Differential Effects of Olanzapine and Risperidone on Cognition in Schizophrenia?
Abstract
Recent studies suggest that novel antipsychotics have positive effects on certain cognitive functions in schizophrenia. The present study investigated this claim by means of saccadic paradigms, which provide a selective index of cognitive function. Thirty-three first-episode schizophrenic patients were randomly assigned to either olanzapine or risperidone treatment and compared with healthy control subjects for three saccadic paradigms. The influence of symptom profile, extrapyramidal symptoms, age, education, gender, hospitalization, and medication dose on cognitive performance was also investigated. Although the two patient groups did not differ from each other in task performance, both patient groups showed substantial problems in inhibitory control of saccades. A high level of education appeared to be protective for this impairment.
Cognitive dysfunctions are considered to be among the core deficits of schizophrenia, since they contribute to disease chronicity, prognosis, and social functioning.1–4 Treatments that ameliorate cognitive dysfunctions, therefore, have important implications for prognosis and long-term outcome. There is increasing interest in the influence of antipsychotic medication on cognition; in particular, the effects of novel antipsychotics (APs) have been the focus of many recent studies (see reviews5,6). The consensus is that novel APs are superior to classical APs with regard to improvement of cognitive function that is independent of improvement in psychopathology. However, novel APs do not have positive effects on all cognitive functions, and some novel APs (in particular clozapine, olanzapine, and risperidone) appear to have variable effects on cognitive processes.5 Further clarification of the effects of these drugs on cognition will have important implications for clinical and scientific progress by facilitating patient management and providing new insights into the pharmacological modulation of neuropsychological function.
Through the precise recording of various spatial and temporal parameters of rapid eye movements, saccadic eye movement paradigms provide a selective index of cognitive function. Cognitive processes that are commonly incorporated in saccadic tasks include visuospatial attention, spatial working memory, and response inhibition.7 A large number of studies have shown saccadic impairments in schizophrenic patients.8–11 In order to understand these impairments, it is useful to make a distinction between visually guided (externally driven) saccades and voluntary (internally driven) saccades (comprising antisaccades, memory-guided saccades, and predictive saccades). The generation of visually guided or reflexive saccades primarily requires the resources of spatial attention and a precise motor program. In contrast, voluntary saccades require in addition higher-order executive functions, such as working memory. A large number of studies have shown that schizophrenic patients perform accurately on visually guided saccades,9,11,12 whereas they have severe problems with voluntary saccades, especially when they have to suppress (inhibit) response tendencies toward novel targets.9,11–13
In contrast to the many studies of neuropsychological test performance, only a few studies have investigated the influence of antipsychotics on (cognitive) saccadic tasks,14 and these have focused primarily on the effects of classical APs. These APs mainly target the dopaminergic nigrostriatal structures that are involved in the generation of saccades. Crawford et al.10 and Hommer et al.14 showed that administration of classical APs resulted in a reduced accuracy of internally driven saccades (in this case, predictive saccades). Crawford et al.10 also found a trend toward more antisaccade inhibition errors. Hutton et al.11 reported reduced amplitudes for antisaccades, along with decreases in latency and the number of inhibition errors. In one of the few studies to examine the effects of novel APs, Burke et al.15 showed that risperidone improved antisaccade performance by reducing the number of inhibition errors. This improvement was predicted by treatment duration. In contrast, Sweeney et al.16 found a detrimental effect of risperidone on visually guided saccades that was manifested in reduced amplitudes, later onset, and changes in peak velocity.
In the present explorative study we investigated the performance of first-episode schizophrenic patients and healthy control subjects on three saccadic tasks: visually guided saccades, antisaccades, and memory-guided saccades. Although several studies have reported robust saccadic abnormalities for chronic schizophrenic patients, only one study has reported abnormalities in first-episode psychotic patients.11 It is important to confirm this finding because this would support the controversial notion of cognitive impairment immediately after disease onset.
Using saccadic tasks that measure psychomotor function, selective attention, visuospatial working memory, and executive functioning, in patients and healthy control subjects, we investigated whether symptom profile, extrapyramidal symptoms, age, education, gender, hospitalization, and medication dose had an influence on performance. We hypothesized that patients would perform worse than control subjects on the antisaccade and memory-guided saccade tasks, but not on the visually guided saccade task.
In addition, we examined whether two novel APs, olanzapine and risperidone, had differential effects on saccades. In reviews of recent research, Meltzer and McGurk5 and Purdon6 concluded that risperidone had positive effects on selective attention (alertness), visuomotor tracking, working memory, motor function, and executive functioning (set shifting), whereas olanzapine had positive effects on attention (reaction time), motor function, visuospatial function, and executive skills. However, risperidone was examined more frequently than olanzapine, and thus its reported beneficial effects on more cognitive domains may be overreported in comparison to the effects of olanzapine. Currently, risperidone appears to be superior to olanzapine with respect to spatial working memory. There is, however, a need for studies conducting a direct comparison between the two drugs. On the basis of these authors' work,5,6 we hypothesized that the risperidone group would perform better than the olanzapine group on saccadic measures of (visuospatial) working memory.
METHODS
Subjects
The study included 33 patients (24 males; 9 females) who had recently experienced their first psychotic episode according to DSM-IV17 and had received a diagnosis within the schizophrenia spectrum (schizophrenia, schizophreniform disorder, schizoaffective disorder). All patients were in a relatively stable phase of their illness and received either olanzapine or risperidone for at least 7 weeks. Mean age was 28.8 (SD=8.3) years and average education was at high school level. The presence of positive and negative symptoms was assessed with the Positive and Negative Symptom Scale (PANSS),18 and severity of extrapyramidal symptoms was judged by the Extrapyramidal Symptom Rating Scale.19 Exclusion criteria were 1) age under 17 or above 60 years; 2) systemic or neurological illness; 3) severe mental retardation; 4) history of alcohol or drug abuse; 5) use of medication other than olanzapine or risperidone; 6) tardive dyskinesia and severe extrapyramidal symptoms; and 7) impaired vision or hearing loss.
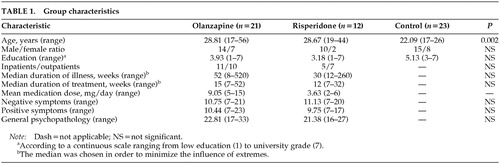
Patients were randomly assigned to either olanzapine or risperidone treatment. The olanzapine group consisted of 21 patients and the risperidone group of 12 patients. Characteristics of these groups are described in Table 1. The relatively small size of the risperidone group was due to exclusion of patients who were on combined drug therapy.
A control group of 23 healthy volunteers (15 males, 8 females), recruited from the local community, was included to evaluate the saccadic performance of patients. These subjects had no history of psychiatric or neurological illness. Moreover, they had no first-degree relatives with a schizophrenia spectrum disorder. Mean age was 22.1 (SD=2.8) years and average education was at high school level. Informed consent was obtained for all subjects included in the study.
Eye Movement Measurement
Subjects were seated 90 cm from a big television screen while their heads were stabilized in a chin rest. Targets (green squares subtending 0.25° of visual angle) were displayed on the screen on four locations at 7.5° and 15° on either side of the central fixation point. In each saccadic task the total number of trials was 48. The experiments were conducted in the dark, and eye movements were recorded by using an infrared limbus reflection device (IRIS, Skalar Delft).
Saccadic Tasks
Visually Guided Saccade Task:
After 800 ms of central fixation, a peripheral target was randomly presented for 1,000 ms to either the left or right side of the fixation point (Figure 1A). Simultaneous with target presentation, a buzzer signal was initiated for 200 ms. Subjects were asked to move their eyes as quickly and accurately as possible to the target location, and afterwards return to central fixation. Intertrial interval was 1,000 ms and trial duration was 2,800 ms.
Antisaccade Task:
After 800 ms of central fixation, a peripheral target was randomly presented for 2,000 ms to either the left or right side of the fixation point (Figure 1B). Simultaneous with target presentation, a buzzer signal was initiated for 200 ms. Subjects were asked to move their eyes to the mirror-image location. Trial duration was 2,800 ms.
Memory-Guided Saccade Task:
After 800 ms of central fixation, a peripheral target was randomly presented for 200 ms to either the left or right side of the fixation point (Figure 1C). The fixation point remained on, and subjects were asked to delay the saccade until the fixation point extinguished (after 500 ms). At the moment of saccade initiation, no information on the previous target location was available. Simultaneous with fixation point offset, a buzzer signal was initiated for 200 ms. Intertrial interval was 3,000 ms and trial duration was 4,500 ms.
Data Analysis
Saccadic analysis was conducted offline, using interactive proprietary software developed at the University of Maastricht. Table 2 shows the saccadic paradigms, performance parameters, and presumed cognitive functions measured.
In the visually guided saccade condition, the latency of the primary saccades was analyzed; we presumed that this condition reflects psychomotor functioning because the task required processing of visuospatial information and transformation of this material into an oculomotor program. We also measured the number of false anticipations. False anticipations are saccades made in advance of the target presentation due to false predictions of alternation between the left and right side (despite the instruction of randomization). The number of false anticipations was thought to reflect executive functioning, since preventing oneself from making erroneous alternating saccades involves the maintenance of task instructions in working memory.
In the antisaccade condition, we analyzed latency and percentage of inhibition errors. An inhibition error was scored when the participant moved his or her eyes reflexively to the peripheral target rather than to the opposite site. Inhibition errors resulted from a failure to suppress the prepotent reflexive saccade to the target. We presumed that latency and inhibition errors were both measures of executive functioning and perhaps also of selective attention, since the task requires the participant to ignore a visual distractor and to maintain in working memory the task instructions in order to inhibit the reflexive response to the target. In addition, the latency of inhibition errors was analyzed.
In the memory-guided saccade condition, we analyzed the accuracy of primary saccades and the “final eye positions” (eye positions after corrective saccades were made). Final eye position was presumed to be an index of spatial working memory.20 We also measured the percentage of inhibition errors and the percentage of errors in delaying the saccade. Delay errors were interpreted as a measure of executive functioning, since the oculomotor program for the saccade, which is prepared immediately after stimulus presentation, should be controlled (inhibited) during the delay period. As a criterion for differentiating between delay errors and inhibition errors, we used the individual mean latency of antisaccade inhibition errors on the antisaccade task. Saccades exceeding this mean latency plus one standard deviation were categorized as delay errors.
In order to examine whether first-episode patients performed worse than healthy control subjects on each of the eleven saccadic measures, the data were analyzed by means of two-tailed t-tests or Mann-Whitney U-tests. Differences between patients using olanzapine or risperidone and healthy control subjects were examined by one-way analysis of variance (ANOVA) followed by post hoc testing (Tukey honestly significant difference; two-tailed tests with significance level set at alpha<0.05). When variables did not resemble the normal distribution, nonparametric Kruskal-Wallis one-way ANOVA was performed, followed by Mann-Whitney U-tests for multiple comparisons. The relationships between various saccadic measures were examined by using parametric and nonparametric correlation analyses for the patient and control groups separately.
RESULTS
First-Episode Patients Versus Healthy Control Subjects
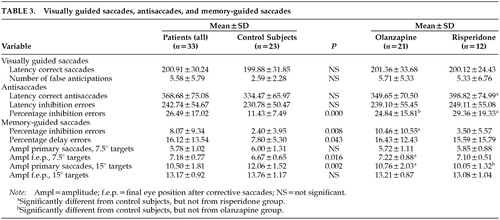
The patient and control groups did not differ significantly for gender and level of education, whereas there was a difference for age (z=–3.60, P=0.000). Because age was found to have an influence on some measures of saccadic performance,21 correlation analysis was performed to reveal significant influences of age on each of the saccadic measures; however, no significant correlations were obtained. Table 3 displays performance means and between-group differences. Compared with control subjects, patients made significantly more inhibition errors on the antisaccade task (t=4.50, df=46.98, P=0.001) and the memory-guided saccade task (z=–2.64, P=0.008). On the memory-guided saccade task, patients also differed significantly from control subjects for the number of delay errors (z=–2.02, P=0.043), the final eye position to small target eccentricities (t=2.50, df=50, P=0.016), and the amplitude of primary saccades to large target eccentricities (t=–3.29, df=50, P=0.002).
Correlations Between the Saccadic Measures
Because of multiple comparisons in the correlation analysis, only two-tailed P-values smaller than 0.01 were considered significant for correlation coefficients. Within the patient group we found significant correlations between the following saccadic measures: latency of visually guided saccades and latency of incorrect antisaccades (r=0.48, P=0.005); latency of correct and incorrect antisaccades (r=0.73, P=0.001); antisaccade inhibition errors and final eye position to small target eccentricities (r=0.52, P=0.003); and amplitudes to small and large target eccentricities (P-values ranging from 0.002 to 0.004).
Within the control group the following saccadic measures were significantly correlated: latency of visually guided saccades and latency of correct antisaccades (r=0.70, P=0.000); latency of visually guided saccades and latency of incorrect antisaccades (r=0.63, P=0.002); and amplitudes to small and large target eccentricities (P-values ranging from 0.000 to 0.003).
Saccadic Performance and Disease-Related Factors
Within the patient group, age, symptom profile (PANSS subscales), extrapyramidal symptoms, duration of illness, medication dose, and duration of medication treatment were not correlated with saccadic performance. However, a significant negative correlation was found between the level of education and the latency of antisaccades, both correct (r=–0.62, P=0.001) and incorrect (r=–0.50, P=0.009). Inpatients and outpatients showed equal performance levels, and also female and male patients did not differ on the tasks.
Olanzapine, Risperidone, and Healthy Control Subjects
The olanzapine, risperidone, and control groups did not differ for gender and level of education, whereas there was a difference for age (χ2=12.95, df=2, P=0.002). The olanzapine and risperidone groups were significantly older than the control group (P=0.000 and P=0.006, respectively). The medication groups were similar for inpatient/outpatient ratio, duration of illness, duration of medication treatment, and severity of positive symptoms, negative symptoms, and general symptoms. No significant differences in the saccadic variables were found between the two medication groups. The olanzapine and risperidone groups, however, differed significantly from control subjects for antisaccade inhibition errors (P=0.007 and P=0.002, respectively) and the amplitudes of primary saccades to large target eccentricities (P=0.045 and P=0.006, respectively). The olanzapine group, in contrast to the risperidone group, differed significantly from control subjects for inhibition errors on the memory-guided task (P=0.002) and the final eye position to small target eccentricities in this task (P=0.048). The risperidone group differed significantly from control subjects for the latency of correct antisaccades (P=0.032).
DISCUSSION
We examined saccadic eye movements in three saccade tasks in first-episode psychotic patients and healthy control subjects. Our patient group performed worse than control subjects on the antisaccade and memory-guided saccade tasks. Impairment was most pronounced for the number of inhibition errors, which is in accordance with previous studies.10,22 Inhibition errors reflect a failure in the control of response tendencies and have been attributed to dorsolateral prefrontal dysfunction.23 In addition, patients showed more delay errors in the memory-guided task. These errors reflect a failure in delaying an already prepared saccadic motor program, which could be interpreted as a failure in executive control. For the memory-guided task, patients also showed significantly reduced amplitudes on targets with large eccentricity, whereas amplitudes on targets with small eccentricity were more accurate than those of control subjects. These findings are difficult to interpret and should first be confirmed by studies using a larger sample.
Within the patient group we found a number of correlations between the various saccadic measures. First, antisaccade inhibition errors and final eye position of small saccades were positively correlated. It is possible that these two measures depend on a common cognitive process; visuospatial working memory is the most likely candidate. Second, whereas none of the demographic and disease-related factors were significantly correlated with saccadic performance, we found a negative correlation between the latency of both correct and incorrect antisaccades and the level of education. Patients with more education performed better than patients with less education. Perhaps intelligence protects patients from poor performance on measures of executive function and attention. This would be in line with the findings of Holthausen et al. (unpublished manuscript), who demonstrated that patients with normal performance on neuropsychological tasks scored higher on intelligence tests than did patients with impaired performance. However, the present negative correlation needs to be confirmed in future studies in which IQ and level of education are established more extensively.
With respect to differential effects of risperidone and olanzapine, no significant group differences on any of the saccadic measures were found. This was surprising and counter to our hypothesis. We predicted superior beneficial effects for risperidone on measures of spatial working memory. In fact, the risperidone group performed even slightly worse on these measures; the amplitudes of memory-guided saccades were less accurate. Previous studies reported reduced accuracy on the memory-guided task after treatment with classical APs,10,14 raising the possibility that our findings were related to medication dose. Risperidone in a high dose has an action profile that resembles that of classical APs. However, our dose range was low and similar to those used in other studies (2 mg to 6 mg per day); thus it seems unlikely that this could explain the discrepancy between our results and the results from previous studies reporting fairly beneficial effects of risperidone on neuropsychological measures of visuospatial working memory.
It seems plausible that olanzapine and risperidone have no differential effects on the cognitive functions targeted in this study. However, future studies in which these drugs are directly compared in a design with baseline measurements are warranted. Additional clues about the influence of novel APs on saccades might also be provided by studies investigating 1) smooth-pursuit eye movements, since during these eye movements the saccadic system is also active, and 2) the effects of single doses of APs in healthy control subjects, since this provides knowledge about the direct effects of APs without interference from disease-related factors. Currently neither type of study has been conducted with novel APs. A comparison of these saccadic data for patients on risperidone and olanzapine with data from studies of classical APs in similar experimental paradigms10,11 is consistent with a beneficial effect of some novel drugs on cognition. However, confirmation of this possibility will have to await direct head-to-head studies comparing the effects of classical and novel APs.
An important factor that might explain the failure to find significant differences between the olanzapine and risperidone groups is the small size of the groups. It seems unlikely, however, that larger groups would have resulted in significant effects, since power analysis revealed that significant results could be obtained with our group sizes. Moreover, many studies have shown that with similar or even smaller group sizes significant differential drug effects could be obtained. The omission of baseline measurements might also have prohibited us from finding differential effects, because with our design we could not establish individual improvement after treatment duration. Nevertheless, we think that the random group assignment in combination with close matching provided a good opportunity to find significant group differences.
In sum, the present study replicates the previous finding that not only chronic schizophrenic patients, but also first-episode patients have substantial problems with cognitive processes incorporated in saccadic tasks. Apparently cognitive abnormalities are already present in an early phase of the disease. Schizophrenic patients are particularly impaired in the inhibitory control of reflexive saccades, although a high level of education appears to be an important protective factor. We also have demonstrated that random assignment to either treatment with risperidone or olanzapine did not result in differential saccadic performance between the groups.
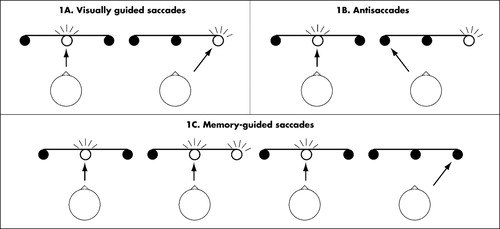
FIGURE 1. Simplified saccadic paradigms.
 |
 |
 |
1 Andreasen NC: The role of the thalamus in schizophrenia. Can J Psychiatry 1997; 42:27-33Crossref, Medline, Google Scholar
2 Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348-357Link, Google Scholar
3 Hemsley DR: Cognitive disturbance as the link between schizophrenic symptoms and their biological basis. Neurol Psychiatry Brain Res 1994; 2:163-170Google Scholar
4 Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321-330Crossref, Medline, Google Scholar
5 Meltzer HY, McGurk SR: The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 1999; 25:233-255Crossref, Medline, Google Scholar
6 Purdon SE: Cognitive improvement in schizophrenia with novel antipsychotic medications. Schizophr Res 1999; 35(suppl):S51-S60Google Scholar
7 Broerse A, Crawford T, den Boer JA: Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia 2001; 39:742-756Crossref, Medline, Google Scholar
8 Thaker GK, Nguyen JA, Tamminga CA: Increased saccadic distractibility in tardive dyskinesia: functional evidence for subcortical GABA dysfunction. Biol Psychiatry 1989; 25:49-59Crossref, Medline, Google Scholar
9 Crawford TJ, Haeger B, Kennard C, et al: Saccadic abnormalities in psychotic patients, I: neuroleptic-free psychotic patients. Psychol Med 1995; 25:461-471Crossref, Medline, Google Scholar
10 Crawford TJ, Haeger B, Kennard C, et al: Saccadic abnormalities in psychotic patients, II: the role of neuroleptic treatment. Psychol Med 1995; 25:473-483Crossref, Medline, Google Scholar
11 Hutton SB, Crawford TJ, Puri BK, et al: Smooth pursuit and saccadic abnormalities in first-episode schizophrenia. Psychol Med 1998; 28:685-692Crossref, Medline, Google Scholar
12 Karoumi B, Ventre-Dominey J, Vighetto A, et al: Saccadic eye movements in schizophrenic patients. Psychiatry Res 1998; 77:9-19Crossref, Medline, Google Scholar
13 McDowell JE, Clementz BA: The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res 1997; 115:333-344Crossref, Medline, Google Scholar
14 Hommer DW, Clem T, Litman R, et al: Maladaptive anticipatory saccades in schizophrenia. Biol Psychiatry 1991; 30:779-794Crossref, Medline, Google Scholar
15 Burke JG, Patel JKM, Morris PK, et al: Risperidone improves antisaccade error rates in schizophrenia (abstract). Schizophr Res 1998; 29:115Crossref, Google Scholar
16 Sweeney JA, Bauer KS, Keshavan MS, et al: Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology 1997; 16:217-228Crossref, Medline, Google Scholar
17 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington DC, American Psychiatric Association, 1994Google Scholar
18 Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-275Crossref, Medline, Google Scholar
19 Simpson GM, Angus JWS: A rating sscale for extrapyramidal side effects. Acta Psychiatr Scand 1970; 212:11-19Crossref, Google Scholar
20 Crawford TJ, Henderson L, Kennard C: Abnormalities of nonvisually guided saccades in Parkinson's disease. Brain 1989; 112:1573-1586Crossref, Medline, Google Scholar
21 Fischer B, Biscaldi M, Gezeck S: On the development of voluntary and reflexive components in human saccade generation. Brain Res 1997; 754:285-297Crossref, Medline, Google Scholar
22 Schultz SK, Andreasen NC: Schizophrenia. Lancet 1999; 353:1425-1430Crossref, Medline, Google Scholar
23 Pierrot-Deseilligny C, Rivaud S, Gaymard B, et al: Cortical control of reflexive visually guided saccades. Brain 1991; 114:1473-1485Crossref, Medline, Google Scholar
24 Holthausen EA, Wiersma D, Van den Bosch RJ: Do schizophrenic patients without neuropsychological deficits still show signs of cognitive decline? (submitted)Google Scholar



