York, Thieme Medical, 1999, pp 187-197Google Scholar
2 Levin HS: Neurobehavioral outcome of closed head injury: implications for clinical trials, in Traumatic Brain Injury: Bioscience and Mechanics, edited by Bandak FA, Eppinger RH, Ommaya AK. Larchmont, NY, Mary Ann Liebert, 1996, pp 105-114Google Scholar
3 Gennarelli TA, Thibault LE, Graham DI: Diffuse axonal injury: an important form of traumatic brain damage. The Neuroscientist 1998; 4:202-215Crossref, Google Scholar
4 Povlishock JT, Christman CW: The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma 1995; 12:555-564Crossref, Medline, Google Scholar
5 Bigler ED: Neuroimaging in pediatric traumatic head injury: diagnostic considerations and relationships to neurobehavioral outcome. J Head Trauma Rehabil 1999; 14:70-87Crossref, Medline, Google Scholar
6 Smith DH, Meaney DF: Axonal damage in traumatic brain injury. Neuroscientist 2000; 6:483-495Crossref, Google Scholar
7 Devinsky O, Morrell MJ, Vogt BA: Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118:279-306Crossref, Medline, Google Scholar
8 Malamud N: Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol 1967; 17:113-123Crossref, Medline, Google Scholar
9 Vogt BA, Finch DM, Olson CR: Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435-443Medline, Google Scholar
10 Carter CS, Mintun MA, Nichols T, et al: Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry 1997; 154:1670-1675Crossref, Medline, Google Scholar
11 Petersen SE, Fox PT, Posner MI, et al: Positron emission tomographic studies of the cortical anatomy of single-word-processing. Nature 1988; 331:585-589Crossref, Medline, Google Scholar
12 Valenstein E, Bowers D, Verfaellie M, et al: Retrosplenial amnesia. Brain 1987; 110:1631-1646Crossref, Medline, Google Scholar
13 Grasby PM, Frith CD, Friston KJ, et al: Functional mapping of brain areas implicated in auditory-verbal memory function. Brain 1993; 116:1-20Crossref, Medline, Google Scholar
14 Wu J, Buchsbaum MS, Gillin JC, et al: Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry 1999; 156:1149-1158Medline, Google Scholar
15 Sutherland RJ, Hoesing JM: Posterior cingulate cortex and spatial memory: a microlimnology analysis, in Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook, edited by Vogt BA, Gabriel M. Boston, Birkhauser, 1993, pp 461-477Google Scholar
16 Vogt BA: Structural organization of cingulate cortex: areas, neurons, and somatodendritic transmitter receptors, in Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook, edited by Vogt BA, Gabriel M. Boston, Birkhauser, 1993, pp 19-45Google Scholar
17 Fontaine A, Azouvi P, Remy P, et al: Functional anatomy of neuropsychological deficits after severe traumatic brain injury. Neurology 1999; 53:1963-1968Crossref, Medline, Google Scholar
18 McAllister TW, Saykin AJ, Flashman LA, et al: Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 1999; 53:1300-1308Crossref, Medline, Google Scholar
19 Saykin AJ, Johnson SC, Flashman LA, et al: Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: an fMRI study. Brain 1999; 122:1963-1971Crossref, Medline, Google Scholar
20 McCrory PR, Berkovic SF: Video analysis of acute motor and convulsive manifestations in sport-related concussion. Neurology 2000; 54:1488-1491Crossref, Medline, Google Scholar
21 Gean AD: Imaging of Head Trauma. New York, Raven, 1994Google Scholar
22 Bigler ED, Blatter DD, Anderson CV, et al: Hippocampal volume in normal aging and traumatic brain injury. AJNR Am J Neuroradiol 1997; 18:11-23Medline, Google Scholar
23 Blatter DD, Bigler ED, Gale SD, et al: MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR Am J Neuroradiol 1997; 18:1-10Medline, Google Scholar
24 Dahmer ER, Shilling MA, Hamilton BB, et al: A model systems database for traumatic brain injury. J Head Trauma Rehabil 1993; 8:12-25Crossref, Google Scholar
25 Gale SD, Johnson SC, Bigler ED, et al: Trauma-induced degenerative changes in brain injury: a morphometric analysis of three patients with preinjury and postinjury MR scans. J Neurotrauma 1995; 12:151-158Crossref, Medline, Google Scholar
26 Blatter DD, Bigler ED, Gale SC, et al: Quantitative volumetric analysis of brain MR: normative database spanning five decades of life. AJNR Am J Neuroradiol 1995; 16:241-251Medline, Google Scholar
27 Bigler ED, Lowry CM, Anderson CV, et al: Dementia, quantitative neuroimaging, and apolipoprotein E genotype. AJNR Am J Neuroradiol 2000; 21:1857-1868Medline, Google Scholar
28 Killiany RJ, Gomez-Isla T, Moss M, et al: Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol 2000; 47:430-439Crossref, Medline, Google Scholar
29 Rasband W: NIH-IMAGE v. 1.55. Washington, DC, U.S. National Institutes of Health, 1994. Accessible at http://rsb.info.nih.gov/nih-image/Google Scholar
30 Ono M, Kubik S, Abernathey CD: Atlas of Cerebral Sulci. New York, Thieme Medical, 1990Google Scholar
31 Gluhbegovic N, Williams TH: The Human Brain: A Photographic Guide. Cambridge, UK, Harper and Row, 1980Google Scholar
32 Roberts MJ, Hanaway J: Atlas of the Human Brain in Section. Philadelphia, Lea and Febiger, 1970Google Scholar
33 Wechsler D: Wechsler Memory Scale-Revised Manual. San Antonio, TX, The Psychological Corporation, 1987Google Scholar
34 Rey A: L'examen clinique en psychologie. Paris, Presses Universitaires de France, 1958Google Scholar
35 Lezak MD: Neuropsychological Assessment, 2nd edition. New York, Oxford University Press, 1983Google Scholar
36 Osterrieth PA: Le teste de copie d'une figure complexe: contribution à l'étude de la perception et la memoire. Archives de Psychologie 1944; 30:286-356Google Scholar
37 Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ, Neuropsychology Press, 1985Google Scholar
38 Beck AT: Beck Depression Inventory Manual. San Antonio, TX, The Psychological Corporation, 1987Google Scholar
39 Beck AT: Beck Anxiety Inventory. San Antonio, TX, The Psychological Corporation, 1990Google Scholar
40 Gale SD, Johnson S, Bigler ED, et al: Nonspecific white matter degeneration following traumatic brain injury. Journal of the International Neuropsychological Society 1995; 1:17-28Crossref, Medline, Google Scholar
41 Clark DL, Boutros NN: The Brain and Behavior: An Introduction to Behavioral Neuroanatomy. Malden, MA, Blackwell Science, 2000Google Scholar
42 Mesulam M-M: Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations, in Principles of Behavioral and Cognitive Neurology, edited by Mesulam M-M. Oxford, UK, Oxford University Press, 2000, pp 1-120Google Scholar
43 Bigler ED: The lesion(s) in traumatic brain injury: implications for clinical neuropsychology. Arch Clin Neuropsychol 2001; 16:95-131Crossref, Medline, Google Scholar
44 Tate DF, Bigler ED: Fornix and hippocampal atrophy in traumatic brain injury. Learning and Memory 2000; 7:442-446Crossref, Medline, Google Scholar
45 Berman KF, Weinberger DR: Neuroimaging studies of schizophrenia, in Neurobiology of Mental Illness, edited by Charney DS, Nestler EJ, Bunney BS. New York, Oxford University Press, 1999, pp 246-257Google Scholar
46 Cummings JL, Coffey CE: Neurological basis of behavior, in Textbook of Geriatric Neuropsychiatry, 2nd edition, edited by Coffey CE, Cummings JL. Washington, DC, American Psychiatric Publishing, 2000, pp 81-105Google Scholar
47 Connor PD: Cingulate, hippocampal, and thalamic degeneration following traumatic brain injury: a quantitative study of magnetic resonance imaging and neuropsychological assessment of memory in psychology. Doctoral dissertation. Provo, UT, Brigham Young University, 1995Google Scholar
48 Long CJ, Pueschel K, Hunter SE: Assessment of the effects of cingulate gyrus lesions by neuropsychological techniques. J Neurosurg 1978; 49:264-271Crossref, Medline, Google Scholar



