Mesial Temporal Structures and Comorbid Anxiety in Refractory Partial Epilepsy
Abstract
We used quantitative magnetic resonance imaging (MRI) to examine the mesial temporal structures in subjects with refractory partial epilepsy and comorbid anxiety and found preservation with a right-sided preponderance. The findings indicate a role for this brain region in the genesis of anxiety.
The role of the mesial temporal structures in the genesis of refractory partial epilepsy1 and psychopathology2 is well recognized.
Previous quantitative magnetic resonance imaging (MRI) studies have shown that the mesial temporal structures are preserved or enlarged in subjects with refractory partial epilepsy and comorbid psychopathology, when compared to those without psychopathology and normal controls.3,4 This contrasts with the decrease in mesial temporal structure volumes that is observed over time in refractory epilepsy and the correlation between the degree of mesial temporal, sclerosis, and the duration of epilepsy.5
Based on our previous findings,3,4 we hypothesized that mesial temporal structure volumes in subjects with refractory partial epilepsy and comorbid anxiety would be preserved when compared to subjects with epilepsy and without psychopathology. We compared mesial temporal structure volumes in patients with refractory partial epilepsy, both with and without comorbid anxiety, as well as normal controls.
METHODS
Subjects resident at the National Society for Epilepsy were interviewed (ESK) using Schedules for Clinical Assessment in Neuropsychiatry (SCAN).6 All data were acquired as part of routine care and are covered by normal clinical consent protocols. Approval for this study was obtained from the relevant ethics committee.
Subjects were rated to have clinical anxiety if SCAN (anxiety) crossed the cut-off, and at least three disabling anxiety symptoms were reported, whether or not ICD-10 diagnostic criteria for anxiety disorders were met. This algorithm was based on both experience that subjects with epilepsy seldom fulfil rigid diagnostic criteria for psychiatric disorder and precedents in psychiatric research for using operationalized rules when clinician ratings do not concur with diagnostic criteria.7
Only subjects with refractory partial epilepsy (with or without secondary generalization) were included. Subjects with primary generalized epilepsy, moderate or severe learning disability, and previous brain surgery were excluded. Subjects with psychopathology other than anxiety were also excluded. A neurologist (M.K.) made the diagnosis of localization related epilepsy based on seizure semiology, electroencephalography (EEG) and MRI scan.
The magnetic resonance (MR) images were obtained on a 1.5 T General Electric (GE) Sigma scanner and volumetric measurements performed with interactive software (MRreg),8 using methods that have been described in detail elsewhere.3,4 The rater (PS) was blind to subject grouping.
Analyses:
Data were analyzed using the Statistical Program for Social Sciences (SPSS) (Windows version 8.0). Subjects were divided into a) no psychopathology group and b) comorbid anxiety group for the purpose of the analysis.
The intrarater reliability was assessed using two different methods to enable comparison with previously published volumetric data: a) the ratio of measured standard deviations to average amygdala volumes and b) the coefficient of repeatability.9,10
We compared corrected amygdala and hippocampus volumes in the two groups using t test. A threshold of p < 0.05 (95% confidence interval) was used to determine significance. Age at onset and duration of epilepsy in the two groups were compared using analysis of variance (ANOVA).
RESULTS
Of 66 subjects who were interviewed, 14 were diagnosed as having clinically significant anxiety based on criteria described herein. Ten of the subjects met inclusion criteria for this study, of which two were excluded for unsatisfactory scan quality. Eight subjects with anxiety and eight others with no psychopathology (patient control group) were matched for age and sex. The two groups were compared with a control group that consisted of 15 volunteers.
The mean age of the anxiety group was 59.1, and that of the no psychopathology group was 50.87 the difference not being statistically significant. There were six males and two females in both groups.
The onset of epilepsy was significantly earlier in the anxiety group than in the no psychopathology group (p < 0.05). Subjects with anxiety also had a significantly longer duration of epilepsy when compared to subjects in the no psychopathology group (p < 0.001).
Amygdala and hippocampal volumes were measured and corrected for intracranial volume, as described.4 The coefficient of variation expressed as a percentage of mean volume was 6.15%. The coefficient of repeatability was 11.2% of the overall mean.
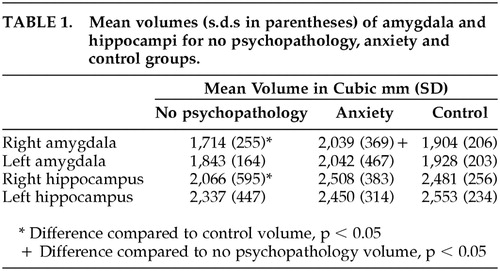
The mean amygdala and hippocampal volumes, along with the standard deviations for both the groups and the controls, are given in Table 1. Amygdala and hippocampal volumes were smaller bilaterally in the no psychopathology group when compared to the control group, although this difference was statistically significant only on the right (r amygdala, t = 1.96, p < 0.05; r. hippocampus, t = 1.73. p ≤ 0.05). For the anxiety group, amygdala and hippocampal volumes were no different from controls. When the anxiety group and the no psychopathology group were compared, mean amygdala volumes in the anxiety group were greater bilaterally, although the difference was statistically significant only on the right (t = p < 0.05).
When subjects in the anxiety group were subdivided based on ICD-10 criteria into those with and without anxiety disorder (four in each group), and mean amygdale volumes were compared among these two groups and the no psychopathology group, a trend of increasing volumes was observed with amygdala being smallest in the no psychopathology group, larger in the clinically significant anxiety group (not meeting ICD-10 criteria for an anxiety disorder), and largest in the anxiety disorder group (meeting ICD-10 criteria). However, the small sample sizes prevent meaningful statistical analysis of these group differences.
DISCUSSION
We have shown that the volume of the amygdala and hippocampus is preserved in subjects with refractory partial epilepsy and anxiety, in contrast to patients with epilepsy and no associated psychopathology. This is despite the significantly earlier seizure onset and longer duration of epilepsy in this group. Our findings are in line with recent literature linking these structures, the amygdala in particular, to generalized anxiety disorder11 and subjective anxiety in social phobia12 and are unlikely therefore to be specific to refractory epilepsy.
Despite the small numbers, our data have shown a laterality difference in mesial temporal structure volumes in patients with comorbid anxiety. Such a right-sided preponderance has been demonstrated in functional imaging studies of subjects with anxiety disorder13,14 and in a volumetric study of the amygdala in generalized anxiety disorder.15 This finding is therefore consistent with the general trend linking emotionality with the nondominant hemisphere and may indicate an expanded role for this hemisphere in the genesis of psychopathology.
Several hypotheses may be considered in attempting to explain these findings. On the one hand, it may be that the presence of comorbid anxiety in epilepsy countermands neuronal loss in the mesial temporal structures, resulting in the preservation of these structures. On the other hand, it is possible that epilepsy with comorbid anxiety is a distinct syndrome associated with preservation or even increase in mesial temporal structure volumes. However, this being a cross-sectional study, one can only speculate about the direction of causality, and prospective cohort studies will need to be performed to clarify these issues. Further, the small sample size and our decision to assess for clinical significance of symptoms, as opposed to diagnosis, limit our ability to subtype anxiety and study its natural history, which we would need to do in order to assess the direction of causality.
The finding of an association between long duration of epilepsy and the presence of comorbid anxiety is of interest. Both neurobiological and psychosocial processes may explain and contribute to this association. From the biological viewpoint, the limbic structures could potentially play a role in the genesis of both disorders (epilepsy and anxiety). On the other hand, a long history of refractory epilepsy would confer significant uncertainty, fear of seizures, restrictions in lifestyle, and considerable social disadvantage on these individuals. Such a climate of psychological adversity is commonly associated with the development of anxiety, thus explaining this association.
Although the sample sizes in our study are small, this is a pilot study design and small sizes, if anything, should obscure significant findings. The methodology has been published earlier and is sound and valid in estimating amygdala, hippocampal, and total brain volumes.3,4 As the rater was blinded to psychopathology variables, we do not expect any rater bias to confound these results. Further, psychopathology was assessed carefully and systematically by an expert, and the study groups are expected to be homogeneous in their selection.
Confounding factors include the location of the seizure focus, other risk factors for epilepsy, and the role of antiepileptic drug therapy, and these have not been accounted for. Additionally, as there are other studies linking psychopathology in epilepsy with mesial temporal sclerosis as opposed to preservation of the mesial temporal structures,16 these findings need to be interpreted with caution.
CONCLUSION
The association we have observed between anxiety and amygdala volumes, especially on the right side, is interesting and probably suggests a role for this structure, in the genesis of anxiety. These findings may be explored further in longitudinal studies involving larger sample sizes that also differentiate between trait and state anxiety.
 |
1 Fish DR, Spencer SS: Clinical correlations: MRI and electroencephalogram. Magn Reson Imaging 1995; 13:1113–1117Crossref, Medline, Google Scholar
2 Stevens JR: Psychiatric aspects of epilepsy. J Clin Psychiatry. 1988; 49(suppl):49–57Google Scholar
3 Tebartz van Elst L, Woermann FG, Lemieux L, et al: Amygdala enlargement in dysthymia—a volumetric study of subjects with temporal lobe epilepsy. Biol Psychiatry 1999; 46:1614–1623Crossref, Medline, Google Scholar
4 Tebartz van Elst L, Woermann FG, Lemieux L, Thompson PJ, et al: Affective aggression in subjects with temporal lobe epilepsy: a quantitative MRI study of the amygdale. Brain 2000; 123:234–243Crossref, Medline, Google Scholar
5 Cendes F, Andermann F, Gloor P, et al: Relationship between atrophy of the amygdala and hippocampus in temporal lobe epilepsy. Brain 1994; 117:739–746Crossref, Medline, Google Scholar
6 Schedules for Clinical Assessment in Neuropsychiatry (SCAN) (1992). World Health Organization, Division of Mental Health, GenevaGoogle Scholar
7 Prince MJ: Measurement in psychiatry. Int. Rev. Psych. 1998; 10(4):264–271Google Scholar
8 Lemieux L, Weishmann UC, Moran NF, et al: The detection and significance of subtle changes in mixed signal brain lesions by serial MRI scan matching and spatial normalization. Med Image Anal 1998; 2:227–242Crossref, Medline, Google Scholar
9 Cendes F, Andermann F, Gloor P, et al: MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 1993; 43:719–725Crossref, Medline, Google Scholar
10 Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1:307–310Crossref, Medline, Google Scholar
11 Chua P, Krams M, Toni I, et al: A functional anatomy of anticipatory anxiety. Neuroimage 1999; 9:563–571Crossref, Medline, Google Scholar
12 Tillfors M, Furmark T, Marteinsdottir I, et al: Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001; 158(8):1220–1226Crossref, Medline, Google Scholar
13 Reiman EM, Raichle, Robins E, Mintun MA, et al: Neuroanatomic correlates of lactate induced anxiety attack. Arch Gen Psychiatry 1989; 46:493–500Crossref, Medline, Google Scholar
14 Nordahl TE, Stein MB, Benkelfat C, et al: Regional cerebral metabolic asymmetries replicated in an independent group of subjects with panic disorders. Biol Psychiatry 1998; 44:998–1006Crossref, Medline, Google Scholar
15 De Bellis, Casey BJ, Dahl RE, et al: A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biol Psychiatry 2000; 48(1):51–57Crossref, Medline, Google Scholar
16 Quiske A, Helmstaedter C, Lux S, et al: Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res 2000; 39(2):121–125Crossref, Medline, Google Scholar



