Effects of Depressed Mood on Objective and Subjective Measures of Attention
Abstract
People with depression report frequent cognitive failures, but objective measures of cognition show mixed results. Some studies show impairment on effortful tasks. The relationship between subjective and objective cognitive failures was studied in 102 “depressed” or “nondepressed” UK servicemen, grouped by Beck Depression Inventory scores with a cutoff score of 10. Participants were administered cognitive tests including the Sustained Attention to Response Task (SART), a laboratory measure of vigilance that has revealed increased attentional lapses in traumatic brain injury patients. The depressed men made more errors on SART than the nondepressed men (P=0.012) but reported much higher incidences of cognitive failures on a standardized questionnaire (P=0.0001). The depressed men's SART reaction times slowed following an error, a pattern different from that of brain-injured subjects. Nonclinical depressed subjects may respond “catastrophically” to errors, heightening the subjective sense of failure and contributing to the strong relationship between subjectively reported cognitive failures and depression.
Complaints of concentration and memory difficulties among people with depression have been well documented. An extensive review of cognitive processing in depression concluded that as people become more depressed, they engage in fewer processes that require attention.1 Depression therefore interferes specifically with effortful processing,2 particularly retrieval,3 and only at its most severe are automatic processing abilities affected.4
Over and above these objective deficits, people with depression invariably complain of concentration and memory problems.5 Depressed patients, including those with chronic fatigue, show a marked discrepancy between subjectively rated poor performance and objective psychometric measures.6,7 This could be because such people negatively appraise their performance (which may be adequate), or because depression causes a noticeable decline in performance, or a combination of the two. The relationship between depression and subjective and objective cognitive problems therefore requires further analysis.
The Cognitive Failures Questionnaire (CFQ)8 was introduced as a valid self-report measure of everyday slips of action. Mahoney et al.9 found that self-reported cognitive failures on the CFQ were strongly related to stress and anxiety, and Wagle et al.10 demonstrated that CFQ scores were similar across inpatients with neuropsychiatric conditions and those with “functional” psychiatric disorders. A new laboratory paradigm that quantifies attentional errors or slips of action, of the sort noted by people with depression, has been described by Robertson et al.11 The SART (Sustained Attention to Response Task) is a continuous performance paradigm in which subjects are required to make frequent responses to nontargets and to withhold a response to rarely presented targets. Robertson et al.11 argued that “slips of action” are the result of brief lapses in sustained attention, defined as “mindful, conscious processing of stimuli” despite their “repetitive, nonarousing qualities.” They found an increase in such slips in subjects with traumatic brain injury in comparison to healthy control subjects, which correlated highly with CFQ scores in both groups of subjects. As well as inappropriate responses, a significant speeding up of reaction times was noted11 in trials leading up to an error, suggesting that errors could be predicted on the basis of performance characteristics. In addition, the brain injury group, unlike control subjects, did not show a significant slowing down of reaction time after an incorrect response was made, indicating possible differences in response style or error monitoring that may have implications for behavior in everyday life.
In this study we measured performance on the SART and its relationships to self-reported cognitive errors on the CFQ,8 ratings of depression on the Beck Depression Inventory (BDI),12 and other cognitive measures in subjects drawn from an ongoing study of military personnel. We predicted that measures of depressed mood, subjective cognitive failures, and objective attentional performance would correlate. In particular, we hypothesized that subjects scoring above a cutoff for mild depression on BDI would make significantly more errors on the SART than subjects scoring below the cutoff. However, we also hypothesized that the strongest association would be between CFQ and BDI scores (and not between SART error scores and BDI scores).
In addition, we sought to explore the pattern of response times in trials before and after errors of omission, compared with response times before correctly withheld responses, in depressed and nondepressed subjects, and to see whether this differed in comparison to the brain-injured patients reported by Robertson et al.11 Such a difference, if uncovered, would imply differing mechanisms of cognitive failures in “functional” and “organic” conditions.
METHODS
Subjects
The subjects of this report were the first 102 males recruited for the second phase of a study investigating the health of UK military personnel. The first phase required subjects to return a postal questionnaire that asked about military experiences including deployment, exposures, illnesses, and symptoms.13 Respondents were classified as well (higher scores) or impaired on the basis of their score on a global measure of physical functioning, the SF-36.14 A random sample of people scoring above and below the cutoff (with those below being oversampled) was then recruited for the second phase. Subjects in this phase were asked to take part in both a medical and a neuropsychological assessment. Approximately half were currently serving in the military. All had served either in the 1991 Gulf War or as peacekeepers in the 1992 Bosnia conflict (or both) or were serving at the time but were deployed to neither theater. Ages ranged between 22 and 58 years.
For the purpose of this study, the subjects were divided into two groups according to whether their depression scores were above or below the standard cutoff for mild depression (10 on the BDI) as recommended by Beck and Steer.12 A cross-tabulation of physical functioning and depression ratings showed no significant findings within this group of subjects; therefore physical functioning (i.e., whether subjects were “well” or “impaired”) was not considered as a factor within this study.
Subjects completed a battery of neuropsychological tests lasting approximately 2.5 hours, which included the measures described below.
Attentional Measures
Sustained Attention to Response Task (SART).11 A computer-administered task in which 225 single digits are presented visually over 4.3 minutes. Each digit is presented for 250 ms followed by a 900-ms mask. Subjects are asked to respond by pressing the mouse for each digit, except on 25 occasions when the number 3 is presented. Here they are asked to withhold a response. The target digit is distributed randomly throughout the 225 trials. Subjects were instructed to give equal importance to accuracy and speed during the task.
Paced Auditory Serial Addition Task (PASAT).15 Sixty single digits are presented auditorily (one digit per 2 seconds). Subjects are instructed to add each digit to the one that comes before it and to give their answer out loud. A score representing the number of correct answers divided by the total time for each trial was calculated.
Stroop Color-Word Test.16 Subjects are first presented with a “color” task, a series of 112 color names in four columns, printed in nonmatching colored ink, and instructed to read the words aloud as quickly and accurately as they can. The “color-word” task is then presented, in which the subject is instructed to say aloud the color of the ink that the word is printed in. Each task is timed and a “Stroop effect” calculated based on the difference in time for each task.
General Cognitive Measures
National Adult Reading Test (NART).17 A reading test of “irregular” words, which gives a stable estimate of premorbid intelligence that is resistant to the effects of psychopathology, including depression.18
Wechsler Adult Intelligence Scale–Revised (WAIS-R).19
Wechsler Memory Scale–Revised (WMS-R),20 Logical Memory and Paired Associates subtests (see Table 1 for subtests).
Cognitive Failures Questionnaire (CFQ).8 A self-report measure of failures in perception, memory, and motor function.
Measure of Psychopathology
Beck Depression Inventory (BDI).12 A self-report questionnaire.
RESULTS
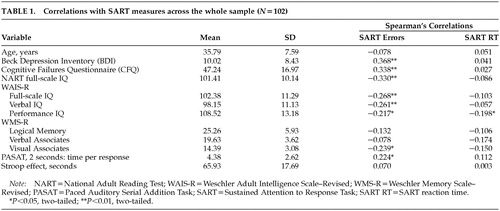
Table 1 shows the means and standard deviations for each variable, and also the relationship of each variable with SART error rate and reaction time (RT) across the whole sample. “SART errors” are the number of errors of commission, that is, pressing in response to a “3” (25 trials), and “SART reaction time” is the overall reaction time for correct presses when digits other than “3” are presented (200 trials). Because SART performance variables were not normally distributed, and because of the unequal group numbers, we used nonparametric statistics for between-group and within-group comparisons and correlations.
SART error rate was more strongly related than SART reaction time to other general cognitive and attentional variables (Spearman's correlations). Correlation coefficients were modest. Age did not significantly correlate with either SART measure. As anticipated, the results show a significant positive correlation between CFQ ratings and error rate on the SART, whereby people who report more attentional failures also make more errors of commission on the SART.
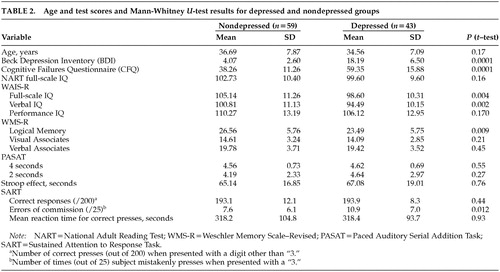
Table 2 shows the comparison of cognitive performance measures for the nondepressed and depressed groups. Subjects scoring less than 10 on the BDI were assigned to the nondepressed group (n=59), and subjects scoring 10 or above were assigned to the depressed group (n=43). The table shows that the depressed group scored significantly higher on the CFQ (i.e., had more failures) and scored significantly lower on the WMS-R Logical Memory immediate recall subtest (i.e., had poorer memory). Although the differences in NART-predicted IQ were not significant, the depressed group showed significantly lower WAIS-R full-scale and verbal IQ scores.
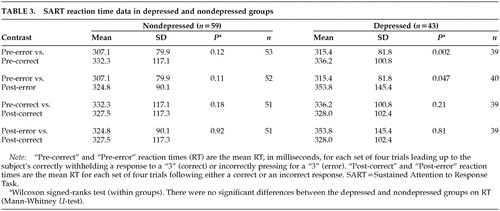
Also shown in Table 2 is the breakdown of depressed and nondepressed group scores for all the SART measures. It is clear that error rate is the only dependent variable that distinguishes the two groups, with subjects scoring above a cutoff for mild depression making more errors of commission (failures to withhold a press).
Figure 1 shows that both the depressed and nondepressed groups tended to speed up their RT in the trials leading up to an error, compared with the trials leading up to a correct response. This difference is significant in the depressed group only. The graph also illustrates that the depressed group showed a significant slowing of RT after an error response (data shown in Table 3).
It was hypothesized that there would be a significant relationship between level of depression and self-reported attentional failures in everyday life. A very high correlation was found (Pearson's r=0.739, P<0.001; Spearman's rho=0.713, P<0.001), indicating that people with higher ratings of depression also rate themselves as having higher incidences of cognitive failure. In the light of such a high correlation coefficient between CFQ and BDI, further analyses were carried out to explore the relationship between these variables and SART error rate.
In Figure 2, the gridlines show the median split for each variable (SART errors=9, CFQ=45). The pattern displayed suggests that although depressed people report many more cognitive failures, this is not necessarily affecting their error rate on the SART. This disjunction receives some support statistically from Spearman's rho correlation coefficient. In the nondepressed group, SART errors correlate with CFQ at 0.28 (P<0.05), but in the depressed group, the same contrast in the same direction fails to reach statistical significance (rho=0.20, not significant). Inspection of the figure suggests that the depressed group had high CFQ scores (i.e., they overestimated their failures) despite only mildly impaired performance. Further analysis using multiple regression revealed that the relationship between CFQ score and SART became nonsignificant (partial correlation coefficient=0.115, P=0.26) when BDI score was included in the model. BDI emerged as the only significant predictor of SART (beta=0.31, SE=0.076, P=0.002).
DISCUSSION
We carried out tests of attention on a nonclinical sample of people with and without symptoms of depression and related these to subjective reports of cognitive failures. The sample was drawn from men who were serving in the UK armed forces in 1991-1992, half of whom are still serving. The mean age of the sample was mid-thirties and mean IQ was around 100. Hence while the homogeneity of the group may be an advantage, it may not be representative of the general population. Nevertheless, the sample does enable inferences to be drawn about the cognitive underpinnings of subjective and objective attentional difficulties in people with mild to moderate depression.
Depression, defined in terms of the conventional albeit somewhat arbitrary cutoff on the BDI, was associated with significant differences on cognitive measures, including most subtests of WAIS-R and the Logical Memory subtest of the WMS-R. This result is consistent with other work on both ambulant and hospitalized depressed patients,21,22 and in particular supports the finding that effortful free-recall tasks are more affected by depression than learning or recognition tasks.3
The whole group showed a greater performance IQ than verbal IQ, perhaps due to lower educational opportunities in some servicemen. Extensive normative data on the SART are so far lacking, but studies on high-IQ and low-IQ normal control subjects do not show significant differences.23 We used three main attentional measures: the PASAT, the Stroop Color-Word Test, and the SART. Errors on the SART, presumed to reflect lapses in sustained attention, were the only measure to differ significantly across depressed and nondepressed subjects, although PASAT measures showed a weak correlation with BDI scores. The SART differs from the PASAT in having a minimal working memory component and a simple motor response. It is possible that more striking deficits in PASAT and other measures occur with more severe disturbances such as those seen in clinical cases of depression and mental fatigue.24 It is important to note here that replication of these findings with a clinically defined group of depressed patients would be an important next step.
One of our aims was to examine whether the pattern of performance in our depressed subjects on the SART differed from that of patients with traumatic brain injury, the group on which the SART was validated. The level of errors in the current sample was highly comparable with the brain injury group studied by Robertson et al.11 The quickening in response time prior to errors of commission was also similar. However, the depressed group showed a pattern not seen in the brain injury group, namely a slowing in RT following an error. This may be regarded as an accentuation of the pattern observed in the control subjects and could be interpreted as a form of “catastrophic” reaction. A similar pattern has been recently observed in depressed patients25 and is very unlike the pattern in brain-injured subjects, who appear not to be affected by their equally poor performance.
This finding is important for two reasons. First, it suggests a different profile of responding from the “organic”patients, contrary to the position reached by Mialet et al.,26 namely that the cognitive deficits seen in depression are nonspecific and do not differentiate from other psychiatric and neurological disorders. Second, it may in part explain the strong relationship between self-reported failures (particularly those measured by the CFQ) and depression noted here and by others.10,27–29 That is, an error or “slip” activates a strong negative reaction, which then leads to additional recruitment of attentional resources and hence a heightened sense of subjective effort. Depression appears to impair sustained attention sufficiently to lead to increased lapses, yet it does not impair accurate monitoring of such lapses or an appreciation of their significance. If this situation generalized to everyday life, it is easy to see how a cycle of minor slips followed by increased concern and self-monitoring and increased attention to performance, and hence further lapses, could ensue.
This proposed “vicious cycle” could also explain the maintenance of other disorders. One study of Persian Gulf War veterans in the United States found a pattern of subjective cognitive complaints and depression in the context of normal cognitive performance.30 Using a similar questionnaire to measure cognitive failures, the authors found that subjective impairment correlated with BDI scores at r=0.58, whereas it correlated with objective cognitive measures at r<0.3.
In summary, we have found that mild to moderate depression, as measured by the BDI, can be associated with quantifiable deficits in sustained attention of a similar order to those found in traumatic brain injury. The pattern of responding, however, is different from such cases in that post-error slowing is a distinctive feature, perhaps indicating increased awareness of errors. This heightened awareness may help to explain the other finding in this group, namely that subjective reports of cognitive failures were very strongly related to mood and rather weakly related to objective performance.
ACKNOWLEDGMENTS
The authors are grateful to Tom Manly and Ian Robertson for expert advice regarding the SART. This study was funded by the U.S. Department of Defense.
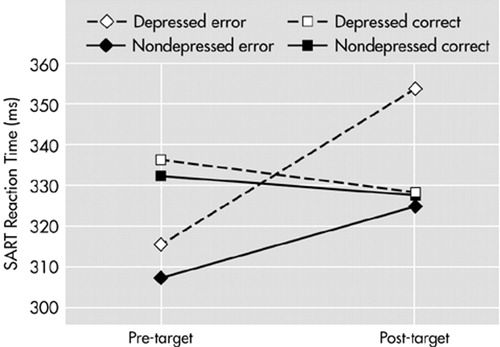
FIGURE 1. Sustained Attention to Response Task (SART) reaction time in milliseconds before and after target presentations, for correct and error responses to target.
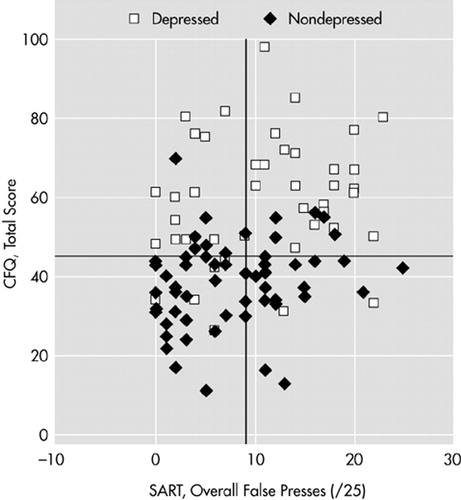
FIGURE 2. Correlation between Sustained Attention to Response Task (SART) errors and the Cognitive Failures Questionnaire (CFQ) within depressed and nondepressed groups. Median scores indicated by lines.
 |
 |
 |
1 Hartlage S, Alloy LB, Vazquez C, et al: Automatic and effortful processing in depression. Psychol Bull 1993; 113:247-278Crossref, Medline, Google Scholar
2 Veiel HO: A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol 1997; 19:587-603Crossref, Medline, Google Scholar
3 Massman PJ, Delis DC, Butters N, et al: The subcortical dysfunction hypothesis of memory deficits in depression: neuropsychological validation in a subgroup of patients. J Clin Exp Neuropsychol 1992; 14:687-706Crossref, Medline, Google Scholar
4 Weingartner H, Cohen RM, Murphey DL: Cognitive processes in depression. Arch Gen Psychiatry 1981; 38:42-47Crossref, Medline, Google Scholar
5 Kahn RL, Zarit SH, Hilbert NM, et al: Memory complaint and impairment in the aged: the effect of depression and altered brain function. Arch Gen Psychiatry 1985; 32:1569-1573Crossref, Google Scholar
6 Cope H, Pernet A, Kendell B, et al: Cognitive functioning and magnetic resonance imaging in chronic fatigue. Br J Psychiatry 1995; 167:86-94Crossref, Medline, Google Scholar
7 Weardon AJ, Appleby L: Research on cognitive complaints and cognitive functioning in patients with chronic fatigue syndrome (CFS): what conclusions can we draw? J Psychosom Res 1996; 41:197-211Crossref, Medline, Google Scholar
8 Broadbent DE, Cooper PF, Fitzgerald P, et al: The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 1982; 21:1-16Crossref, Medline, Google Scholar
9 Mahoney AM, Dalby JT, King MC: Cognitive failures and stress. Psychol Rep 1998; 82:1432-1434Crossref, Medline, Google Scholar
10 Wagle AC, Berrios GE, Ho L: The Cognitive Failures Questionnaire in psychiatry. Compr Psychiatry 1999; 40:478-484 Crossref, Medline, Google Scholar
11 Robertson IH, Manly T, Andrade J, et al: “Oops!”: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia 1997; 35:747-758Crossref, Medline, Google Scholar
12 Beck AT, Steer RA: Beck Depression Inventory. San Antonio, TX, The Psychological Corporation, 1993Google Scholar
13 Unwin C, Blatchley N, Coker W, et al: Health of UK servicemen who served in the Persian Gulf War. Lancet 1999; 353:169-182Crossref, Medline, Google Scholar
14 Ware JE Jr, Snow KK, Kosinski M, et al: SF-36 Health Survey: Manual and Interpretation Guide (B1-B5). Boston, The Health Institute, New England Medical Center, 1993Google Scholar
15 Gronwall DMA, Wrightson P: Delayed recovery of intellectual function after minor head injury. Lancet 1975; ii(7874):1452Google Scholar
16 Trenerry MR, Crosson B, Deboe J, et al WR: Stroop Neuropsychological Screening Test. Lutz, FL, Psychological Assessment Resources, 1989Google Scholar
17 Nelson HE, Willison J: National Adult Reading Test (NART), 2nd ed. Windsor, UK, NFER-Nelson, 1991Google Scholar
18 Crawford JR, Besson JA, Parker DM, et al: Estimation of premorbid intellectual status in depression. Br J Clin Psychol 1987; 26:313-314Crossref, Medline, Google Scholar
19 Wechsler DA: Wechsler Adult Intelligence Scale-Revised. New York, The Psychological Corporation, 1981Google Scholar
20 Wechsler D: Wechsler Memory Scale-Revised Manual. San Antonio, TX, The Psychological Corporation, 1987Google Scholar
21 Brown RG, Scott LC, Bench CJ, et al: Cognitive function in depression: its relationship to the presence and severity of intellectual decline. Psychol Med 1994; 24:829-847Crossref, Medline, Google Scholar
22 Purcell R, Maruff P, Kyrios M, et al: Neuropsychological function in young patients with unipolar major depression. Psychol Med 1997; 27:1277-1285Crossref, Medline, Google Scholar
23 Manly T, Robertson IH, Galloway M, et al: The absent mind: further investigations of sustained attention to response. Neuropsychologia 1999; 37:661-670Crossref, Medline, Google Scholar
24 Johnson SK, Lange G, DeLuca J, et al: The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis and depression. Appl Neuropsychol 1997; 4:145-153Crossref, Medline, Google Scholar
25 Elliott R, Sahakian BJ, McKay AP, et al: Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med 1996; 26:975-989Crossref, Medline, Google Scholar
26 Mialet JP, Pope HG, Yurgelun-Todd D: Impaired attention in depressive states: a non-specific deficit? Psychol Med 1996; 26:1009-1020Crossref, Medline, Google Scholar
27 Merckelbach H, Muris P, Nijman H, et al: Self-reported cognitive failures and neurotic symptomology. Personality and Individual Differences 1996; 20:715-724Crossref, Google Scholar
28 Van Gorp WG, Satz P, Hinkin C, et al: Metacognition in HIV-1 seropositive asymptomatic individuals: self-ratings versus objective neuropsychological performance. J Clin Exp Neuropsychol 1992; 13:812-819Crossref, Google Scholar
29 Gass CS, Apple C: Cognitive complaints in closed-head injury: relationship to memory test performance and emotional disturbance. J Clin Exp Neuropsychol 1997; 19:290-299Crossref, Medline, Google Scholar
30 Binder LM, Storzbach D, Anger W, et al: Subjective cognitive complaints, affective distress, and objective cognitive performance in Persian Gulf War veterans. Arch Clin Neuropsychol 1999; 14:531-536Crossref, Medline, Google Scholar



