Accelerated Memory Decline in Alzheimer's Disease With Apolipoprotein ϵ4 Allele
Abstract
To investigate a possible effect of the apolipoprotein (APOE) ϵ4 allele on memory decline in Alzheimer's disease (AD), we examined 64 AD patients with the APOE ϵ3/3, ϵ3/4, or ϵ4/4 allele using the Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog) and its subtests at the initial examination and at the 1-year follow-up visit. One-year changes in the scores of the Word Recall subtest, Word Recognition subtest, and total ADAS-Cog were significantly correlated with the number of APOE ϵ4 alleles after controlling for the effects of age, sex, education, test interval, and baseline scores. Findings revealed that APOE ϵ4 allele is related to an accelerated memory decline in AD.
The apolipoprotein (APOE) ϵ4 allele is a well-known risk factor for developing Alzheimer's disease (AD) and lowers the age at onset in a dose-dependent fashion1–4 (i.e., the risk for developing AD becomes bigger and the age at onset becomes lower as the number of APOE ϵ4 alleles increase). These facts suggest that the APOE ϵ4 allele accelerates the degenerative process for developing AD and leads to the hypothesis that cognitive decline in AD should progress more rapidly in patients who carry this allele. However, this latter issue (i.e., whether the APOE ϵ4 allele is associated with a faster rate of cognitive decline) remains open to debate. While some studies have found such a relationship,5,6 others have not,7–18 and some studies have even reported a converse effect.19,20
Among the elderly, both those who are healthy21 and those who suffer from mild cognitive impairment,22 the APOE ϵ4 allele has been reported to be associated with greater memory decline. Longitudinal change in hippocampal volume is also reportedly greater in ϵ4-positive subjects than in ϵ4-negative subjects in nondemented elderly23 and in patients with AD.18 Several cross-sectional studies of patients with AD have reported that the APOE ϵ4 allele is associated with poorer memory function24,25 and more severe atrophic changes24,26–29 and hypometabolism30 in the medial temporal lobe structures. At autopsy, the APOE ϵ4 allele is also reported to be associated with greater densities of Aβ deposition, senile plaques, and neurofibrillary tangles, especially in the hippocampus.31,32 These longitudinal and cross-sectional studies suggest that the APOE ϵ4 allele mainly affects the medial temporal lobe and memory function. If so, the possible effect of APOE ϵ4 allele on the rate of cognitive decline may be detected in one neuropsychological test but not in others. It is noteworthy that most studies using the Mini-Mental State Examination (MMSE),33 which is one of the most common tests, to examine the effect of the APOE ϵ4 allele on the rate of cognitive decline7–9,11–14 failed to find a positive effect. The failure might be explained by the property of MMSE, which is highly susceptible to floor effects in the memory items on the test.
Another possible explanation for the failure to demonstrate the effect of the APOE ϵ4 allele on the rate of cognitive decline in previous studies is the disregard for the dose effect of the APOE ϵ4 allele (ϵ3/3, ϵ3/4, or ϵ4/4 alleles). The dose effect of the APOE ϵ4 allele on developing AD has been reported in several studies.1–4 A dose effect of the APOE ϵ4 allele is highly expected in the rate of cognitive decline in AD. Most studies based on the presence/absence of the APOE ϵ4 allele7,9,11,14,15–18 have also failed to demonstrate the effect of the APOE ϵ4 allele on the rate of cognitive decline. An analysis in which a dose effect is taken into consideration would be more sensitive to detect the effect of the APOE ϵ4 allele on cognitive decline than an analysis based on its presence or absence.
The Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog), in which approximately 40% of the possible total points pertained to memory items,34 is very sensitive to memory dysfunction and commonly used in clinical drug trials. The aim of this longitudinal study was to elucidate the possible effect of the APOE ϵ4 allele on the rate of cognitive decline using the ADAS-Cog and its subtests. Additionally, we used a sensitive statistical method in order to take the dose effect into consideration.
METHODS
This study was conducted at the infirmary of the Hyogo Institute for Aging Brain and Cognitive Disorders (HI-ABCD), a research-oriented hospital for dementia. All procedures followed the 1993 Clinical Study Guidelines of the Ethical Committee of HI-ABCD, and were approved by the Institutional Review Board. Written, informed consent was obtained from all participants and their caregivers according to the Declaration of Human Rights, Helsinki, 1975.
Subjects
Among patients who were given short-term admission into the infirmary of the HI-ABCD for the examination and management of cognitive impairments from August 1993 to October 1999, we selected 64 AD patients aged 60 or older who fulfilled the criteria of the National Institute of Neurological Disease and Stroke/Alzheimer's Disease and Related Disorders Association for probable AD35 and had any one of the APOE ϵ3/3, ϵ3/4, or ϵ4/4 alleles. They were given follow-up ADAS-Cog tests after an interval of 1-year (11-13 months). At the initial examination, all patients were examined by both neurologists and psychiatrists using standardized medical history inquiries, neurological examinations, routine laboratory tests, standard neuropsychological examinations, electroencephalography, magnetic resonance imaging (MRI) of the brain, magnetic resonance (MR) angiography of the neck and head, and cerebral perfusion/metabolism studies using position emission tomography (PET) or single photon emission tomography (SPECT), which were all incorporated in the diagnosis. None of the patients had any other medical illnesses that might cause cognitive impairment, including thyroid diseases, vitamin deficiencies, and malignant diseases, or the complication of developmental abnormalities, mental diseases, substance abuse, or significant neurological antecedents such as brain traumas, brain tumors, epilepsies, and inflammatory diseases. None of the patients showed focal brain lesions on MR imaging, including lacunar infarcts, hematoma, and obvious autosomal dominant transmission traits. We began conducting the study before the use of donepezil hydrochloride in Japan. (Donepezil hydrochloride is the only anti-AD drug that is presently being approved for use in Japan). None of the patients received any anti-AD drugs during the follow-up period.
Cognitive Measures
To evaluate cognitive change, ADAS-Cog was administered by trained psychometrists at the initial examination and at the 1-year (11-13 months) follow-up visit. ADAS-Cog is scored by errors (with a total error score range of 0 to 70). Therefore, a higher score indicates a poorer performance. The test consists of the following 11 subtests: Word Recall, Word Recognition, Orientation, Recall of Test Instructions, Following Commands, Naming Objects and Fingers, Word Finding Difficulty, Spoken Language Ability, Comprehension, Construction, and Praxis. The maximum error point in each subtest is shown in Table 2. We also calculated the subtotal of the language subtests, such as Following Commands, Naming Objects and Fingers, Word Finding Difficulty, Spoken Language Ability, and Comprehension.
Determination of APOE Genotype
The detailed method for APOE genotyping is described elsewhere.36 In brief, genomic DNA was extracted from peripheral blood with a Genomix deoxyribonucleic acid (DNA) extraction kit (Talent Corp., Trieste, Italy) according to the manufacturer's protocol. The APOE genotype was determined using polymerase chain reaction-restriction fragment length polymorphism according to the procedure described by Wenham et al.37
Statistical Analyses
The differences in the demographic variables and baseline ADAS-Cog scores among these three APOE genotype groups were tested by the Kruskal-Wallis test, Spearman rank correlation coefficients, and the χ2 test. Since a dose-effect of the APOE ϵ4 alleles is postulated,1–4 we tested the relationship between 1-year changes of scores of ADAS-Cog and its subtests and the number of APOE ϵ4 alleles by using the Spearman rank correlation coefficients before and after controlling for the effects of age, sex, education, test interval, and baseline scores. The significance level was set at p > 0.05. All statistical analyses were conducted on SAS release 6.10 (SAS Institute Inc., Cary, NC).
RESULTS
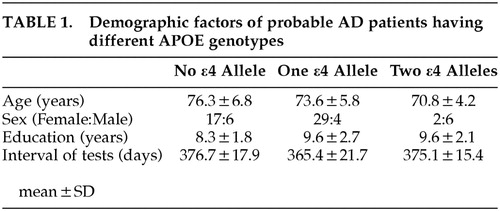
The mean age of the patients was 74.2 ± 6.2 (SD) years for 48 women and 16 men. The mean educational level was 9.1 ± 2.4 years, and the mean interval of tests was 370.7 ± 20.2 days. Twenty-three patients had APOE ϵ3/3, 33 had APOE ϵ3/4, and eight had ϵ4/4 allele. Table 1 summarizes the demographic factors of each APOE genotype. Age showed a significant negative correlation with APOE ϵ4 dose (rs = -0.28, p = 0.02). The sex ratio of the patients with APOE ϵ4/4 significantly differed from that of the other two genotype patients groups (χ2 (df = 2) = 13.6, p = 0.011). Males were predominant in the patients with the APOE ϵ4/4, whereas females were predominant in the patients with the APOE ϵ3/3 and 3/4. There were no significant differences in the educational level or in the test interval among the three groups of patients.
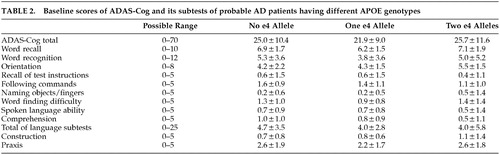
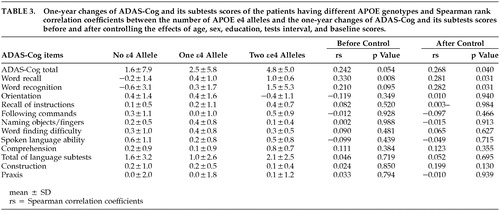
The baseline scores of ADAS-Cog and its subtests were not significantly different among the three groups (Table 2). Table 3 summarizes the 1-year changes of ADAS-Cog and its subtests scores of each genotype group and shows the Spearman rank correlation coefficients between these changes and the number of APOE ϵ4 alleles before and after controlling the effects of the possible confounders including age, sex, education, test interval, and baseline scores. A significant positive correlation was noted between the number of APOE ϵ4 alleles and the one-year change of the Word Recall subtest score. This correlation remained significant after controlling the effects of possible confounders. The 1-year changes of the Word Recognition subtest score and of the total ADAS-Cog score were significantly correlated with the number of APOE ϵ4 alleles after controlling the effects of the possible confounders. None of the other subtests, including the subtotal score of the language subtests, showed significant correlation with the number of APOE ϵ4 alleles.
DISCUSSION
The present study demonstrated that the number of APOE ϵ4 alleles was significantly correlated with cognitive decline in AD over 1 year, as measured by the ADAS-Cog total score and memory subtest scores. The rates of decline of the subtests of language, construction, and praxis disturbances were not correlated with the number of APOE ϵ4 alleles. In our subjects, mean ages negatively correlated with APOE ϵ4 number and probably reflected the effects of APOE ϵ4 on disease onset; the proportion of males was different among the APOE ϵ4 status groups. However, the different ages and sex distributions among the APOE ϵ4 status groups are not likely to account for the effect of APOE ϵ4 allele on the rate of the test performance decline because statistically controlling these variables did not alter the results. Furthermore, the rate of cognitive decline in AD patients, as measured by the ADAS-Cog, is reportedly independent of sex and age.38 Our finding is compatible with the findings in previous cross-sectional studies,24–30 which reported that poorer memory function and more severe medial temporal atrophy and hypometabolism are associated with the APOE ϵ4 allele. Our finding is also compatible with our previous longitudinal MRI volumetric study, which demonstrated that the APOE ϵ4 allele is associated with rapid progression of hippocampal atrophy in AD patients.18 Memory decline is reportedly found in subjects carrying the APOE ϵ4 allele, even before developing dementia.21,22 Taken together, these results suggest that the effect of the APOE ϵ4 allele on the cognitive disturbances in AD patients is domain specific (i.e., the effect is predominant in memory functions and is found both before and after the development of dementia).
It is noteworthy that the rate of progression of the total ADAS-Cog score was also significantly influenced by the APOE genotypes after controlling the effects of age, sex, education, test interval, and baseline scores. Because memory tests account for almost 40% of the ADAS-Cog total score, this result is not surprising. ADAS-Cog has been used as the principal measure of cognitive outcome in clinical trials of potential treatments for AD and is even recommended to be used as the primary outcome measure by the U.S. Food and Drug Administration.39 Our findings suggest that the effects of the APOE ϵ4 allele on the change of ADAS-Cog scores should be taken into consideration in clinical trials and longitudinal studies.
In conclusion, the APOE ϵ4 allele plays an important role not only in the development of AD but also in the progression of certain aspects of cognitive functions. The APOE ϵ4 allele is significantly associated with an accelerated memory decline in AD in a dose-dependent fashion. Although the effect of the APOE ϵ4 on the decline of ADAS-Cog is modest, the longitudinal change of ADAS-Cog would be substantially affected by the number of APOE ϵ4 alleles. Therefore, the effect of the APOE ϵ4 allele on ADAS-Cog should be considered in longitudinal studies, including clinical drug trials. The APOE ϵ4 allele would be recommended to be determined as a significant confounding variable affecting patients' cognitive outcome in clinical trials.
 |
 |
 |
1 Corder EH, Saunders AM, Strittmatter WJ, et al: Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993; 261:921–923Crossref, Medline, Google Scholar
2 Poirier J, Davignon J, Bouthillier D, et al: Apolipoprotein E polymorphism and Alzheimer's disease. Lancet 1993; 342:697–699Crossref, Medline, Google Scholar
3 Lucotte G, Turpin JC, Landais P: Apolipoprotein E-ϵ4 allele doses in late-onset Alzheimer's disease. Ann Neurol 1994; 36:681–682Crossref, Medline, Google Scholar
4 Blacker D, Haines JL, Rodes L, et al: ApoE-4 and age at onset of Alzheimer's disease: The NIMH Genetics Initiative. Neurology 1997; 48:139–147Crossref, Medline, Google Scholar
5 Craft S, Teri L, Edland SD, et al: Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer's disease. Neurology 1998; 51:149–153Crossref, Medline, Google Scholar
6 Kanai M, Shizuka M, Urakami K, et al: Apolipoprotein E4 accelerates dementia and increases cerebrospinal fluid tau levels in Alzheimer's disease. Neurosci Lett 1999; 267:65–68Crossref, Medline, Google Scholar
7 Basun H, Grut M, Winblad B, et al: Apolipoprotein epsilon 4 allele and disease progression in patients with late-onset Alzheimer's disease. Neurosci Lett 1995; 183:32–34Crossref, Medline, Google Scholar
8 Dal Forno G, Rasmusson DX, Brandt J, et al: Apolipoprotein E genotype and rate of decline in probable Alzheimer's disease. Arch Neurol 1996; 53:345–350Crossref, Medline, Google Scholar
9 Kurz A, Egensperger R, Haupt M, et al: Apolipoprotein E epsilon 4 allele, cognitive decline, and deterioration of everyday performance in Alzheimer's disease. Neurology 1996; 47:440–443Crossref, Medline, Google Scholar
10 Growdon JH, Locascio JJ, Corkin S, et al: Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology 1996; 47:444–448Crossref, Medline, Google Scholar
11 Holmes C, Levy R, McLoughlin DM, et al: Apolipoprotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer's disease. J Neurol Neurosurg Psychiatry 1996; 61:580–583Crossref, Medline, Google Scholar
12 Murphy GM Jr, Taylor J, Kraemer HC, et al: No association between apolipoprotein E epsilon 4 allele and rate of decline in Alzheimer's disease. Am J Psychiatry 1997; 154:603–608Crossref, Medline, Google Scholar
13 Lehtovirta M, Kuikka J, Helisalmi S, et al: Longitudinal SPECT study in Alzheimer's disease: relation to apolipoprotein E polymorphism. J Neurol Neurosurg Psychiatry 1998; 64:742–746Crossref, Medline, Google Scholar
14 Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, et al: Apolipoprotein E genotype and progression of Alzheimer's disease: the Rotterdam Study. J Neurol 1999; 246:304–308Crossref, Medline, Google Scholar
15 Farlow MR, Cyrus PA, Nadel A, et al: Metrifonate treatment of AD: influence of APOE genotype. Neurology 1999; 53:2010–2016Crossref, Medline, Google Scholar
16 Raskind MA, Peskind ER, Wessel T, et al: Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. Neurology 2000; 54:2261–2268Crossref, Medline, Google Scholar
17 Aerssens J, Raeymaekers P, Lilienfeld S, et al: APOE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer's disease. Dement Geriatr Cogn Disord 2001; 12:69–77 Crossref, Medline, Google Scholar
18 Mori E, Lee K, Yasuda M, et al: Accelerated hippocampal atrophy in Alzheimer's disease with Apolipoprotein E ϵ4 allele. Ann Neurol 2002; 51:209–214Crossref, Medline, Google Scholar
19 Frisoni GB, Govoni S, Geroldi C, et al: Gene dose of the epsilon 4 allele of apolipoprotein E and disease progression in sporadic late-onset Alzheimer's disease. Ann Neurol 1995; 37:596–604Crossref, Medline, Google Scholar
20 Stern Y, Brandt J, Albert M, et al: The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer's disease. Ann Neurol 1997; 41:615–620Crossref, Medline, Google Scholar
21 Mayeux R, Small SA, Tang M, et al: Memory performance in healthy elderly without Alzheimer's disease: effects of time and apolipoprotein-E. Neurobiol Aging 2001; 22:683–689Crossref, Medline, Google Scholar
22 Dik MG, Jonker C, Bouter LM, et al: APOE-epsilon4 is associated with memory decline in cognitively impaired elderly. Neurology 2000; 54:1492–1497Crossref, Medline, Google Scholar
23 Moffat SD, Szekely CA, Zonderman AB, et al: Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 2000; 55:134–136Crossref, Medline, Google Scholar
24 Lehtovirta M, Laakso MP, Soininen H, et al: Volumes of hippocampus, amygdala and frontal lobe in Alzheimer's patients with different apolipoprotein E genotypes. Neuroscience 1995; 67:65–72Crossref, Medline, Google Scholar
25 Lehtovirta M, Soininen H, Helisalmi S, et al: Clinical and neuropsychological characteristics in familial and sporadic Alzheimer's disease: relation to apolipoprotein E polymorphism. Neurology 1996; 46:413–419Crossref, Medline, Google Scholar
26 Lehtovirta M, Soininen H, Laakso MP, et al: SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E ϵ4 allele. J Neurol Neurosurg Psychiatry 1996; 60:644–649Crossref, Medline, Google Scholar
27 Geroldi C, Pihlajamaki M, Laakso MP, et al: APOE-epsilon4 is associated with less frontal and more medial temporal lobe atrophy in AD. Neurology 1999; 53:1825–1832Crossref, Medline, Google Scholar
28 Bigler ED, Lowry CM, Anderson CV, et al: Dementia, quantitative neuroimaging, and apolipoprotein E genotype. AJNR Am J Neuroradiol 2000; 21:1857–1868Medline, Google Scholar
29 Hashimoto M, Yasuda M, Tanimukai S, et al: Apolipoprotein E ϵ4 and the pattern of regional brain atrophy in Alzheimer's disease. Neurology 2001; 57:1461–1466Crossref, Medline, Google Scholar
30 Hirono N, Hashimoto M, Yasuda M, et al: The effect of APOE e4 allele on cerebral glucose metabolism in AD is a function of age at onset. Neurology 2002; 58:743–750Crossref, Medline, Google Scholar
31 Schmechel DE, Saunders AM, Strittmatter WJ, et al: Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993; 90:9649–9653Crossref, Medline, Google Scholar
32 Berg L, McKeel DW Jr, Miller JP, et al: Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol 1998; 55:326–335Crossref, Medline, Google Scholar
33 Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state for the clinician. J Pschiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
34 Mohs RC, Rosen WG, Davis KL: The Alzheimer's Disease Assessment Scale; An instrument for assessing treatment efficacy. Psychopharmacol Bull 1983; 19:448–450Medline, Google Scholar
35 McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984; 34:939–944Crossref, Medline, Google Scholar
36 Yasuda M, Maeda K, Shimada K, et al: Apolipoprotein E ϵ4 allele and gender difference in risk of Alzheimer's disease. Alzheimer Res 1995; 1:77–81Google Scholar
37 Wenham PR, Price WH, Blandell G: Apolipoprotein E genotyping by one-stage PCR. Lancet 1991; 337:1158–1159Crossref, Medline, Google Scholar
38 Stern RG, Mohs RC, Davidson M, et al: A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry 1994; 151:390–396Crossref, Medline, Google Scholar
39 Mayeux R, Sano M: Treatment of Alzheimer's disease. N Engl J Med 1999; 341:1670–1679Crossref, Medline, Google Scholar



