Nulliparity and Late Menopause Are Associated With Decreased Cognitive Decline
Abstract
Changes in Mini-Mental State Examination (MMSE) scores were examined over a median of 12.8 years in a population of 361 community-dwelling postmenopausal women who had never received estrogen replacement therapy. In a linear regression model that took into account age, education, race, surgical versus natural menopause, use of birth control pills, and MMSE score at baseline, it was found that nulliparous women and women who went through menopause later in life had significantly less cognitive decline. These results suggest that greater lifetime exposure to endogenous estrogen may be associated with less age-related cognitive decline.
Reproductive events such as menarche, pregnancy, and menopause influence a woman's risk for a number of diseases. For example, the incidence of breast cancer is higher in women who have longer estrogen exposure due to early menarche, late menopause, or nulliparity.1,2 Conversely, a naturally high exposure to estrogen over a lifetime may decrease the chance of developing osteoporosis.3 Some studies also indicate that estrogen influences the risk for age-related diseases of the central nervous system. A large body of basic science research has provided possible mechanisms by which estrogen might influence brain aging. These include modulation of acetylcholine4 and other brain neurotransmitters, enhanced dendritic sprouting,5 interaction with trophic factors,6 prevention of cerebral ischemia,7 protection against oxidative stress,8 and modulation of lipoprotein isoforms.9 Clinical studies of estrogen replacement therapies have shown mixed results, with the two published prospective studies demonstrating a reduced risk of Alzheimer's disease (AD) with hormone therapy10,11 and retrospective studies yielding mixed results.12–15 Current data do not support the use of hormone replacement therapy (HRT) in the treatment of AD.16–19 Studies of HRT on age-related decline in particular cognitive domains have also produced mixed results,20–23 with positive studies showing an appreciably consistent effect on verbal memory.21,23–25
Little is known about the effects of endogenous estrogens on cognition across the lifespan. A few studies have assessed the risk of developing Alzheimer's disease associated with reproductive events that affect lifetime endogenous estrogen exposure. One study showed a significantly increased risk of Alzheimer's disease associated with advanced age at menarche, but not advanced age at menopause.26 Another found the opposite pattern of results,13 and two others found no effect for either factor.11,27 Studies of the effect of reproductive factors on cognition in nondemented postmenopausal women have involved small samples (i.e., around 100 women), and suggest that reproductive events associated with higher lifelong estrogen exposure may be associated with enhanced cognition. Two of the studies reported that early menopause is associated with poorer cognitive function,28,29 and one also reported that earlier menarche is associated with enhanced cognitive functioning.29 To our knowledge, however, there are no reports on the effects of reproductive events on longitudinal change in cognitive function among postmenopausal women.
We investigated the hypothesis that reproductive events indicative of increased endogenous estrogen exposure would be associated with less decline in cognitive function. Specifically, we examined the effects of nulliparity, age at menopause, use of oral contraceptive pills, and surgical menopause on change in Mini-Mental State Examination (MMSE) score after a median of 12.8 years in a community-based sample of 361 postmenopausal women. To control for the effects of exogenous estrogens, we included only postmenopausal women who had never received estrogen replacement therapy (ERT). The sample was drawn from a population of adult household residents of East Baltimore surveyed in 1981 and between 1993 and 1996 as part of the Epidemiologic Catchment Area study (ECA). We hypothesized that nulliparity and later age at menopause would be associated with less cognitive decline.
METHOD
Subjects
Participants were selected from the general population of residents of East Baltimore who were surveyed systematically in the Baltimore arm of the ECA study.30 The original goals of the ECA study were to investigate the epidemiology of mental disorders, including cognitive decline, in the general population. Wave 1 was performed in 1981, wave 2 in 1982, and wave 3 between 1993 and 1996. The ECA program and its Baltimore arm have been described in detail elsewhere.30,31 The average age and education for the 1,920 participants in wave 3 of the Baltimore arm of the ECA were 53.5±16.3 (SD) years and 11.6 ± 2.6 years, respectively; 37% were men, 34.7% black, and 65.3% nonblack (mostly white, non-Hispanic). From an original sample of 1,198 female participants in the wave 3 Baltimore arm of the ECA, 448 women were not postmenopausal, 182 had used estrogens, and 256 did not complete the MMSE at waves 1 and 3. Data from these individuals were excluded. The implications of loss to follow-up have been discussed elsewhere.31 The final sample consisted of 361 women, with a mean age at wave 3 of 63.4 ± 14.3 years (range, 31 to 94 years) and 10.5 ± 2.7 years of education (range 0 to 17 years). The sample was 34.9% black. The mean age at menopause was 43.4 ± 8.9 years; 44.6% of the women had surgical menopause, and 25.8% had used oral contraceptives. Thus, the racial composition of the study group and the original ECA group were similar, but the final sample was older (63.4 years vs. 53.5 years), and had less education (10.5 years vs. 11.6 years).
Measures of Cognitive Function and Decline
The MMSE, a commonly used test of cognitive function,32 was administered to participants at waves 1 and 3 of the ECA survey. The MMSE is a list of 30 questions and tasks, covering orientation to time and place, word registration and recall, attention and calculation, language, and visual construction. The test normally takes 5 to 10 minutes to administer. The MMSE is one of the tests recommended by the National Institute of Neurological Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association to document the diagnosis of Alzheimer's disease, but it is not considered sufficient to serve as the sole criterion for determining dementia.33 Construct validation studies demonstrate that MMSE scores are highly correlated with scores obtained from other tests of cognitive function.33 The MMSE has been used as a measure to track cognitive decline in large populations, including that of the Baltimore ECA.30,31 These studies demonstrated that changes in MMSE are influenced by multiple factors, including education, race, and baseline MMSE score.31 Trained interviewers administered the MMSE in the homes of the interviewees. The median time between exams was 12.8 years. Difference scores (DMMSE) were calculated by subtracting the score on the MMSE at wave 1 from the MMSE at wave 3.
Aspects of Reproductive Function
In wave 3, participants were asked several questions about their reproductive history, including whether they had given birth to a live infant, their age at menopause, whether they underwent surgical menopause, whether they used oral contraceptives, and whether they received ERT. Not recorded were type of oral contraceptive, type of surgery, and information about pregnancies that did not result in a live birth. For descriptive purposes, age at menopause was divided into three groups, with menopause occurring at ages 40 and below, ages 41 to 50, and ages 51 and older.
Sociodemographic Variables
Information about sociodemographic variables associated with cognitive decline was recorded at wave 1.31 For descriptive purposes, age at wave 3 was grouped as follows: 18–30, 31–40, 41–50, 51–60, 61–70, and 71 and up. Race was divided into black and nonblack; the sample was mostly white non-Hispanics. Education was categorized into five groups by years of education according to common educational landmarks: grade school (0–8 years), some high school (9–11 years), completed high school or the equivalent (12 years or a General Equivalency Diploma [GED]), some college (13–15 years), and completed college (16 years or more).
Analyses
A linear stepwise regression model was estimated, with an inclusion criterion of P<0.05. The dependent variable was dMMSE, and the independent variables were age, race, educational level, MMSE score at wave 1, history of live births, oral contraceptive use, age at menopause, and whether menopause was caused by surgery. Age and age at menopause were coded as continuous variables. Race was coded 0 for nonblack and 1 for black. Education was coded 1 through 5, corresponding to the five education levels described above. Use of oral contraceptives, whether a participant had given birth, and whether menopause was induced by surgery were each coded either 1 for no or 2 for yes. A similar regression analysis was conducted for MMSE score at wave 3. Cofactors that were shown to be significant in the regression equations were entered into linear models to yield adjusted MMSE and dMMSE scores. Calculations were made for two-tailed RESULTS.
Results
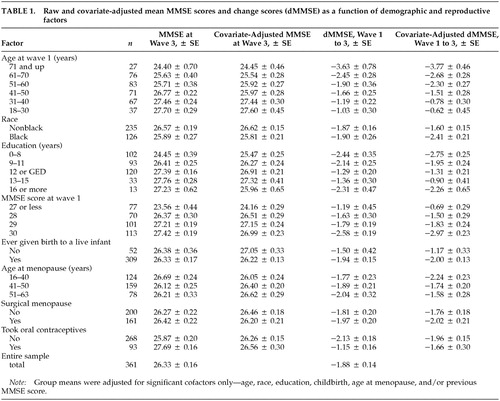
Table 1 lists the mean MMSE score at wave 3, the mean dMMSE, and the MMSE and dMMSE scores adjusted for age, race, education, childbirth status, age at menopause, and previous MMSE score (dMMSE only) for each of the subgroups.
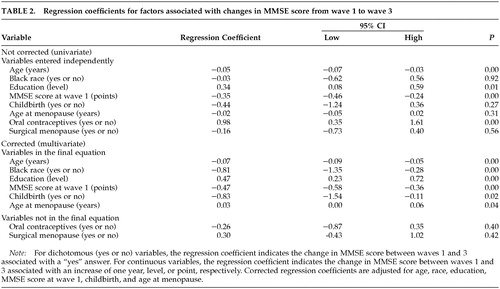
Several variables were associated with changes in MMSE scores at follow-up. A stepwise linear regression model was estimated that was significantly (F=20.27, P<0.0001) associated with dMMSE. Multiple R for the model was 0.51, and the adjusted R2 was 0.24. Table 2 lists the variables that independently were associated with change in MMSE as well as the variables that were associated with the change after adjustment for the other variables in the equation. As shown in the table, having given birth to a live infant, earlier menopause, older age, lower education, black race, and higher MMSE scores at wave 1 were associated with a significantly greater decline in MMSE score between wave 1 and wave 3. Use of oral contraceptives and surgical menopause were not significant predictors of dMMSE.
DISCUSSION
We investigated the effects of reproductive factors indicative of increased lifelong endogenous estrogen exposure—age at menopause, nulliparity, oral contraceptive use, and surgical menopause—on longitudinal change in MMSE scores in a community-based sample of 361 postmenopausal women who had never received ERT. The primary findings of interest were that late menopause and nulliparity were associated with less cognitive decline. These results suggest that in addition to affecting a woman's risk for such age-related diseases as osteoporosis and breast cancer, certain reproductive events may influence age-related changes in the central nervous system.
To our knowledge, these are the first published data demonstrating a relationship between parity and cognitive decline. One previous investigation examined parity and cognition in a sample of 87 nondemented postmenopausal women from an Alzheimer's disease research center, 57% of whom were receiving estrogen replacement.29 A composite measure of global cognitive functioning was significantly correlated with an index score of endogenous and exogenous estrogen exposure derived from a variety of reproductive factors and medication history.29 No relationship was observed between parity and MMSE score or any other individual cognitive measure, although the authors noted a negative association between age at menopause and a measure of working memory. Our study had greater statistical power to detect an effect of parity and excluded women receiving ERT, which may modulate any detrimental effects of parity on cognitive function.
A relationship between age at menopause and cognitive function has been observed previously in younger samples of women. A study of 103 patients (mean age, 52 years) who were referred to an endocrine clinic for menopausal complaints found evidence of poorer verbal memory associated with early age at menopause and with surgical menopause.28 Verbal memory was negatively associated with years since surgery and positively associated with age at surgery. Another study showed that cognitive performances in childhood and at age 43 were positively associated with age at menopause.34
The findings presented here suggest that age at menopause predicts the rate of cognitive decline later in life, after age 60. Although we found no evidence of cognitive decline associated with surgical menopause in our older sample of women, we observed the largest declines in cognitive function among women who were ages 16–40 at menopause, almost all of whom underwent surgical menopause. This suggests that early surgical menopause, at least in the absence of estrogen replacement, may be associated with poorer cognitive outcomes later in life.
In this study, the effect of age at menopause became clear only after adjustment for age, education, oral contraceptive use, and other factors. Without these adjustments, early menopause was more closely, although not significantly, associated with less cognitive decline. However, this appears to be an artifact of our data. On average, the women in our sample who underwent menopause later were older at the time of the follow-up assessment than their counterparts who underwent earlier menopause. (The mean ages for the three groups with early, normal, and late menopause were 55.2 ± 1.4, 66.9 ± 1.0, and 69.3 ± 1.1 years, respectively.) Exposure to estrogen has been shown to increase life expectancy.35 The effect of age at menopause on life expectancy was not the subject of this study, but the greater average age of the late-menopause group might reflect the benefits of increased estrogen exposure for these women. Other explanations are, of course, also possible.
Both age at menopause and nulliparity were associated with approximately an excess 1-point drop in MMSE performance over an average interval of 12.8 years. A 1-point change is considered small and of negligible clinical importance at the individual level, but at the population level the change reflects a sum of normal and abnormal age-related changes. Detectable changes in MMSE scores at the population level are moderately to highly specific predictors of impairment.33 In our study, however, only total MMSE scores were examined. Some authors have suggested that in normal individuals, the most common errors in the MMSE occur in recall, calculation, and orientation to time. Impairments in language items may be more useful in distinguishing between normal subjects and those with dementia.33
We were unable to differentiate cognitive decline that was due to normal age-related changes from that due to dementia because formal diagnoses of dementia were not made. The effects of nulliparity and age at menopause on dementia are uncertain. Previous studies of the effects of reproductive factors on risk for Alzheimer's disease have produced mixed results.10–15 Additional research is needed to clarify whether measurable and statistically significant changes in dMMSE associated with age at menopause and nulliparity signify normal or abnormal age-related changes or a combination of the two.
Data for this study were taken from responses in a study commissioned primarily for the purposes of studying mental health.30 Thus, the questions asked were often geared toward psychosocial aspects of the participants' lives rather than to record a detailed reproductive history. Many of the hormone-related questions that one would ideally like answered, such as age at menarche, pregnancies that did not result in live births, menstrual cycle history, symptoms at menopause, types and effects of reproductive surgery, abortion, lactation, and other possible confounders that could influence hormonal function, were not part of the interview. Age at menopause was judged by self-reports rather than medical records, and no hormone concentrations were measured. Thus, given the number of potential confounders, no definitive conclusions can drawn about why nulliparity and late menopause were associated with less cognitive decline in this population. However, the findings of this study did fit with predictions based on the effects of endogenous estrogens, and thus it is worth speculating on how endogenous hormones might be involved.
Menopause is associated with drops in levels of estrogens, progestins, and androgens, with simultaneous alterations in receptors and in the ratios of these classes of hormones.36–38 Although confounding characteristics, such as health factors, of women undergoing surgical menopause at a young age may account for the observed cognitive decline, demonstrations of improved memory among surgical menopausal women after treatment with ERT24,39 suggest that the relationship could be mediated by estrogen deficiency. Data on ERT in postmenopausal women have been inconsistent, however. If endogenous estrogen were the critical factor underlying the relationship observed in our study between age at menopause and cognitive decline, this might indicate that the lifetime exposure to the hormone, rather than just the postmenopausal replacement, is critical for the neuroprotective effects.
The hormonal changes in pregnancy and childbirth are more complex than those in menopause. A marked increase in estrogen level occurs during pregnancy, followed by a decline after childbirth that lasts about one year; lactation further decreases estrogen levels.40 High progesterone levels antagonize many estrogenic effects during pregnancy.41,42 Parity influences estrogen levels later in life.43–45 During the follicular phases of the menstrual cycle nulliparous women produce higher estrogen levels than parous women.40,43 The difference in absolute levels of estrogen continues after menopause.45 Overall, the effect of nulliparity seems to be a higher lifetime exposure to estrogen, which could provide one possible explanation for the effects seen on cognitive decline.
The question of which, if any, hormonal changes underlie the effects we observed will require further study. Some success has been achieved in combining a history of estrogen-altering events into indices29 and using other physiologic phenomena known to be influenced by estrogen, such as bone mineral density,28 to predict cognitive ability. Studies that provide both detailed reproductive histories and good longitudinal data on cognitive decline are lacking, however. This study provides an important next step toward the understanding of how reproductive events, hormonal concentrations, and cognitive function are interrelated. Hopefully these findings will spur investigations in which detailed reproductive histories and careful measurements of endogenous hormone levels can be correlated with cognitive changes.
One final note should be made about the possible social implications of these findings. This study reports on an aspect of reproductive function over which women have some degree of control, namely, having children. Although we believe that women should be made aware of all the possible effects their decisions may have, we strongly caution against influencing reproductive choices on the basis of these findings. Our study was limited to postmenopausal community residents who had never received ERT. Study of this population is useful for examining the effects of the natural effects of estrogen; however, it is an unusual population in many respects, and with the increasing popularity of ERT as well as many other sociodemographic changes, this study population is unlikely to accurately represent the future of women currently in their childbearing years. Follow-up studies will be required to confirm the effects of reproductive events on cognitive decline in different populations before definitive conclusions can be drawn.
ACKNOWLEDGMENTS
Supported by grant 1RO1-MH-47447 for the Baltimore Epidemiologic Catchment Area study follow-up. The authors thank the American Federation of Aging Research for scholarship support and Jeannie-Marrie Sheppard, B.A., for analytic support.
 |
 |
1 Trichopoulos D, MacMahon B, Cole P: Menopause and breast cancer risk. J Natl Cancer Inst 1972; 48:605-613Medline, Google Scholar
2 Ramon JM, Escriba JM, Casas I, et al: Age at first full-term pregnancy, lactation, and parity and risk of breast cancer: a case-control study in Spain. Eur J Epidemiol 1996; 12:449-453Crossref, Medline, Google Scholar
3 Isaia GC, Di Stefano M, Ardissone P, et al: Senile osteoporosis: pathophysiology and therapeutic perspectives. Aging (Milan) 1997; 9:77-78Medline, Google Scholar
4 Luine V, Park D, Joh T, et al: Immunochemical demonstration of increased choline acetyltransferase concentration in rat preoptic area after estradiol administration. Brain Res 1980; 191:273-277Crossref, Medline, Google Scholar
5 Gould E, Woolley CS, Frankfurt M, et al: Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J of Neurosci 1990; 10:1286-1291Crossref, Medline, Google Scholar
6 Toran-Allerand CD, Miranda RC, Bentham WDL, et al: Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal forebrain. Proceedings of the National Academy of Sciences 1992; 89:4668-4672Crossref, Medline, Google Scholar
7 Gangar KF, Vyas S, Whitehead M, et al: Pulsatility index in internal carotid artery in relation to transdermal oestradiol and time since menopause. Lancet 1991; 338:839-842Crossref, Medline, Google Scholar
8 Mooradian AD: Antioxidant properties of steroids. J Steroid Biochem Mol Biol 1993; 45:509-511Crossref, Medline, Google Scholar
9 Srivastava RA, Bhasin N, Srivastava N: Apolipoprotein E gene expression in various tissues of mouse and regulation by estrogen. Biochem Mol Biol Int 1996; 38:91-101Medline, Google Scholar
10 Tang M, Jacobs D, Stern Y, et al: Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet 1996; 348:429-432Crossref, Medline, Google Scholar
11 Kawas C, Resnick S, Morrison A, et al: A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology 1997; 48:1517-1521Crossref, Medline, Google Scholar
12 Henderson VW, Paganini-Hill A, Emanuel CK, et al: Estrogen replacement therapy in older women. Arch Neurol 1994; 51:896-900Crossref, Medline, Google Scholar
13 Baldereschi M, Di-Carlo A, Lepore V, et al: Estrogen-replacement therapy and Alzheimer's disease in the Italian Longitudinal Study on Aging. Neurology 1998; 50:996-1002Crossref, Medline, Google Scholar
14 Brenner DE, Kukull WA, Stergachis A, et al: Postmenopausal estrogen therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol 1994; 140:262-267Crossref, Medline, Google Scholar
15 Broe GA, Henderson AS, Creasey H, et al: A case-control study of Alzheimer's disease in Australia. Neurology 1990; 40:1698-1707Crossref, Medline, Google Scholar
16 Henderson VW, Paganini-Hill A, Miller BL, et al: Estrogen for Alzheimer's disease in women. Neurology 2000; 54:295-301Crossref, Medline, Google Scholar
17 Mulnard RA, Cotman CW, Kawas C, et al: Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease. JAMA 2000; 283:1007-1015Crossref, Medline, Google Scholar
18 Wang PN, Liao SQ, Liu RS, et al: Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology 2000; 54:2061-2066Crossref, Medline, Google Scholar
19 Asthana S, Baker LD, Craft S: High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology 2001; 57:605-612Crossref, Medline, Google Scholar
20 Resnick SM, Metter EJ, Zonderman AB: Estrogen replacement therapy and longitudinal decline in visual memory: a possible protective effect? Neurology 1997; 49:1491-1497Crossref, Medline, Google Scholar
21 Jacobs DM, Tang MX, Stern Y, et al: Cognitive function in nondemented older women who took estrogen after menopause. Neurology 1998; 50:368-373Crossref, Medline, Google Scholar
22 Barrett-Connor E, Kritz-Silverstein D: Estrogen replacement therapy and cognitive function in older women. JAMA 1993; 269:2637-2641Crossref, Medline, Google Scholar
23 Maki P, Zonderman A, Resnick S: Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psych 2001; 158:227-233Crossref, Medline, Google Scholar
24 Sherwin BB: Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 1988; 13:345-357Crossref, Medline, Google Scholar
25 Sherwin BB: Can estrogen keep you smart? Evidence from clinical studies. J Psych Neurosci 1999; 24:315-321Medline, Google Scholar
26 Paganini-Hill A, Henderson VW: Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol 1994; 140:256-261Crossref, Medline, Google Scholar
27 Waring SC, Rocca WA, Petersen RC, et al: Postmenopausal estrogen replacement therapy and risk of AD. Neurology 1999; 52:965-970Crossref, Medline, Google Scholar
28 Nappi RE, Sinforiani E, Mauri M, et al: Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest 1999; 47:29-36Crossref, Medline, Google Scholar
29 Smith CA, McCleary CA, Murdock GA, et al: Lifelong estrogen exposure and cognitive performance in elderly women. Brain Cogn 1999; 39:203-218Crossref, Medline, Google Scholar
30 Eaton W, Kessler L: Epidemiologic Field Methods in Psychiatry: The NIMH Epidemiologic Catchment Area Program. Orlando, FL, Academic Press, 1985Google Scholar
31 Lyketsos CG, Chen LS, Anthony JC: Cognitive decline in adulthood: an 11.5-year follow-up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry 1999; 156:58-65Crossref, Medline, Google Scholar
32 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar
33 Tombaugh TN, McIntyre NJ: The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc 1992; 40:922-935Crossref, Medline, Google Scholar
34 Richards M, Kuh D, Hardy R, et al: Lifetime cognitive function and timing of the natural menopause. Neurology 1999; 53:308-314Crossref, Medline, Google Scholar
35 Zubialde JP, Lawler F, Clemenson N: Estimated gains in life expectancy with use of postmenopausal estrogen therapy: a decision analysis. J of Fam Pract 1993; 36:271-280Medline, Google Scholar
36 McEwen BS, Alves SE: Estrogen actions in the central nervous system. Endocr Rev 1999; 20:279-307Medline, Google Scholar
37 al-Azzawi F: Endocrinological aspects of the menopause. Br Med Bull 1992; 48:262-275Crossref, Medline, Google Scholar
38 Amso NN, Crow J, Shaw RW: Comparative immunohistochemical study of oestrogen and progesterone receptors in the fallopian tube and uterus at different stages of the menstrual cycle and the menopause. Hum Reprod 1994; 9:1027-1037Crossref, Medline, Google Scholar
39 Phillips SM, Sherwin BB: Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology 1992; 17:485-495Crossref, Medline, Google Scholar
40 Petrakis NL, Wrensch MR, Ernster VL, et al: Influence of pregnancy and lactation on serum and breast fluid estrogen levels: implications for breast cancer risk. Int J Cancer 1987; 40:587-591Crossref, Medline, Google Scholar
41 Bernard A, Duffek L, Torok I, et al: Progesterone and oestradiol levels and cytoplasmic receptor concentrations in the human myometrium at term, before labour and during labour. Acta Physiol Hung 1988; 71:507-510Medline, Google Scholar
42 Noci I, Borri P, Periti E, et al: Decidual progesterone and estrogen receptors in the first trimester of pregnancy. Ann N Y Acad Sci 1994; 734:26-32Crossref, Medline, Google Scholar
43 Bernstein L, Pike MC, Ross RK, et al: Estrogen and sex hormone-binding globulin levels in nulliparous and parous women. J Natl Cancer Inst 1985; 74:741-745Medline, Google Scholar
44 Dorgan JF, Reichman ME, Judd JT, et al: Relationships of age and reproductive characteristics with plasma estrogens and androgens in premenopausal women. Cancer Epidemiol Biomarkers Prev 1995; 4:381-386Medline, Google Scholar
45 Trichopoulos D, Brown J, MacMahon B: Urine estrogens and breast cancer risk factors among post-menopausal women. Int J Cancer 1987; 40:721-725Crossref, Medline, Google Scholar



