Identification of HIV-Associated Progressive Multifocal Leukoencephalopathy
The human immunodeficiency virus (HIV) and resulting acquired immune deficiency syndrome (AIDS) is a leading cause of death worldwide. A recent estimate from the United Nations Programme on HIV/AIDS indicates that 40 million people worldwide have HIV/AIDS.1 The advent of highly active antiretroviral therapy (HAART) has certainly increased survival. However, central nervous system (CNS) disorders remain a significant cause of morbidity and mortality in HIV/AIDS-infected patients. Current statistics indicate that 39% to 70% of all AIDS patients develop neurological disorders, and these are the initial manifestations of AIDS in 7% to 20%.2 A large retrospective study of 450 AIDS autopsy cases (1984–1999) found that the brain continues to be second only to lung in pathology, with 28% to 38% of cases having CNS findings.3 Commonly occurring conditions include toxoplasma encephalitis, cryptococcal meningitis, primary central nervous system lymphoma, HIV-associated dementia, progressive multifocal leukoencephalopathy (PML), and other viral and opportunistic infections. Most authors agree that only some conditions have decreased with HAART. The incidence of PML has not changed over time, whereas some opportunistic infections such as toxoplasmosis and cytomegalovirus have had as much as a 50% decline.3 Currently PML is estimated to occur in 1% to 10% of AIDS patients, and in one study it occurred within one year of death in 91.7% of patients who were infected.4,5
A good brief overview of PML, with historical references, can be found in the review of Berger and Major.4 This disorder is a multifocal demyelinating disease that is caused by a reactivation of the dormant JC virus (JCV), a DNA-containing polyomavirus. It was first described by a German neuropathologist, Hallervorden, in 1930. It was described as a syndrome in 1958 by Åstrom and Richardson and identified as a viral disease in 1965 by Zu-Rhein. The virus was isolated in 1971 by Padgett and Walker.4,6 It was a rare disorder in immunocompromised patients until the AIDS epidemic, when its incidence greatly expanded. PML cases increased 400% in the decade between 1984 and 1994.7 The rate varies considerably by country, being low in the United States (2% to 5%) and higher in most European studies (4% to 11%).7 Additionally, PML was documented more often in intravenous drug users than non–drug users.3 When PML develops in conjunction with HIV, it is particularly devastating, with survival times in the absence of treatment of only a few months.8 Most adults (70% to 90%) have IgG antibodies to JCV by middle age.4,9 The initial infection has not been identified, but one proposal is that the acute infection may be in the tonsils.4 JCV is also present in the kidneys.10,11
Because the disease is multifocal, symptoms will depend on the areas demyelinated; however, common complaints are present.4 Weakness, disturbances of speech, cognitive abnormalities, headaches, gait disorders, seizures, sensory loss, and visual impairments (field loss, diplopia) are reported. Limb weakness was estimated in more than 50% of cases; cognitive and gait disorders in 25% to 33%; seizures in 10%. HIV-associated dementia and other CNS disorders can certainly have similar presentations. It is therefore necessary to quickly identify this rapidly fatal disease in the least possibly invasive way. Recent improvements in cerebrospinal fluid (CSF) testing and newer imaging techniques are replacing the older biopsy method.
PATHOLOGY
Recent laboratory findings culminated in a proposed cycle for the development and progression of PML.10,11 The virus has an icosahedral capsid covering a double-stranded DNA, which can be divided into three regions: early and late coding regions and a regulatory (noncoding) region. JCV DNA is present in the peripheral blood lymphocytes in more than 95% of PML cases. Activated B cells cross the blood–brain barrier. Immunohistochemical staining of tissue samples indicates that JCV is predominantly found in the oligodendrocytes, while HIV-1 is primarily found in the microglia, monocytes, and astrocytes.10–12 Controversy remains as to whether or not glia can be a latent site for harborization. Once the virus is inside the CNS, replication begins in the glial cells. Recent theory, greatly simplified, is that the HIV-infected cells produce Tat, a transregulatory protein.10,11 Tat is released from its host cell and taken up by the neighboring oligodendrocytes. Tat then induces the JCV promoter. Thus, JCV replication is increased. Host cells are lysed, and the cycle continues. Additionally, Tat induces the production of several cytotoxic proteins, including tumor necrosis factor, interleukin-1, and transforming growth factor β, that not only destroy neighboring cells but also stimulate further viral production.
Classic histological examination indicates extensive demyelinated plaques, oligodendrocytes with eosinophilic intranuclear JCV inclusions, and atypical hypertrophic astrocytes (Figure 1A, 1B, and 1C).10,11 Less commonly, microglial nodules, perivascular lymphocytic cuffing, and cavity formation are found. Mossakowski and Zelman7 autopsied 20 cases of PML. They found that PML was coexistent with other opportunistic AIDS-related brain diseases in 14 of the 20 cases. They divided the results into early, atypical, and late forms. The early form had multiple scattered foci, commonly near the corticosubcortical junctions or deep white matter. The oligodendrocytes were consistently abnormal, with the classic enlarged metachromatic nuclei. The astrocytes were mildly enlarged. The atypical form was more unifocal, with the classic findings in the glia. The late, severe group had much more demyelination, with bilateral, asymmetric lesions in the cerebrum and, less commonly, the brainstem and cerebellum. The glia were very abnormal, with JCV virions within the oligodendrocytes, hypertrophic astrocytes, cavitary lesions, and macrophage/lymphocytic invasion. The authors also noted that in their experience, PML destruction is more severe in AIDS than in other immunocompromising diseases, citing a synergy with the HIV virus itself.
DIAGNOSTIC ADVANCES
Definitive diagnosis of PML can be made only by brain biopsy. However, this is invasive and can have associated with it occasional morbidity (hemorrhage or infection within 30 days), rare mortality, or lesion of brain circuitry. In a recent review of brain biopsies of 435 patients (47 local and 388 by literature review), Skolasky et al.13 determined that biopsies have an 88% definitive diagnostic yield. However, morbidity and mortality were high and varied from site to site. Morbidity ranged from 3.3% to 30.8% (average 8.4%); mortality ranged from 0% to 5.3% (average 2.9%). In this survey, 25% of patients were diagnosed with PML. Currently, noninvasive techniques are increasing. Radiologic imaging and CSF analysis are emerging as potential tools to replace biopsy in many cases.
Magnetic Resonance Imaging
Both PML and HIV encephalopathy are associated with nonenhancing lesions in the cerebral white matter that show little or no mass effect and are hyperintense on T2-weighted and FLAIR magnetic resonance (MR) images.8,14 However, PML can be differentiated from HIV encephalopathy because the PML lesions are commonly hypointense on T1-weighted MR images, while in pure HIV infection, lesions are isointense. Serial imaging indicates the PML lesions become more hypointense as the disease progresses; markedly low signal intensity may indicate particularly aggressive infection.8 In addition, PML lesions are focal and, if bilateral, they are asymmetric. They are located predominantly in the subcortical white matter just below the cortical ribbon, involve the arcuate (U) fibers (although brainstem and cerebellar lesions are also found), and present little or no atrophy.15 In contrast, white matter lesions in HIV encephalopathy are diffuse and symmetric and do not involve the arcuate fibers. In addition, both cortical and subcortical (particularly caudate) atrophy are common.8,12,14
A new type of MR imaging, magnetization transfer (MT) imaging, appears to be much more sensitive than standard MR to the demyelinating process that occurs in PML. MT imaging is based on the cross-relaxation between protons that are essentially immobile because they are bound to tissue (“solid-like” pool) and free protons (“liquid-like” pool), and thus it provides different information than is available from standard clinical MR images.16–18 Most of the signal used to create any MR image comes from the free pool of protons. For MT imaging, specially designed saturation pulses are included in the imaging sequence to alter the magnetization of the bound protons. This in turn causes a decrease in the signal coming from the free protons and thus changes the signal intensity on the resulting MR image. Most commonly, this is expressed as the change in signal intensity relative to the normal MR image (magnetization transfer ratio, MTR, usually expressed as a percentage). The decrease in signal in the saturated image is large for complex tissues (gray matter, white matter) and small for areas that are mostly fluid (CSF, blood). The MTR is greater for white matter than for gray matter because of the high membrane content. MT imaging is sensitive to any process that disrupts the structural integrity of tissue, particularly the myelin sheath and axon.19–21
Two studies have shown that MT imaging can distinguish the demyelinating lesions of PML from both the diffuse lesions of HIV encephalopathy and nonspecific white matter lesions that may be present (Figure 2, left and right).22,23 There were differences between the two studies in both methodology and patient selection. In one study, the pure HIV group all had cognitive deficits, while in the other, none did. Nevertheless, the MT imaging results were similar. Both found the average MTR for normal white matter to be 47% to 48%. Both found the MTR of PML lesions to be profoundly decreased (average MTR 22% to 26%). White matter lesions related purely to HIV, with or without accompanying cognitive deficits, had mildly reduced MTR (average MTR 38% to 40%). These results indicate that MT imaging has the potential to distinguish the destructive demyelinating lesions of PML from the edematous lesions of HIV. Thus, it shows promise for differential diagnosis, prognosis, or monitoring of therapy by serial assessment.
Magnetic Resonance Spectroscopy
Proton (1H) MR spectroscopy provides measures of several neurochemicals related to neuronal viability. Signal is present from N-acetylaspartate (NAA, located primarily in neurons, a marker for neuron and axon viability), choline (Cho, principally phosphatidyl choline, a membrane constituent), and creatine (Cr, used as an internal standard because its level is usually stable). An additional peak may be present from lactate (Lac, metabolite associated with inflammation or neuronal mitochondrial dysfunction and related to activated anaerobic glycolytic metabolism). If a short echo-time acquisition is used, it is possible to observe myoinositol (MI, putative glial marker). The absolute amount of signal in each spectral peak is highly dependent on the acquisition parameters, so for comparison purposes the signals of interest (NAA, Cho) are commonly expressed relative to Cr. It is also possible to measure absolute metabolite concentrations, but the acquisition is more complex. The Cho/Cr peak reflects membrane metabolism and has been found to be elevated both during degradation (demyelination) and rapid synthesis. The NAA/Cr peak reflects neuronal and axonal density. Studies vary considerably in design and methodology, including differences in how spectra are acquired, what part of brain (lesion or normal appearing) was examined, and the types of spectral comparisons done. Nevertheless, the results are in reasonable agreement across studies.
In early HIV, when clinical symptoms are absent or mild, NAA/Cr is unchanged (92% to 98% of normal value).24–26 In late-stage HIV, when clinical symptoms are severe, NAA/Cr decreases (62% to 84% of normal value).24–28 Decreased NAA/Cr is found both in lesions and in normal-appearing brain.24–30 These results are compatible with pathological findings that neuronal loss (which should result in a decrease in NAA/Cr) occurs in the late stage of HIV.31 It is possible that in some cases decreased NAA/Cr indicates neuronal dysfunction rather than destruction. A return to normal levels was found concomitant with resolution of clinical symptoms following treatment with zidovudine in one study.32 In contrast, Cho/Cr elevations were similar within studies in the presence and absence of symptoms and in the presence or absence of lesions on diagnostic images, although the size of the increase varied across studies.24–26 This elevation may reflect an increase in membrane density due to glial changes and/or inflammatory cells.24 These results suggest that the Cho peak of the spectrum may indicate the presence of subclinical HIV. MI and MI/Cr of normal-appearing white matter are also elevated in early HIV, but higher elevations are correlated with more severe clinical symptoms.30 Thus increased MI may also indicate subclinical HIV, perhaps reflecting glial activation in response to inflammation.
Only a few studies have included patients with PML.28,33,34 Both groups reporting on PML patients have found substantial decreases in NAA, whether measured in relation to Cr, to the contralateral NAA value, or by absolute quantification. This decrease is consistent with the destructive nature of the PML infection. Both groups have also found Cho to be elevated, perhaps reflecting myelin destruction. Lactate was present in 75% of spectra, also consistent with the severe nature of PML.28,34 Both increases and decreases in MI were found in the one study that measured it.33 Interestingly, the study that used absolute quantification found that Cr was decreased.34 If this is a general finding, it will require reevaluation of studies in which ratios were used.
Only one study has directly compared spectral changes in pure HIV with those in PML (Figure 3).28 The results suggest that this technique may be helpful in differentiating PML from other entities. PML lesions had a more profound decrease in NAA than those associated with HIV encephalopathy, whereas lesions in HIV encephalopathy had a more consistent increase in Cho. In addition, PML lesions had an increased lactate peak.
CSF Analysis
JCV can be detected by polymerase chain reaction (PCR) testing of the CSF.35 A recent review summarized the sensitivity as 90% to 100%, with a specificity of 92% to 100%.9 However, other reports indicate that sensitivity may be lower in some circumstances.8,23,28 PCR testing for JCV has potential as a prognostic tool because lower survival correlates with higher levels of JCV in the CSF.9,36,37 Patients with a JCV load<4.7 log (log copies per ml of CSF) survived much longer than those with log>4.7 at time of diagnosis.36,37 In one report, JCV load did not correlate with lesion load as measured from T2-weighted MR images.37 In this study of 12 patients, the volume of lesions did not correlate with JCV load even when controlled for CD4 count, treatment with HAART, plasma HIV load, age, and location of lesions. Three of the 12 patients had more than one viral load measured. Two of the three had longitudinal correlation of viral load with lesion volume, and the third was inverse. The authors recommend further longitudinal examinations in larger patient groups for any correlations with lesion volume to be found. It might also be interesting to quantify lesion load by using MT imaging.
CONCLUSION
As newer imaging techniques and CSF testing become the mainstay of PML identification, it will be important for treatment to improve also. In some cases, HAART alone has been effective treatment for PML. However, Miralles et al.38 report that approximately one-third of patients die despite treatment and improved CD4 cell counts (>200 cells/mm3). Additionally, PML can develop following initiation of HAART, with a paradoxical worsening of inflammatory changes that are not typical for untreated PML.39,40 It has been suggested that improved immune system function may be important in activating latent infection.39 In these cases, at least, adjunct therapy is required. Cidofovir, an antiviral agent, has shown some promise in this regard.39,41 Serial assessment with some combination of MT imaging, MR spectroscopy, and CSF testing may provide a more rapid and reliable tailoring of individual therapy, thus affecting management and prognosis. It is very likely, too, that MT imaging and MR spectroscopy will provide similarly important insights into other demyelinating conditions.
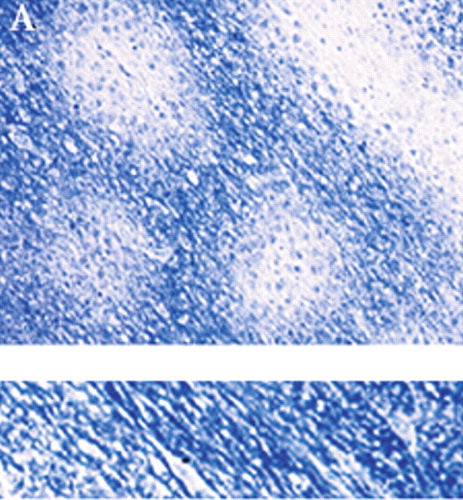
Figure 1A. The histological characteristics of progressive multifocal leukoencephalopathy (PML) include destruction of myelin (A, myelin-stained section of subcortical white matter), loss of oligodendrocytes (B, enlarged oligodendrocytes harboring intranuclear eosinophilic inclusion bodies, at arrow), and bizarre reactive astrocytes (C, transformed bizarre reactive astrocyte). Companion normal images are shown beneath each panel for comparison.
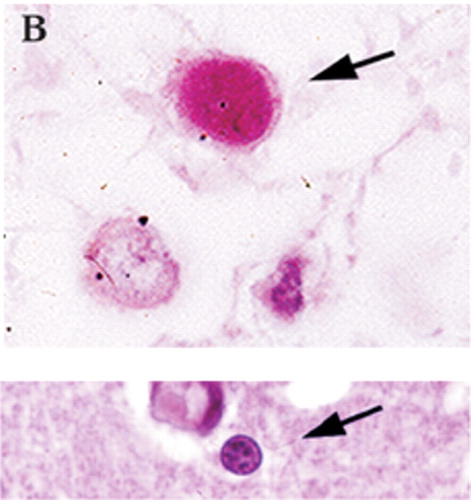
Figure 1B. The histological characteristics of PML include destruction of myelin (A, myelin-stained section of subcortical white matter), loss of oligodendrocytes (B, enlarged oligodendrocytes harboring intranuclear eosinophilic inclusion bodies, at arrow), and bizarre reactive astrocytes (C, transformed bizarre reactive astrocyte). Companion normal images are shown beneath each panel for comparison.
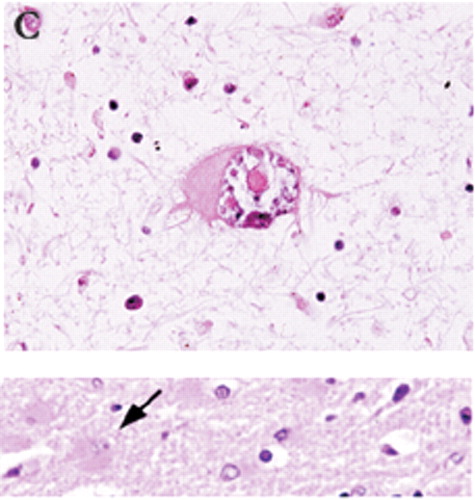
Figure 1C. The histological characteristics of PML include destruction of myelin (A, myelin-stained section of subcortical white matter), loss of oligodendrocytes (B, enlarged oligodendrocytes harboring intranuclear eosinophilic inclusion bodies, at arrow), and bizarre reactive astrocytes (C, transformed bizarre reactive astrocyte). Companion normal images are shown beneath each panel for comparison.
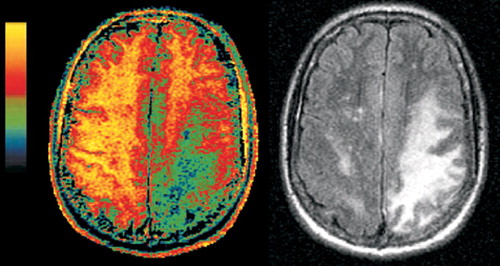
Figure 2A. Companion magnetization transfer ratio (MTR, color) and FLAIR (grayscale) magnetic resonance images of PML (left set) and HIV-white matter lesions (right set). Although the areas of abnormality in the white matter look quite similar on the FLAIR images, they are clearly different on the MTR images. The MTR of the PML lesions is deeply decreased (green and blue areas in left set of images), consistent with the demyelinating nature of the disease. The lesions in HIV infection show very little decrease in MTR, as would be expected based on the less destructive nature of this pathology.
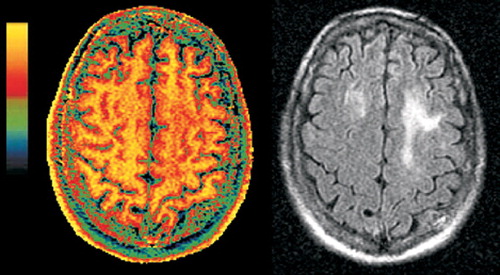
Figure 2B. Companion magnetization transfer ratio (MTR, color) and FLAIR (grayscale) magnetic resonance images of PML (left set) and HIV-white matter lesions (right set). Although the areas of abnormality in the white matter look quite similar on the FLAIR images, they are clearly different on the MTR images. The MTR of the PML lesions is deeply decreased (green and blue areas in left set of images), consistent with the demyelinating nature of the disease. The lesions in HIV infection show very little decrease in MTR, as would be expected based on the less destructive nature of this pathology.
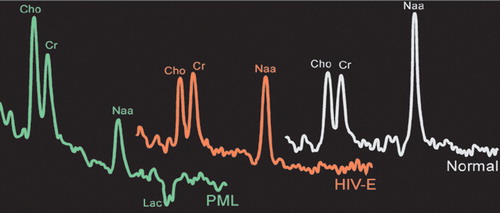
Figure 3. Illustrations of proton (1H) magnetic resonance spectra acquired from normal brain (white), a white matter lesion in a patient with pure HIV infection (red), and a PML lesion (green). Spectra have been scaled so that creatine (Cr) peaks are similar heights. Note that the N-acetylaspartate (NAA) peak is decreased in both HIV and PML.
1 Stover J, Walker N, Garnett GP, et al: Can we reverse the HIV/AIDS pandemic with an expanded response? Lancet 2002; 360:73-77Crossref, Medline, Google Scholar
2 Maschke M, Kastrup O, Esser S, et al: Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J Neurol Neurosurg Psychiatry 2000; 69:376-380Crossref, Medline, Google Scholar
3 Jellinger KA, Setinek U, Drlicek M, et al: Neuropathology and general autopsy findings in AIDS during the last 15 years. Acta Neuropathol 2000; 100:213-220Crossref, Medline, Google Scholar
4 Berger JR, Major EO: Progressive multifocal leukoencephalopathy. Semin Neurol 1999; 19:193-200Crossref, Medline, Google Scholar
5 Welch K, Morse A, and the Adult Spectrum of Disease Project in New Orleans: The clinical profile of end-stage AIDS in the era of highly active antiretroviral therapy. AIDS Patient Care and STDs 2002; 16:75-81Crossref, Medline, Google Scholar
6 Henson JW, Louis DN: Edward Peirson Richardson Jr (1918-1998) and the discovery of PML. J Neurovirol 1999; 5:325-326Crossref, Medline, Google Scholar
7 Mossakowski MJ, Zelman IB: Pathomorphological variations of the AIDS-associated progressive multifocal leukoencephalopathy. Folia Neuropathol 2000; 38:91-100Medline, Google Scholar
8 Post MJ, Yiannoutsos C, Simpson D, et al: Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AJNR Am J Neuroradiol 1999; 20:1896-1906Medline, Google Scholar
9 Mamidi A, DeSimone JA, Pomerantz RJ: Central nervous system infections in individuals with HIV-1 infection. J Neurovirol 2002; 8:158-167Crossref, Medline, Google Scholar
10 Sweet TM, Del Valle L, Khalili K: Molecular biology and immunoregulation of human neurotropic JC virus in CNS. J Cell Physiol 2002; 191:249-256Crossref, Medline, Google Scholar
11 Del Valle L, Croul S, Morgello S, et al: Detection of HIV-1 Tat and JVC capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol 2000; 6:221-228Crossref, Medline, Google Scholar
12 Paul R, Cohen R, Navia B, et al: Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neurosci Biobehav Rev 2002; 26:353-359Crossref, Medline, Google Scholar
13 Skolasky RL, Dal Pan GJ, Olivi A, et al: HIV-associated primary CNS lymorbidity and utility of brain biopsy. J Neurol Sci 1999; 163:32-38Crossref, Medline, Google Scholar
14 Sarrazin JL, Soulie D, Derosier C, et al: [MRI aspects of progressive multifocal leukoencephalopathy] (French). J Neuroradiol 1995; 22:172-179Medline, Google Scholar
15 Kastrup O, Maschke M, Diener HC, et al: Progressive multifocal leukoencephalopathy limited to the brain stem. Neuroradiology 2002; 44:227-229Crossref, Medline, Google Scholar
16 Dousset V, Armand JP, Huot P, et al: Magnetization transfer imaging in AIDS-related brain diseases. Neuroimaging Clin N Am 1997; 7:447-460Medline, Google Scholar
17 Van Buchem MA, Tofts PS: Magnetization transfer imaging. Neuroimaging Clin N Am 2000; 10:771-788Medline, Google Scholar
18 Filippi M, Grossman RI: MRI techniques to monitor MS evolution: the present and the future. Neurology 2002; 58:1147-1153Crossref, Medline, Google Scholar
19 Dousset V, Grossman RI, et al: Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992; 182:483-491Crossref, Medline, Google Scholar
20 Lexa FJ, Grossman RI, Rosenquist AC: MR of wallerian degeneration in the feline visual system: characterization by magnetization transfer rate with histopathologic correlation. AJNR Am J Neuroradiol 1994; 15:201-212Medline, Google Scholar
21 Dousset V, Brochet B, Vital A, et al: Lysolecithin-induced demyelination in primates: preliminary in vivo study with MR and magnetization transfer. AJNR Am J Neuroradiol 1995; 16:225-231Medline, Google Scholar
22 Dousset V, Armand JP, Lacoste D, et al: Magnetization transfer study of HIV encephalitis and progressive multifocal leukoencephalopathy. AJNR Am J Neuroradiol 1997; 18:895-901Medline, Google Scholar
23 Ernst T, Chang L, Witt M, et al: Progressive multifocal leukoencephalopathy and human immunodeficiency virus-associated white matter lesions in AIDS: magnetization transfer MR imaging. Radiology 1999; 210:539-543Crossref, Medline, Google Scholar
24 Menon DK, Ainsworth JG, Cox IJ, et al: Proton MR spectroscopy of the brain in AIDS dementia complex. J Comput Assist Tomogr 1992; 16:538-542Crossref, Medline, Google Scholar
25 Chong WK, Sweeney B, Wilkinson ID, et al: Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology 1993; 188:119-124Crossref, Medline, Google Scholar
26 Tracey I, Carr CA, Guimaraes AR, et al: Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: a proton magnetic resonance spectroscopic study. Neurology 1996; 46:783-788Crossref, Medline, Google Scholar
27 Jarvik JG, Lenkinski RE, Grossman RI, et al: Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlation. Radiology 1993; 186:739-744Crossref, Medline, Google Scholar
28 Simone IL, Federico F, Tortorella C, et al: Localised 1H-MR spectroscopy for metabolic characterisation of diffuse and focal brain lesions in patients infected with HIV. J Neurol Neurosurg Psychiatry 1998; 64:516-523Crossref, Medline, Google Scholar
29 Menon DK, Baudouin CJ, Tomlinson D, et al: Proton MR spectroscopy and imaging of the brain in AIDS: evidence of neuronal loss in regions that appear normal with imaging. J Comput Assist Tomogr 1990; 14:882-885Crossref, Medline, Google Scholar
30 Chang L, Ernst T, Leonido-Yee M, et al: Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999; 52:100-108Crossref, Medline, Google Scholar
31 Everall IP, Luthert PJ, Lantos PL: Neuronal loss in the frontal cortex in HIV infection. Lancet 1991; 337:1119-1121Crossref, Medline, Google Scholar
32 Vion-Dury J, Nicoli F, Salvan AM, et al: Reversal of brain metabolic alterations with zidovudine detected by proton localised magnetic resonance spectroscopy. Lancet 1995; 345:60-61Crossref, Medline, Google Scholar
33 Chang L, Miller BL, McBride D, et al: Brain lesions in patients with AIDS: H-1 MR spectroscopy. Radiology 1995; 197:525-531Crossref, Medline, Google Scholar
34 Chang L, Ernst T, Tornatore C, et al: Metabolite abnormalities in progressive multifocal leukoencephalopathy by proton magnetic resonance spectroscopy. Neurology 1997; 48:836-845Crossref, Medline, Google Scholar
35 Giri JA, Gregoresky J, Silguero P, et al: Polyoma virus JC DNA detection by polymerase chain reaction in CSF of HIV infected patients with suspected progressive multifocal leukoencephalopathy. American Clinical Laboratory 2001; 20:33-35Medline, Google Scholar
36 De Luca A, Giancola ML, Ammassari A, et al: The effect of potent antiretroviral therapy and JC virus load in cerebrospinal fluid on clinical outcome of patients with AIDS-associated progressive multifocal leukoencephalopathy. J Infect Dis 2000; 182:1077-1083Crossref, Medline, Google Scholar
37 Garcia de Viedma D, Diaz Infantes M, Miralles P, et al: JC virus load in progressive multifocal leukoencephalopathy: analysis of the correlation between the viral burden in cerebrospinal fluid, patient survival, and the volume of neurological lesions. Clin Infect Dis 2002; 34:1568-1575Crossref, Medline, Google Scholar
38 Miralles P, Berenguer J, Lacruz C, et al: Inflammatory reactions in progressive multifocal leukoencephalopathy after highly active antiretroviral therapy. AIDS 2001; 15:1900-1902Crossref, Medline, Google Scholar
39 Razonable RR, Aksamit AJ, Wright AJ, et al: Cidofovir treatment of progressive multifocal leukoencephalopathy in a patient receiving highly active antiretroviral therapy. Mayo Clin Proc 2001; 76:1171-1175Crossref, Medline, Google Scholar
40 Clifford DB, Yiannoutsos C, Glicksman M, et al: HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999; 52:623-625Crossref, Medline, Google Scholar
41 Segarra-Newnham M, Vodolo KM: Use of cidofovir in progressive multifocal leukoencephalopathy. Ann Pharmacother 2001; 35:741-744Crossref, Medline, Google Scholar



