Regional Cerebral Blood Flow and Cognitive Deficits in Chronic Lyme Disease
Abstract
This study examined brain functioning in patients with Lyme encephalopathy. Eleven patients underwent neuropsychological tests and Xenon133-regional cerebral blood flow (rCBF) studies, using an external detector system. Each rCBF scan was age- and sex-matched to two archival, normal controls. While few differences were noted on gray-matter flow indices (ISI, fg), Lyme patients demonstrated significant flow reductions in white matter index (k2) (p=.004), particularly in the posterior temporal and parietal lobes bilaterally (p=.003). Flow reductions in white matter areas were significantly associated with deficits in memory (r=.66, p=.027) and visuospatial organization (r=.62, p=.041). Results suggest that Lyme encephalopathy may be a disease primarily affecting the cerebral white matter.
Caused by the tick-borne spirochete Borrelia burgdorferi, Lyme disease presents early as a flu-like illness that follows an erythema migrans rash. The disease presents later as a multisystem ailment that affects the joints, heart, muscles, and peripheral and central nervous system (CNS).1 A mild to severe encephalopathy is most common among patients who have persistent neurologic symptoms following infection with Borrelia burgdorferi. Encephalopathy in these patients is characterized by disturbances in memory, attention, verbal fluency, and processing speed, and it is often accompanied by irritability, fatigue, sensory hyperacuities, and sleep disturbance.2,3 Despite the marked disability associated with Lyme encephalopathy, little is known about whether the disease process affects primarily the cortical gray, subcortical gray, or cerebral white matter.
Although patients who have early central neurologic involvement usually show evidence of cerebrospinal fluid (CSF) abnormalities, as many as 20%–40% of patients with this particular manifestation of encephalopathy have no detectable spinal fluid abnormalities on routine analysis,4 despite active CNS infection. Magnetic resonance imaging (MRI) scans reveal punctate white matter lesions on T2 weighted images in approximately 50%–70% of the cases with Lyme meningitis, encephalitis, or encephalomyelitis;5,6 but the rate drops to 15%–40% of patients with Lyme encephalopathy.7,8 Low sensitivity of the CSF analysis and MRI scan in patients with chronic Lyme encephalopathy makes it difficult for the clinician who is seeking to distinguish cognitive problems that are secondary to a primary psychiatric disturbance from cognitive problems due to Lyme disease. Such a distinction is of critical importance in guiding the course of future treatment.
Since regional cerebral blood flow (rCBF) has been shown to be useful as a measure of global and focal flow deficits in Alzheimer’s disease,9,10 major depressive disorder,11 multi-infarct dementia, and Pick's disease,12 we explored the hypothesis that rCBF assessments might also be useful in furthering our understanding of the pathophysiological effects of Lyme disease on the brain. Subsequently, three specific questions were raised. First, do the perfusion patterns of patients with cognitive symptoms and a history of Lyme disease differ from age- and sex-matched controls? Second, if a perfusion deficit is observed, is it focal, or is it distributed more diffusely in the cerebral gray or white matter? Third, is there a correlation between neuropsychological deficits on objective testing and rCBF measures?
METHODS
Institutional review board approval was obtained for this study. All patients gave written informed consent.
Patients
Subjects with persistent cognitive problems after at least 4 weeks of IV antibiotic therapy for Lyme disease were recruited. All patients also were required to meet the following criteria: 1) exposure to a Lyme endemic area; 2) a history of physician-diagnosed erythema migrans and/or a positive serologic test for Lyme disease; 3) a history of clinical symptoms typical of Lyme disease affecting the cardiac, neurologic, and/or articular systems; and 4) current objective cognitive impairment. Because this study was designed prior to the establishment of the two-tiered serologic testing method recommended by the Centers for Disease Control (CDC) in 1995, our serologic criteria consisted of the prior CDC standard of either a reactive enzyme linked immunosorbent assay (ELISA) or a reactive Western blot. Patients were considered to have objective cognitive impairment if they met any of the following four criteria: 1) a 15-point (1 standard deviation) difference between the Wechsler Verbal Memory and Verbal Intelligence Quotient (IQ), between the Wechsler Visual Memory and Verbal (IQ), or between the Wechsler General Memory and the Total IQ (n=5);13,14 2) a Buschke Selective Reminding Test Total Recall or a Consistent Long-Term Retrieval score that was 1.5 SD or more below the published mean for that individual's age group (n=7);15 3) a Controlled Verbal Fluency Test score that was 1.5 SD or more below the published mean (n=2);16 and/or 4) an inability to complete at least four of six categories on the Wisconsin Card Sort (n=2).17 Despite having received what is generally recognized as adequate treatment for CNS Lyme Disease, all patients were considered to have persistent encephalopathy as demonstrated by objective cognitive impairment.
Controls
From our archival normal control rCBF database, two age- and sex-matched controls were selected for each case of Lyme disease. Controls had rCBF studies but not neuropsychological assessments.
Regional Cerebral Blood Flow Assessment
Regional Cerebral blood flow was assessed using the 133Xe-inhalation technique, with cortical counts obtained from 32 NaI (T1) scintillation detectors (16 per cerebral hemisphere), with the Novo Diagnostic Systems Cerebrograph, Model 32c.18 This method has a resolution of 26 mm on the cortical surface. All procedures were performed in a dark, silent room, with the subjects' eyes closed. All scan data were reviewed for evidence of movement, respiration, or other artifact, and no subjects were excluded on these grounds. Studies were completed between 1993 and 1996.
Raw detector counts combined with the input function were used to generate four flow measures at each detector site. The first of these measures, the Initial Slope Index (Model 2 ISI),19,20 is an integrated flow measure that is influenced most by perfusion of gray matter. The second and third measures are based on well-established four-compartment modeling of flow volumes, which produce estimates of faster-clearing (fg) and slower-clearing (k2) compartments.19–22 These compartments are primarily affected by flow to gray matter (fg) or white matter (k2), respectively.23,24 Because the k2 index is more susceptible to artifact when overall flow rates are low, a ratio of fast to slow clearing flows was also computed (wg). This ratio is less susceptible to distortion under low flow conditions and can be used to confirm results obtained for either of the compartment indices (fg or k2; see 18,24).
Analyses of rCBF data were achieved in two steps. In the first step, averaged, whole brain flows were evaluated according to the four different CBF parameters: Initial Slope Index (ISI), fg, k2, and wg. Second, a detector-by-detector analysis was undertaken. In this analysis, raw counts at each detector were first “normalized” for each subject by dividing by the average flow for all detectors (mean flow/32). Normalized detector values were then used in a repeated measures multivariate analysis of variance (MANOVA) to compare flow topography between Lyme patients and controls. In the MANOVA, disease status was the between-subjects variable, and hemisphere (2 levels) and detector site (16 levels) were the within-subjects variables. An average F statistic was computed across all variables for each factor in the MANOVA. Normalized data at individual detectors were compared between groups using t tests if significant disease-status effects were found in the overall MANOVA. This analysis was performed for each of the four perfusion indices.
Neuropsychological Measures
The assessments included the Weschsler Adult Intelligence Scale (WAIS), the Wechsler Memory Scale (WMS), the Buschke Selective Reminding Test (SRT), the Controlled Verbal Fluency Test, the Wisconsin Card Sort, the Beck Depression Inventory,25 and the Zung Anxiety Scale.26 Since no neuropsychological data were available for controls, patient data were examined for consistency (e.g., the degree to which performance levels on different tests differed) and in comparison to published norms. Pearson correlations between rCBF data and neuropsychological performance data were examined.
RESULTS
Subjects
The average age of the 11 Lyme disease patients was 44.2 years (SD 12.4; range 19–63). The duration of symptoms was 59.5 months (SD 57.5; range 6–170), and the length of time since diagnosis was 18.9 months (SD 17.1; range 2–60). The duration of prior oral antibiotic treatment was 7.9 months (SD 7.3; range 0–19), and the duration of prior IV antibiotic treatment was 2.0 months (SD 1.1; range 1–4). The symptom history since the onset of Lyme disease included the following: memory loss (11/11), excessive fatigue (11/11), sleep disturbance (11/11), arthralgias (11/11), word-finding problems (10/11), headaches (9/11), radiculopathy (8/11), irritability and mood lability (8/11), recalled tick bite (5/11), physician diagnosed erythema migrans (4/11), and arthritis (4/11). Five of the 11 patients had had prior spinal taps, with 3 of the 5 revealing abnormal CSF results (2 ELISA positive for Borrelia burgdorferi and 1 with elevated protein). Eight of the 11 had had prior MRI scans, 4 of whom had abnormal results (one or more white matter hyperintensities).
Controls and patients had similar mean age, sex distribution, blood hemoglobin, pulse, and end-tidal pCO2 concentration. The controls differed from patients only in their systolic blood pressure, with both groups in a normal range. (Table 1). Exploratory correlational and covariance analyses were completed using the systolic blood pressure variable, but it was inconsistently related to blood flow measures and did not appear to exert any influence on group rCBF differences.
Regional Cerebral Blood Flow. Patients' and controls' mean flows for three rCBF parameters (ISI, fg, or k2) did not differ significantly, but significant differences in mean flow for the wg ratio were observed (Table 1). A higher wg suggests an imbalance between fast and slow clearing flows that requires further investigation. Detector-by-detector analyses were then carried out for each of the four rCBF parameters.
For the ISI measure, no effect was found for disease status alone (F[1,31] < .001, p=.999), which is consistent with the global CBF findings. However, a significant three-way interaction was found for disease status in hemisphere by detector site (F[15,465]=1.79, p=.033), indicating that patients and controls differed at specific detectors within an identifiable hemisphere. Analysis of individual normalized detector values (ISI parameter) revealed that patients and controls differed significantly at only two sites in the left hemisphere. Patients had lower relative CBF at one anterior, dorsolateral detector (F2: t[31]=2.11, p=.043), and higher relative CBF at the most posterior detector in the occipital lobe (O2: t[31]=−2.97, p=.006). This finding must be viewed with an awareness that, at an alpha level of .05, two of 32 detectors might differ by chance.
A similar omnibus analysis of the fg data produced no significant differences related to disease status in the overall MANOVA (for disease status: F[1,31] < .001, p=.999; for disease status by detector: F[15,465]=1.40, p=.141; for disease status by hemisphere: F[1,31]=.37, p=.550; for disease status by detector by hemisphere: F[15,465]=1.05, p=.403).
Analysis of the k2 data, on the other hand, produced a significant disease status by detector interaction (F[15,465]=2.30, p=.004), indicating that patients differed from controls at specific detector sites bilaterally. In the analysis of the individual detector sites, patients showed higher relative white matter CBF at a number of frontal detectors (F1 on the right: t[31]=−2.17, p=.038; F3 on the left: t[31]=−2.41, p=.022); and one occipital detector (O2 on the left: t[31]=−2.43, p=.021). They had consistently lower CBF at posterior temporal and parietal detectors (P4 on right: t[31]=2.12, p=.042 and T3 on the left: t[31]=2.59, p=.015). Overall, this measure of white matter CBF was consistently lower in posterior temporal and parietal regions on both sides of the patients' brain. If relative flows were averaged in these regions (T3, P1, P2, P3, P4), patients had lower flows both bilaterally (t[31]=3.20, p=.003) and on each side of the brain individually (right: t[31]=2.20, p=.035; left: t[31]=4.15, p < .001). Thus, in the patients' cortex there appear to be broad regions in which white matter CBF was reduced. Patient/control differences in k2 are illustrated pictorially in Figure 1.
The reduction in slow-clearing flow among the patients was confirmed in the analysis of the wg ratio at individual detector sites, where there was a main effect for disease status in the overall MANOVA (F[1,31]=5.08, p= .031). Significant patient/control differences (all t[31] > 2.04, p < .05) are found at 19 of the 32 detector sites (F1, F2, F3, F4, C1, C2, P1, P2, P3, P4 on right, F1, F3, C1, C2, T1, T3, P1, P2, P3 on left). All are in the direction of patients having a higher wg.
Flow deficits do not appear to be a reflection of the small white matter hyperintensities that were observed in some subjects on MRI, since a comparison of those with (n=4) and without (n=4) hyperintensities revealed no differences. Overall, higher wg in patients appears to reflect a reduction in slow-clearing flows, since there was no evidence of any elevation in fast-clearing flows at any detector site. Normalized values of k2 appear higher in anterior regions, but this appears to be an artifact of the normalization procedure: when large areas of cortex are poorly perfused (e.g., posterior regions in patients), relative flow to other areas—as a percent of the total—will be elevated.27
Neuropsychological Findings
On the WAIS-R, the Lyme patients' IQs fell within a normal range and were generally consistent (Table 2). Clinically significant reductions in verbal memory were noted, on both the WMS-R and the Buschke Selective Reminding Test. For the group as a whole, both Verbal Fluency and Wisconsin Card Sort performance were within normal limits.
On the Beck Depression Inventory, the mean of 16.4 (SD 12.8) placed the Lyme patients in the “mild depression” severity category. Although the majority of patients had scores consistent with either “none” or “mild” depression (7/11), two cases had borderline-moderate depression, and two had more severe depressive scores. On the Zung Anxiety Index, the mean of 55.6 (SD 11.3) was consistent with moderate anxiety for the group as a whole. Two patients had scores indicative of no anxiety, five had scores indicative of minimal to moderate anxiety, and four had scores consistent with marked to severe anxiety.
In the analysis of neuropsychological performance and rCBF, both the Buschke Total score and the Consistent Long Term Retrieval Score, were correlated with mean flow for k2 (r=.66, p=.027 for Total; r=.68, p=.022 for CLTR). In other words, improved cognitive performance was associated with improved white matter flow. This flow measure was also correlated with the degree of reduction in WAIS-R Block Design relative to Vocabulary score (r=.62, p=.041). Correlations with the averaged bilateral posterior temporal/parietal flow were similar but did not reach significance. The reduction in WAIS-R Digit Symbol was, however, significantly correlated with this k2 measure on the left side (r=.63, p=.037). Consistent associations were not observed with the wg measure. However, a trend-level negative association was noted between rCBF parameters and indices of premorbid intellectual functioning, and lower global flows were associated with higher estimates of premorbid intelligence (e.g., ISI mean flow and Verbal IQ, r=−.58, p=.062) and a tendency toward higher levels of education (r=−.45, p=.163). Both factors would attenuate the expression of neuropsychological deficits.28 With a sample this small, there is inadequate power to statistically control for their effects on performance across an entire battery.
Tests scores that correlated with mean flow for k2 were not associated with either Beck or Zung scores, and neither the mean flow for k2 nor the average flow to temporal and parietal regions was correlated with the Beck or Zung scores. If the Beck and Zung scores are partialled out of the association between test scores and rCBF, significant correlations are essentially unchanged. With both Beck and Zung scores partialled out, the Buschke total score is correlated with k2 mean flow (rpartial=.68, p=.042); Block Design deviation from Vocabulary is correlated with k2 mean flow (rpartial=.70, p=.036); and Digit Symbol deviation from Vocabulary is correlated with left-sided temporal-parietal flow (rpartial=.67, p=.048). Depression and anxiety do not appear to mediate the association between test scores and flow measures.
DISCUSSION
What is striking in this study is the finding that there is an alteration of presumed white matter blood flow in the brains of patients who have chronic Lyme Disease and complain of cognitive deficits. Each of the 11 patients in our sample reported ongoing mild to severe cognitive problems that were confirmed on objective neuropsychological testing. As a group, when compared to published norms, the Lyme patients had significant deficits in verbal memory, which was demonstrated using both the Wechsler Memory Scale and the Buschke Selective Reminding Test. Memory deficits such as these are typically seen in samples of patients with Lyme encephalopathy.2,29
Lyme patients did not differ from controls on the standard, gray matter-weighted rCBF index, which is the ISI. However, when CBF was separated into faster-clearing and slower-clearing components, the patients showed abnormalities that were restricted to slower-clearing flows, reflecting diminished white matter perfusion.18 Therefore, Lyme-related perfusion deficits, which were evident in a large portion of posterior cortex, are more likely to affect white matter. This is one of the first studies to find differences that are restricted to slow-clearing flows. Compartment modeling has generally been used to characterize decrements in fast-clearing flow associated with gray matter degeneration.10,24 The k2 index can be unstable in low flow conditions,18 but findings with k2 were confirmed by results obtained with both fg and the wg ratio. If k2 were reduced by artifact, the fg would more than likely have been reduced as well; and the wg ratio would not have differed from that of controls. A noteworthy observation is that differences in wg in this small sample of Lyme patients appear to be more widespread than differences in k2. The k2 index tends to be less sensitive than other flow indices because it varies across a smaller range of values and may actually understate the level of deficit in white-matter flows that is suggested by the wg ratio. Further studies using three-dimensional tomographic imaging techniques are clearly needed in order to resolve this discrepancy, particularly because the resolution of these techniques is less than half that of the method used here.
Global reductions in k2 were significantly correlated with the magnitude of cognitive deficits in Lyme patients, specifically in memory (Buschke Total Recall and CLTR) and visuospatial organization (Block Design). An association was also found between left-sided temporal-parietal white matter flow and poorer Digit Symbol performance. These correlational analyses were limited by the small size of the patient sample and the unusual distribution of premorbid intelligence in this sample (highest estimated premorbid intelligence in those with worst perfusion measures). Further studies with larger samples are needed in order to delineate the association between perfusion abnormalities and cognitive impairment. Because our sample size was small, the likelihood of finding statistically significant group differences was reduced (a Type II error), the possibility that the sample was unusual increased (Type I error), and the generalizability of our findings is limited.
We are well aware of the complexity of the wg and k2 findings and the difficulty of their interpretation. While some k2 results may be due to “slippage” or other artifacts, constructing an artifactual explanation that would affect the patients and not the controls is difficult. The face validity of our results is supported by two additional factors. First, as reviewed below, the literature is consistent with a neuropathological process affecting predominantly white matter. Second, the significant correlations of k2 reductions with neuropsychological test performance indicate that our physiological observations are meaningfully related to disease severity.
A similar impression regarding white matter involvement was reached in the Logigian et al SPECT study.7 In their study, hypoperfusion of the subcortical basal ganglia and white matter was a common feature in 13 patients with Lyme encephalopathy. Given that B burgdorferi has been found to preferentially injure oligodendrocytes when rat brain has been cultured in vitro, white matter hypoperfusion might reflect injury to the oligodendrocyte resulting in a secondary deafferentation of the cortical structures. A postmortem case report of a man with rapidly progressive dementia after well-documented Lyme disease is consistent with a primarily subcortical localization for the damage that may occur in patients with Lyme encephalopathy. In this case report, neuropathology revealed severe subcortical neuronal loss, neuronophagia, and gliosis, primarily in the substantia nigra and the thalalmus; but only mild cortical pathology was observed.30
Myelinated tracts comprise nearly half of the volume of adult cerebral hemispheres, and they connect gray matter structures throughout the brain.31 Diffuse white matter pathology can disrupt these ubiquitous gray matter connections and could account for deficits in neurobehavioral functions that rely upon multiple networks of interconnected neurons. Such functions would include attention, memory, visuospatial ability, complex cognition, and emotional status. A variety of primarily cerebral white matter disorders are associated with neuropsychiatric disturbances, including multiple sclerosis, Binswanger’s disease, traumatic brain injury, acquired immune deficiency syndrome (AIDS) dementia complex, and normal pressure hydrocephalus. Impaired retrieval but preserved procedural memory and encoding are most characteristic of the dementia associated with white matter disease.31 Most studies that involve larger groups of patients with Lyme encephalopathy have identified deficits in consistent, long-term retrieval but not in actual storage or in procedural memory32—findings consistent with white matter involvement. White matter disease may have a greater potential for recovery than gray matter disease, perhaps because neuronal loss is less common. Spontaneous remission can occur in Multiple Sclerosis, and resolution of MRI white matter hyperintensities, after antibiotic treatment, has been observed in Lyme disease.33
In conclusion, this cerebral blood flow study using Xenon133 demonstrated that patients with persistent Lyme encephalopathy have areas of decreased perfusion that appear to affect primarily the cerebral white matter. This decreased perfusion is associated with cognitive impairment. Future functional imaging studies that use more sophisticated tools (such as PET and/or fMRI) to examine biological and behavioral challenges need to focus on delineating white matter abnormalities in order to better characterize the pathophysiology of Lyme encephalopathy.
ACKNOWLEDGMENTS
Dr. Fallon received support from a New York State Psychiatric Institute Research Support Grant & from the Lyme Disease Association to conduct this study.
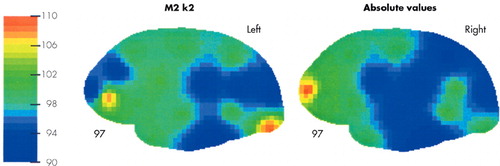
FIGURE 1. Relative white matter clearance (k2) in Lyme patients versus controls
Note: Patient flows mapped as a percentage of control flows at each detector site
 |
 |
1 Burgdorfer W: Lyme borreliosis: ten years after the discovery of the etiologic agent, Borrelia burgdoreri. Infection 1991; 4:257–262Crossref, Google Scholar
2 Logigian EL, Kaplan RF, Steere AC: Chronic neurologic manifestations of Lyme Disease. N Engl J Med 1990; 323:1438–1444Crossref, Medline, Google Scholar
3 Fallon BA, Nields JA, Burrascano JJ, et al: The neuropsychiatric manifestations of Lyme borreliosis. Psychiatr Q 1992; 63:95–115Crossref, Medline, Google Scholar
4 Coyle PK, Schutzer SE, Deng Z, et al: Detection of Bb-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 1995; 45:2010–2015Crossref, Medline, Google Scholar
5 Kruger H, Heim E, Schuknecht B, et al: Acute and chronic neuroborreliosis with and without CNS involvement: a clinical, MRI, and HLA study of 27 cases. J Neurol 1991; 238:271–280Medline, Google Scholar
6 Halperin JJ, Volkman DJ, Wu P: CNS abnormalities in Lyme neuroborreliosis. Neurology 1991; 41:1571–1582Crossref, Medline, Google Scholar
7 Logigian EL, Johnson KA, Kijewski MF, et al: Reversible cerebral hypoperfusion in Lyme encephalopathy. Neurology 1997; 49:1661–1670Crossref, Medline, Google Scholar
8 Halperin JJ, Luft BJ, Anand AK, et al: Lyme neuroborreliosis: CNS manifestations. Neurology 1989; 39:753–759Crossref, Medline, Google Scholar
9 Prohovnik I, Mayeux R, Sackeim HA, et al. Cerebral perfusion as a diagnostic marker of early Alzheimer's disease. Neurology 1988; 38:931–937Crossref, Medline, Google Scholar
10 Prohovnik I, Smith G, Sackeim HA, et al: Gray-matter degeneration in presenile Alzheimer’s disease. Ann Neurol 1989; 25:117–124Crossref, Medline, Google Scholar
11 Sackeim HA, Prohovnik I, Moeller JR, et al: Regional cerebral blood flow in mood disorders. I Comparison of major depressives and normal controls at rest. Arch Gen Psychiatry 1990; 47:60–70Crossref, Medline, Google Scholar
12 Risberg J, Gustafson L: Xe cerebral blood flow in dementia and in neuropsychiatry research, in Magistretti P, Edited by Functional radionuclide imaging of the brain. New York: Raven Press, 1983Google Scholar
13 Wechsler D: Wechsler Adult Intelligence Scale manual. San Antonio, TX: The Psychological Corporation, 1974Google Scholar
14 Wechsler D: Wechsler Memory Scale—Revised. San Antonio, TX, The Psychological Corporation. Harcourt Brace Jovanovich, 1987Google Scholar
15 Buschke H, Fuld PA: Evaluation of storage, retention, and retrieval in disordered memory and learning. Neurology 11, 1019–1025Google Scholar
16 Bechtoldt HP, Benton AL, Fogel ML: An application of factor analysis in neuropsychology. Psychological Record 1962 12: 147–156Google Scholar
17 Berg EA: A simple objective treatment for measuring flexibility in thinking. J Gen Psychol 1948 39:15–22 Google Scholar
18 Prohovnik I: Data quality, integrity, and interpretation, in Handbood of Regional Cerebral Blood Flow. Knezevic S, Maximilian VA, Mubrin Z, Prohovnik I, Wade J, Edited by Hillsdale, NJ: Lawrence Erlbaum, 1988Google Scholar
19 Prohovnik I, Knudsen E, Risberg J: Accuracy of models and algorithms for determination of fast-compartment flow by non-invasive 133-Xe clearance, in Functional radionuclide imaging of the brain, Magistretti P, Edited by New York: Raven Press, 1983, pp 87–115Google Scholar
20 Prohovnik I, Knudsen E, Risberg J: Theoretical evaluation and simulation of the initial slope index for noninvasive rCBF, in Cerebral Blood Flow and Metabolism Measurement, Hartmann A, Hoyer S, Edited by Berlin: Springer-Verlag, 1985, pp 56–60Google Scholar
21 Ingvar DH, Cronqvist S, Ekberg R, et al: Normal values of regional cerebral blood flow in man, including flow and weight estimates of gray and white matter. A preliminary study. Acta Neurol Scand Suppl 1965; 14:72–78Medline, Google Scholar
22 Obrist WD, Thompson HK, Wang HS: Regional cerebral blood flow estimated by 133-Xe inhalation. Stroke 1975; 6:245–256Crossref, Medline, Google Scholar
23 MacInnes WD, Golden CJ, Gillen RW, et al: Aging, regional cerebral blood flow, and neuropsychological functioning. J Am Geriatr Soc 1984; 32:712–718Crossref, Medline, Google Scholar
24 Alexander GE, Prohovnik I, Sackeim HA, Stern Y, Mayeux R: J Neuropsychiatry Clin Neurosci 1995; 7:188–96.Google Scholar
25 Beck AT, Ward CH, Mendelson M, et al: An inventory for measuring depression. Arch Gen Psychiatry 1961 4:561–571Google Scholar
26 Zung WW. A rating instrument for anxiety disorders. Psychosomatics 197112:371–379Google Scholar
27 Keilp JG, Prohovnik I. Intellectual decline predicts the parietal perfusion deficit in Alzheimer's disease. J Nucl Med 1995; 36:1347–1354Medline, Google Scholar
28 Stern Y, Alexander GE, Prohovnik K, et al: Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol 1992; 32:371–375Crossref, Medline, Google Scholar
29 Krupp LB, Masur D, Schwartz J, et al: Cognitive functioning in late Lyme Borreliosis. Arch Neurology 1991; 48:1125–1129Crossref, Medline, Google Scholar
30 Waniek C, Prohovnik I, Kaufman MA, et al: Rapidly progressive frontal-type dementia associated with Lyme disease. J Neuropsychiatry 1995; 7:345–347Link, Google Scholar
31 Filley CM: The behavioral neurology of cerebral white matter. Neurology 1998; 50:1535–1540Crossref, Medline, Google Scholar
32 Kaplan RF, Jones-Woodward L: Lyme encephalopathy: a neuropsychological perspective. Semin in Neurol 1997; 17:31–37Crossref, Medline, Google Scholar
33 Fallon BA, Kochevar JM, Gaito A, et al: The underdiagnosis of neuropsychiatric Lyme Disease in children and adults. The Psychiatric Clin North Am 1998; 21:693–703Crossref, Medline, Google Scholar



